Translate this page into:
Management of T cell responses by anesthetic drugs-propofol & isoflurane in perioperative breast cancer patients: A prospective hospital-based study
#,*Equal contribution
For correspondence: Dr Dona Sinha, Department of Receptor Biology and Tumor Metastasis, Chittaranjan National Cancer Institute, Kolkata 700 026, West Bengal, India e-mail: donasinha2012@gmail.com
-
Received: ,
Accepted: ,
Read UPDATED-ARTICLE associated with this - 10.25259/ijmr_2382_23_ER
Abstract
Background & objectives
The choice of anesthetic for better perioperative conservation of immune responses has always been contentious. This study investigated the differential impact of the intravenous anesthetic, propofol, and the volatile anesthetic, isoflurane on the T cell immune responses, if any, among individuals going through perioperative breast cancer.
Methods
Perioperative blood samples (preoperative, intraoperative and postoperative) collected from participants with breast cancer in two arms namely isoflurane arm (n=50) and the propofol arm (n=50) were analyzed for T cell immune response using flow cytometry and ELISA. The interactions of anesthetics with CD4/CD8 were probed with molecular docking and molecular dynamic (MD) simulations.
Results
Linear mixed model analysis showed that isoflurane in comparison to propofol inhibited CD4+ helper (Th) [β-coefficient: -8.75; 95% CI: -13.00 to -4.51] and CD19+ B cell (β: -7.51; 95% CI: -15.46 to 0.44) frequencies during the intraoperative period in perioperative breast cancer patients. Further, interleukin (IL)-10 and IL-12 were significantly increased during the intra- and postoperative periods in the isoflurane group as compared to the propofol group. Molecular docking (MD) validated propofol’s better binding energy with CD4/CD8 than isoflurane. MD simulations propagated that in contrast to isoflurane, propofol formed a more compact and stabilized structure with CD4/CD8, