Translate this page into:
Management of thromboembolic disorders during pregnancy in resource-constrained settings: An Indian perspective
For correspondence: Dr Pankaj Malhotra, Department of Internal Medicine, Postgraduate Institute of Medical Education & Research, Chandigarh 160 012, India e-mail: malhotrapankaj@hotmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Venous thromboembolism (VTE) in pregnancy and resulting thrombotic disorders are increasingly being recognized as an important cause of maternal morbidity and mortality. The diagnosis of VTE during pregnancy has an impact on current as well as future foeto-maternal outcomes. Whereas algorithms to manage VTEs during pregnancy in developed countries exist, these are difficult to implement in resource-constraint settings. In this narrative review, we discuss strategies that can be applied in daily clinical practice by obstetricians and haematologists dealing with these disorders in the country.
Keywords
Anticoagulation
APLA
pregnancy
resource-constrained
thrombosis
venous thromboembolism
Management of thrombotic disorders poses a unique challenge during pregnancy. The risk of VTE (venous thromboembolism) in pregnancy is increased to five times in the antepartum and 60 times in postpartum period as compared to age matched non-pregnant women1,2. Studies in western population have reported incidence rates of thromboembolism (TE) ranging from 18-95 events per 100,000 woman-years during pregnancy3. VTE has been the direct and leading cause of maternal death in the UK since 19854. The available figures from India suggest that 0.1 per cent of pregnant women present with symptomatic deep vein thrombosis (DVT)5. Deaths due to pulmonary embolism (PE) account for 3.75 per cent of the total maternal mortality in India, while this proportion is three times higher in the western populations6,7; the lower incidence in India is probably due to underreporting of VTE. However, the prevalence of VTE is likely to increase in India due to increasing age of pregnancy, increasing prevalence of obesity and use of artificial reproductive techniques8. In this review, the approach to the management of pregnancy-associated VTE in the Indian setting is discussed.
The incidence of VTE during pregnancy is reportedly equal in all trimesters9. Approximately two third of all pregnancy associated VTE occur antepartum. If DVT is left untreated, it progresses to pulmonary embolism (PE) in 15-27 per cent of cases, which can be fatal in 15-30 per cent of cases3. In the context of pregnancy, death can occur within half an hour of an embolic event in 66 per cent of cases10,11. Therefore, a high index of suspicion is required for diagnosing VTE. DVT in pregnancy involves the left lower limb in 90 per cent of cases and is more likely to be proximal12. Upper extremity DVT is generally seen in the context of ovarian hyperstimulation syndrome and the use of assisted reproduction techniques. The incidence of cerebral venous thrombosis (CVT) is 3.7/1000 obstetric admissions and commonly occurs in the third trimester and puerperium. CVT contributes up to 20 per cent of the VTE-associated maternal mortality4,13.
Diagnosis of VTE during pregnancy
Diagnosis of VTE starts with a clinical suspicion. The differential diagnosis of DVT includes a ruptured Baker’s cyst, muscle injury, cellulitis and acute post-thrombotic syndrome. The modified Wells pre-test probability score, that is often used in non-pregnant setting, is not validated in pregnancy. Similarly, the isolated use of D-dimer testing to rule out VTE is not recommended in pregnancy as D-dimer levels are generally high during pregnancy. A clinical prediction rule called the LEFt rule [symptoms in the left leg (L): 1 point], [calf circumference difference equal or greater than 2 cm oedema (E): 1 point] and [first-trimester presentation (Ft): 1 point] has been developed for pregnancy setting but currently lacks prospective validation. The rule helps in accurately classifying pregnant women with increasing proneness of DVT; however, further investigations should be done to rule out a DVT. In patients who are suspected to have DVT as per symptoms or the LEFt rule, compression duplex Doppler is the investigation of choice. If the initial Doppler is negative, serial duplex Doppler can diagnose VTE in additional 24 per cent of cases.
As majority of the lower limb DVT involves the sapheno-femoral veins, Doppler of the sapheno-femoral veins suffices in most cases. In patients who present with unilateral buttock pain and swelling of whole limb, Doppler of the iliac veins is recommended to rule out iliac vein thrombosis. Routine use of magnetic resonance venography in pregnancy for diagnosing VTE may not be feasible considering the cost and limited availability in resource constraint settings. Therefore, serial compression Doppler for the diagnosis of VTE in pregnancy as per LEFt rule is recommended for most healthcare settings across the country.
Clinicians are often confronted with the question of foeto-maternal risk due to radiation exposure required for the definitive diagnosis of PE in pregnancy. Beyond the first trimester, chest X-ray is useful as it can exclude other aetiologies in a resource-constraint setting. Similarly, an ECG too, if abnormal, can give important information. An algorithm called YEARS can help in making treatment decision13. The pregnancy-adapted YEARS algorithm utilizes three clinical parameters: (i) clinical signs of DVT, (ii) haemoptysis and (iii) the possibility of PE being the most likely diagnosis in conjunction with the D-dimer levels. PE is ruled out if none of the three criteria are met and the D-dimer level is less than 1000 ng/ml or if one or more of the three criteria are met and the D-dimer level is <500 ng/ml. Patients in whom PE cannot be ruled out based on YEARS algorithm need further imaging.
The radiation exposure of a single X-ray, ventilation-perfusion (V/Q) scan and CT pulmonary angiogram (CT PA) is far below the suggested accepted maximal cumulative threshold for foetal radiation exposure. The foetal radiation dose associated with V/Q scanning is slightly higher when compared to CTPA (around 0.5 mGy and 0.1 mGy, respectively). Furthermore, the availability of V/Q scan may be an issue in resource-constraint settings while CTPA facilities are more readily available. A recent meta-analysis on these two modalities concluded that both are equally safe during pregnancy14. Therefore, CTPA for diagnosis of PE during pregnancy will be a better option for most health facilities in India.
Therapy of VTE in pregnancy
Site of treatment: The treatment of uncomplicated VTE of non-pregnant patients is generally done on an outpatient basis. Recommendations for pregnant patients, however, differ and most guidelines recommend admission for the initial period in patients with acute VTE12,15. The need for admitting pregnant patients with VTE is an extrapolation of the criteria suggested for the general population and is enumerated in Table I.
| Suspected pulmonary embolism based on YEARS algorithm |
| Massive DVT, e.g., entire limb swelling, acrocyanosis and inferior vena cava thrombosis |
| Increased bleeding risk, e.g., presence of thrombocytopenia/coagulopathy |
| Severe pain which warrants parenteral analgesia |
| Presence of other systemic or obstetric comorbidities that merit admission |
| Poor social support/needs time to learn to self-inject heparin/distant stay from tertiary care setting |
DVT, deep-vein thrombosis; IVC, inferior vena cava
Choice and duration of anticoagulation: The treatment of VTE in pregnancy should consider the effect of therapy on maternal and foetal well-being, as well as the ease of administration. Low molecular weight heparin (LMWH) is the agent of choice for VTE in pregnancy. Most studies comparing once daily dosing with twice-daily dosing of LMWH did not show a significant difference in the risk of bleeding or recurrence/progression of VTE16. Therefore, once daily higher dose or twice daily dose of LMWH can be given based on patient preference. Dosing of LMWH for treatment differs according to the schedule used - 1.5 mg/kg subcutaneously once a day versus 1 mg/kg subcutaneously twice a day. The measurement of anti-factor Xa (anti-FXa) tests is not recommended routinely as they may be unreliable and not available in most centres. Besides, there are no established therapeutic ranges in pregnancy. Therefore, anti-FXa level monitoring is warranted only in patients who have renal impairment, concomitant malignancy or extremes of weight. If monitored, anti-FXa levels are initially checked after one week of commencing LMWH and weekly thereafter to target a peak anti-FXa activity of 0.5-1.2 U/ml17. Dosing recommendations for LMWH according to weight are provided in Supplementary Table.
| Weight (kg) | Enoxaparin | Dalteparin |
|---|---|---|
| <50 | 40 mg sc BD or 60 mg sc OD | 5000 IU sc BD or 10,000 IU sc OD |
| 50-69 | 60 mg sc BD or 90 mg sc OD | 6000 IU sc BD or 12,000 IU sc OD |
| 70-89 | 80 mg sc BD or 120 mg sc OD | 8000 IU sc BD or 160,00 IU sc OD |
| 90-109 | 100 mg sc BD or 150 mg sc OD | 10,000 IU sc BD or 20,000 IU sc OD |
| 110-125 | 120 mg sc BD or 180 mg sc OD | 12,000 IU sc BD or 24,000 IU sc OD |
| >125 | Requires expert consultation for dosing with measurement of Factor-Xa levels | Requires expert consultation for dosing with measurement of Factor-Xa levels |
BD, twice daily; OD, once daily
The cost of LMWH is 10 times higher than that of unfractionated heparin (UFH), and therefore, UFH may be the only option for some patients in a resource-constraint setting18. Besides, UFH has a short t1/2 and is completely reversed with protamine. However, both subcutaneous and intravenous UFHs require regular activated partial thromboplastin time (aPTT) monitoring. The advantage of low cost of UFH is offset by the need for regular aPTT monitoring, higher risk of osteoporosis and higher risk of heparin-induced thrombocytopenia. UFH is preferred in patients nearing term who had DVT or PE within the last two weeks. Table II summarizes the salient features of various anticoagulants in the pregnancy setting.
| Anticoagulant | Pregnancy implications | Dose | Management of labour | Advantage | Disadvantage |
|---|---|---|---|---|---|
| Unfractionated heparin | Safe during pregnancy | 333 U/kg sc stat f/b 250 U/kg sc BD | May be taken for surgery if >6 h have passed since the last dose | Cheap, safe in pregnancy Use in renal failure or in late trimester prior to operative delivery | Subcutaneous injections, needs aPTT monitoring |
| Low molecular weight heparin | Safe during pregnancy | 1.5 mg/kg sc OD or 1 mg/kg sc BD of enoxaparin* | To stop LMWH at least 12-24 h prior to surgery | No monitoring required, drug of choice for anticoagulation during pregnancy | Subcutaneous injections, cannot be given in renal failure, relatively expensive |
| Fondaparinux19 | Minimal transplacental transfer | 7.5 mg sc OD | To stop 24 h prior to labour, switch to LMWH one week prior to EDD | Can be used in allergy to LMWH or Heparin-induced thrombocytopenia20 | Expensive, limited pregnancy data |
| Warfarin | Teratogenic | To maintain INR b/w 2-3; preferably <5 mg OD | To withhold five days prior to labour | Cheap, oral drug, use after counselling in extreme resource constraint after first trimester, can be used during lactation | Teratogenicity, neurologic sequelae in foetus |
| NOACs | Minimal data in pregnancy | Oral drugs, do not require monitoring | Difficult reversal, questionable foetal safety |
*Dosing needs to be adjusted to body weight as pregnancy progresse. NOACs, novel oral anticoagulants; EDD, expected date of delivery; LMWH, low-molecular-weight heparin; aPTT, activated partial thromboplastin time; INR, international normalised ratio; OD, once daily; BD, twice daily
Vitamin K antagonists (VKA), e.g. warfarin and acitrom, are known to cross the placenta and increase the risk of miscarriage, stillbirth, embryopathy (nasal hypoplasia or stippled epiphyses), central nervous system abnormalities and maternal and foetal haemorrhage21. Women in childbearing age receiving warfarin should be warned about these teratogenic risks and advised to use secure methods of contraception while on VKAs. While most western guidelines advocate conversion to therapeutic once daily LMWH prior to conception, this may be difficult to implement in a resource-constraint setting. The alternative option is to counsel women on VKAs to get an early pregnancy test within five weeks from the last menstrual period and switch to LMWH/UFH if pregnancy is confirmed. Meta-analysis on maternal and foetal outcomes of pregnant women in patients with mechanical heart valves has concluded that the adverse effects of VKAs on foetal development appear to be limited to early gestation, with <3 per cent incidence of warfarin embryopathy at doses of ≤5 mg daily that increase to >30 per cent incidence of foetal loss or embryopathy if warfarin dose is >5 mg/day in the first trimester22. Situations of resource constraint are not infrequent in India, and patients with VTE may not be able to continue injectable heparin throughout the pregnancy. Extrapolating the evidence of VKA use in pregnancy with mechanical heart valves, warfarin may be used beyond 13 wk of gestation until the middle of the third trimester after discussion with the patients23. However, as warfarin results in anticoagulated foetus, there is a high risk of foetal intracranial haemorrhage if a vaginal delivery is attempted in women anticoagulated with warfarin. As very low levels of VKAs are secreted in breast milk, maternal VKAs pose little risk to breastfed infants24. Termination of pregnancy is not warranted if VKAs are discontinued within the first six weeks of gestation as the risk of warfarin embryopathy is mostly eliminated in such cases25.
Direct oral anticoagulants (DOACs) cross the placental barrier and are also secreted in breast milk26. Currently, there is insufficient evidence to recommend the routine use of DOACs during pregnancy. However, accidental exposure to DOACs in pregnancy does not warrant a termination. Rather a switch to LMWH is advisable27. In patients presenting with the first episode of VTE during pregnancy without a known thrombophilia state, anticoagulation is continued throughout the pregnancy and up to six weeks postpartum until at least three months of treatment has been given11.
Management of pulmonary embolism in pregnancy
Besides anticoagulation, the requirement for systemic thrombolytic therapy needs to be carefully assessed in patients with pulmonary thromboembolism (PTE). Evidence regarding the benefits and bleeding risks is limited to case reports and case series28. Thrombolysis in pregnancy is associated with 22 per cent risk of major bleeding, nine per cent risk of foetal death and a 39 per cent incidence of preterm delivery29. Extrapolating the data from non-pregnant population, the benefits of thrombolysis may outweigh the risks in patients who are haemodynamically unstable. Most guidelines recommend thrombolysis only in patients with life threatening acute PE, often with the involvement of multidisciplinary care teams15,30,31. In patients who are haemodynamically stable with sub-massive PTE, continuation of anticoagulation with monitoring of vital haemodynamic parameters, ECG and daily echocardiography is recommended17.
Management of labour
As soon as the patient is in labour, heparin (LMWH or UFH) should be stopped immediately. Regional anaesthetic or analgesic technique should be used only after 12 h of prophylactic and 24 h of therapeutic LMWH; these techniques can be used after six hours of stopping UFH. Patients who have had a recent DVT (within two weeks of expected date of delivery) have high recurrence rates of VTE and hence an elective delivery should be considered. These patients can be put on intravenous UFH with monitoring of APTT. UFH is discontinued 4-6 h before planned delivery27,32. Prophylactic dose LMWH can be resumed six hours post-caesarean section or vaginal delivery if surgical haemostasis is judged adequate. Therapeutic dose of LMWH or oral warfarin can be started after 48 h. If there are concerns of postpartum haemorrhage, anticoagulation can be delayed up to 12 h after normal delivery or 24 h after caesarean section.
At discharge, the patient may be transitioned to oral anticoagulation with VKAs, which are safe during breastfeeding. An overlap of LMWH and VKAs of at least five days or till therapeutic INR is achieved is needed.
Management of pregnancy with previous VTE
Recurrent thrombosis accounts for one in five of all VTE events in pregnancy4. The risk is highest in the postpartum period. The risk of recurrence is assessed based on the following factors: body mass index >30 kg/m2, age >35 yr, parity ≥3, smoker, presence of gross varicose veins, current pre-eclampsia, immobility, family history of unprovoked/oestrogen-provoked VTE in first degree relative and multiple pregnancy. At the first antenatal visit, patients should be educated about the warning signs of VTE (leg pain, asymmetrical limb swelling or sudden shortness of breath). In patients with thrombophilia or past episode of VTE due to an oestrogen related risk factor or pregnancy, patients should receive prophylactic anticoagulation during the antepartum and postpartum periods, while patients with previous VTE due to other provoking transient risk factors do not need routine anticoagulation throughout the pregnancy. However, patients with more than two episodes of VTE should be given therapeutic anticoagulation throughout the pregnancy and six weeks postpartum. Women already on anticoagulation with a VKA or DOAC should be switched to LMWH or UFH as soon as the pregnancy is confirmed. This should be done as early as possible once a pregnancy is suspected.
Pregnancy and underlying thrombophilic states
The presence of both inherited and acquired thrombophilic states is associated with an increased risk of VTE during pregnancy as well as poor obstetric outcomes in the form of early and late foetal loss, intrauterine growth retardation and pre-eclampsia33. An inherited thrombophilic predisposition is seen in 60-70 per cent of pregnant patients presenting with an unprovoked DVT34. Factor V and prothrombin mutations are the two most common disorders seen in the general population, as well as those with VTE34. The highest risk for VTE during pregnancy is associated with homozygous Factor V Leiden mutations [odds ratio: 34.4; 95% confidence interval (CI): 9.86-120.05] followed by homozygous prothrombin G20210A mutations (odds ratio: 26.36; 95% CI: 1.24-559.29)33. However, prothrombin gene mutations are rarely seen in the Indian population35. Anticoagulant factor deficiencies such as antithrombin, protein C and protein S deficiency are also associated with an increased risk of thrombosis during pregnancy11.
The most common acquired thrombophilic disorder associated with VTE and poor pregnancy outcomes is antiphospholipid antibody syndrome (APS). APS is associated with an increased risk of thrombosis, with nearly 30 per cent of patients developing a VTE over a 10 yr period36. The risk of thrombosis in asymptomatic carriers of aPL antibodies is highest in those with triple antibody positivity (i.e. lupus anticoagulant, anticardiolipin antibody and anti-beta2-GPI antibody)37. The occurrence of lupus anticoagulant in the blood on two occasions at least 12 wk apart or more than two antibodies or persistently high titres (>40 GPL units measured 12 wk apart) of aPL is considered a high risk aPL profile38.
Indian studies show variable results in terms of prevalence of inherited and acquired thrombophilia during pregnancy. A study from western India found that 37.5 per cent of patients with DVT during pregnancy were positive for aPL antibodies5. A similar study in patients with recurrent pregnancy loss found a prevalence of aPL antibodies and inherited thrombophilia to the tune of 38 and 41 per cent, respectively39.
Indications of thrombophilia screen
Most guidelines recommend against thrombophilia screening after an episode of unprovoked VTE in the general population40. A cost-effectiveness analysis of universal thrombophilia screening versus selective screening in patients with a prior history or family history of VTE revealed that universal thrombophilia screening in all pregnant patients was associated with a reduced occurrence of VTE. However, it was not as cost-effective as compared to selective screening41. Testing should ideally be performed before pregnancy as levels of various proteins (such as protein S) are altered during pregnancy11. Patients with a history suggestive of obstetric APS should be screened by aPL antibody testing. Additionally, all pregnant women already diagnosed with systemic lupus erythematosus should be screened for APS due to the high prevalence of aPL antibodies in them42. The Royal College of Obstetrics and Gynaecology (RCOG) guidelines recommend APS testing of all women of childbearing age with an unprovoked previous VTE11. Given the resource-constrained setting usually faced by clinicians in India, as well as the limited availability of testing for inherited thrombophilia, a limited screening strategy for patients with a prior VTE or a family history of VTE is recommended. The authors’ recommendations for screening for inherited thrombophilia are tabulated in Table III.
| Scenario | History and exam | Complete blood count | APLA testing | Protein C/S deficiency | Factor V Leiden mutation | AT 3 deficiency |
|---|---|---|---|---|---|---|
| Normal pregnancy | ✓ | ✓ | ✖ | ✖ | ✖ | ✖ |
| Previous provoked VTE during pregnancy/oestrogen exposure | ✓ | ✓ | ✖ | ✖ | ✖ | ✖ |
| Previous provoked VTE not related to pregnancy/oestrogen exposure | ✓ | ✓ | ✖ | ✖ | ✖ | ✖ |
| Previous unprovoked VTE | ✓ | ✓ | ✓ | ✖ | ✓ | ✓ |
| Family history of unprovoked VTE at age <40 yr in first-degree relative | ✓ | ✓ | ✖ | ✓ | ✓ | ✓ |
| Family history of known inherited thrombophilia | ✓ | ✓ | Check for same thrombophilia | |||
| Previous bad obstetric history but no VTE | ✓ | ✓ | ✓ | ✖ | ✖ | ✖ |
| Known SLE/connective tissue disorder | ✓ | ✓ | ✓ | ✖ | ✖ | ✖ |
| Recurrent or/second episode of VTE | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
VTE, venous thromboembolic; SLE, systemic lupus erythematosus; APLA, anti-phospholipid antibody; C/S, protein C or S; AT, anti-thrombin
Management of pregnancy with underlying thrombophilia
Table IV summarizes the recommendations from the American College of Chest Physicians (ACCP) and the RCOG for patients with inherited thrombophilia and pregnancy. For the Indian scenario, patients with a prior VTE and no evidence of APS should receive prophylactic anticoagulation in the antenatal period and till six weeks postpartum. Patients with only a family history of VTE should be screened for inherited thrombophilia, and those with a high risk thrombophilia should receive both antepartum and postpartum prophylactic anticoagulation. In patients with a family history of VTE and no high risk thrombophilia and no other risk factor for VTE, watchful observation is sufficient.
| Antenatal | Postpartum | ||
|---|---|---|---|
| ACCP guidelines32 | Prior VTE | Low risk of recurrence* - clinical vigilance High risk of recurrence* - prophylactic LMWH | 6 wk of prophylactic/intermediate dose LMWH/VKA |
| No prior VTE; high-risk# thrombophilia with family history | Prophylactic/intermediate dose LMWH | 6 wk of prophylactic/intermediate dose LMWH/VKA | |
| No prior VTE; high-risk thrombophilia without family history | Clinical vigilance | 6 wkof prophylactic/intermediate dose LMWH/VKA | |
| No prior VTE; low-risk thrombophilia with family history | Clinical vigilance | 6 wk of prophylactic/intermediate dose LMWH/VKA | |
| No prior VTE; low-risk thrombophilia without family history | Clinical vigilance | Clinical vigilance | |
| RCOG guidelines11 | Prior VTE | Prophylactic LMWH** | Prophylactic LMWH for 6 wk |
| Prior VTE with AT deficiency | Higher dose LMWH | Higher dose LMWH for 6 wk | |
| No prior VTE; high-risk# thrombophilia | Consider prophylactic LMWH | Prophylactic LMWH for 6 wk | |
| No prior VTE; low-risk thrombophilia | ≥3 other risk factors*** - Prophylactic LMWH Two other risk factors - Prophylactic LMWH from 28 wk One other risk factor - Clinical vigilance | ≥2 other risk factors or family history - Prophylactic LMWH One other risk factor - 10 days of prophylactic LMWH |
#High risk thrombophilia in ACCP guidelines - Homozygous Factor V Leiden and prothrombin mutations; High risk thrombophilia in RCOG guidelines - protein C, protein S and AT deficiency, homozygous Factor V Leiden and prothrombin mutations; *Low risk of recurrence - Single episode of VTE associated with a transient risk factor (not related to pregnancy or oestrogen use); High risk of recurrence - Single unprovoked VTE; pregnancy/oestrogen use associated VTE; multiple prior unprovoked VTE; **Except those with a single episode of VTE related to previous surgery and no other risk factors; ***Other risk factors include - BMI >30 kg/m2, age >35 yr, parity ≥3, smoker, gross varicose veins, current pre-eclampsia, immobility, family history of unprovoked/oestrogen-provoked VTE in first degree relative, multiple pregnancy, IVF/ART. ACCP, American College of Chest Physicians; VTE, venous thromboembolic; LMWH, low-molecular-weight heparin; VKA, vitamin K antagonists; RCOG, Royal College of Obstetrics and Gynecology; AT, anti-thrombin
Table V summarizes the recommendations for the management of APS during pregnancy. Multiple meta-analyses have shown that heparin + low-dose aspirin is associated with better foetal outcomes than aspirin alone and is the recommended treatment during pregnancy43-45.
| EULAR guidelines | ACCP guidelines | RCOG guidelines | |
|---|---|---|---|
| Prior history of VTE with proven APS | LDA + therapeutic LMWH during antenatal and postpartum period | ||
| Proven obstetric APS with no prior history of VTE | LDA + prophylactic LMWH in the antenatal and postpartum period | - | |
| Serological APS with symptoms but not meeting clinical criteria | LDA ± prophylactic LMWH may be considered on individual basis | - | - |
| Serological APS only (no history of thrombosis/pregnancy complications) | LDA may be considered in high-risk aPL profile | - | May consider for prophylaxis if other risk factors present and persistent aPL positivity |
LDA, low dose aspirin; LMWH, low molecular weight heparin; APS, antiphospholipid antibody syndrome; aPL, anti-phospholip
Future directions
Prevention and treatment of VTE during pregnancy requires considering multiple inter-playing factors for decision-making process. This review is an attempt to simplify the approach and summarize the available recommendations through the algorithm and flow charts given in Figs 1 and 2.
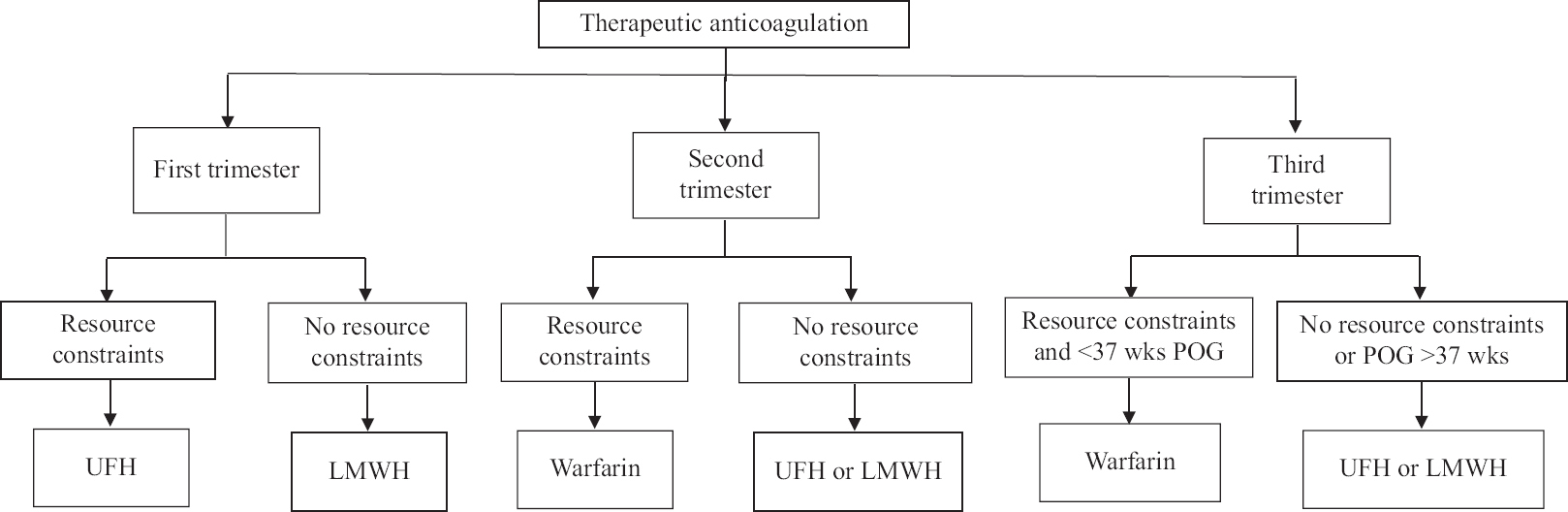
- Algorithm for approach to a pregnant patient with venous thromboembolism. POG, period of gestation; UFH, unfractionated heparin; LMWH, low molecular weight heparin
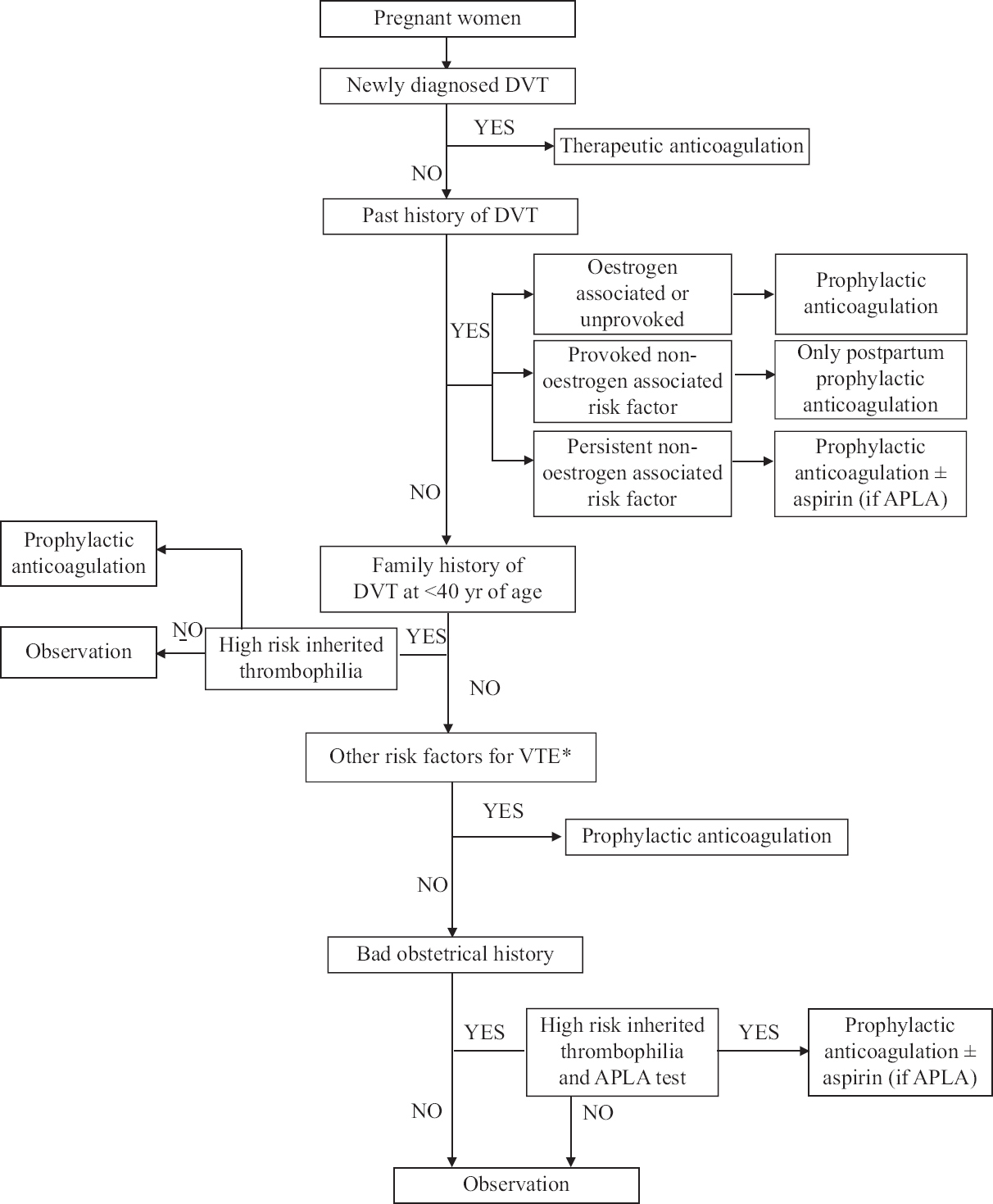
- Treatment options for treatment of venous thromboembolism in pregnancy. DVT, deep vein thrombosis; VTE, venous thromboebolism; ALPA, anti phospholipid antibody syndrome
Therapeutic strategies should be based on the foeto-maternal risks of thrombosis per se, period of gestation and feasibility of various pharmacological and mechanical options. Currently, there are several knowledge gaps as follows regarding the management of VTE in pregnancy in an LMIC setting: the best form of contraception in these patients and the safety of hormonal pills, the best choice of anticoagulation given the demerits of parenteral heparins, best imaging modalities for diagnosing acute PE and the cost-effectiveness of prophylactic anticoagulation in patients at risk of VTE.
There is also a need for the establishment of multidisciplinary response teams comprising obstetricians, haematologists, intensivists and intervention radiologists and patient counsellors to facilitate team-based rapid decisions at the institutional level.
Financial support and sponsorship
None.
Conflicts of interest
None.
References
- Pregnancy, the postpartum period and prothrombotic defects: Risk of venous thrombosis in the MEGA study. J Thromb Haemost. 2008;6:632-7.
- [Google Scholar]
- Incidence, clinical characteristics, and timing of objectively diagnosed venous thromboembolism during pregnancy. Obstet Gynecol. 1999;94:730-4.
- [Google Scholar]
- Deep venous thrombosis in the antenatal period in a large cohort of pregnancies from western India. Thromb J. 2007;5:9.
- [Google Scholar]
- Venous thromboembolism as a cause of severe maternal morbidity and mortality in the United States. Semin Perinatol. 2019;43:200-4.
- [Google Scholar]
- Maternal mortality and its causes in a tertiary center. J Obstet Gynaecol India. 2012;62:168-71.
- [Google Scholar]
- Venous thromboembolism in obese pregnant women: Approach to diagnosis and management. Ginekol Pol. 2017;88:453-9.
- [Google Scholar]
- Deep venous thrombosis in pregnancy: Incidence, pathogenesis and endovascular management. Cardiovasc Diagn Ther. 2017;7:S309-19.
- [Google Scholar]
- Pulmonary thrombo-embolism in pregnancy: Diagnosis and management. Breathe (Sheff). 2015;11:282-9.
- [Google Scholar]
- Obstetric cerebral venous thrombosis –A clinical dilemma. J Clin Diagn Res. 2019;13:QC10-3.
- [Google Scholar]
- Computed tomography pulmonary angiography versus ventilation-perfusion lung scanning for diagnosing pulmonary embolism during pregnancy: A systematic review and meta-analysis. Haematologica. 2019;104:176-88.
- [Google Scholar]
- Practice bulletin no. 123: Thromboembolism in pregnancy. Obstet Gynecol. 2011;118:718-29.
- [Google Scholar]
- Once versus twice daily low molecular weight heparin for the initial treatment of venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD003074.
- [Google Scholar]
- American Society of Hematology 2018 guidelines for management of venous thromboembolism: Venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2:3317-59.
- [Google Scholar]
- Randomised controlled trial for efficacy of unfractionated heparin (UFH) versus low molecular weight heparin (LMWH) in thrombo-prophylaxis. J Assoc Physicians India. 2013;61:882-6.
- [Google Scholar]
- Management and outcome of heparin-induced thrombocytopenia in pregnancy: A systematic review. Cardiovasc Hematol Agents Med Chem. 2015;13:92-7.
- [Google Scholar]
- The “warfarin window”in pregnancy: The importance of half-life. J Obstet Gynaecol Can. 2010;32:988-9.
- [Google Scholar]
- Maternal and fetal outcomes of anticoagulation in pregnant women with mechanical heart valves. J Am Coll Cardiol. 2017;69:2681-91.
- [Google Scholar]
- Oral anticoagulants: Current Indian scenario. In: Muruganathan A, ed. Medicine update. Vol 25. New Delhi: Jaypee; 2013. p. :410-3.
- [Google Scholar]
- Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J Thromb Thrombolysis. 2016;41:92-128.
- [Google Scholar]
- Direct-acting oral anticoagulants (DOACs) in pregnancy: New insight from VigiBase® . Sci Rep. 2019;9:7236.
- [Google Scholar]
- Management of direct oral anticoagulants in women of childbearing potential: Guidance from the SSC of the ISTH: Reply. J Thromb Haemost. 2017;15:195-7.
- [Google Scholar]
- Use of thrombolytic agents to treat pulmonary embolism in pregnancy. Rev Obstet Gynecol. 2013;6:182-4.
- [Google Scholar]
- Thrombolysis in pregnancy: A literature review. J Matern Fetal Neonatal Med. 2019;32:2418-28.
- [Google Scholar]
- Recommendations for the prevention of pregnancy-associated venous thromboembolism. Aust N Z J Obstet Gynaecol. 2012;52:3-13.
- [Google Scholar]
- Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36:527-53.
- [Google Scholar]
- VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed.: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:S691-736.
- [Google Scholar]
- The inherited thrombophilias: Genetics, epidemiology, and laboratory evaluation. Best Pract Res Clin Obstet Gynaecol. 2003;17:397-411.
- [Google Scholar]
- Prothrombin G20210A is not prevalent in North India. J Thromb Haemost. 2003;1:2253-4.
- [Google Scholar]
- Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: A multicentre prospective study of 1000 patients. Ann Rheum Dis. 2015;74:1011-8.
- [Google Scholar]
- Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: A multicenter prospective study. Blood. 2011;118:4714-8.
- [Google Scholar]
- EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78:1296-304.
- [Google Scholar]
- Inherited and acquired thrombophilia in Indian women experiencing unexplained recurrent pregnancy loss. Blood Cells Mol Dis. 2015;55:200-5.
- [Google Scholar]
- Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315-52.
- [Google Scholar]
- Screening for thrombophilia in high-risk situations: Systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study. Health Technol Assess. 2006;10:1-110.
- [Google Scholar]
- Combination of heparin and aspirin is superior to aspirin alone in enhancing live births in patients with recurrent pregnancy loss and positive anti-phospholipid antibodies: A meta-analysis of randomized controlled trials and meta-regression. Rheumatology. 2009;49:281-8.
- [Google Scholar]
- Heparin treatment in antiphospholipid syndrome with recurrent pregnancy loss: A systematic review and meta-analysis. Obstet Gynecol. 2010;115:1256-62.
- [Google Scholar]
- Comparison of therapeutic interventions for recurrent pregnancy loss in association with antiphospholipid syndrome: A systematic review and network meta-analysis. Am J Reprod Immunol. 2020;83:e13219.
- [Google Scholar]






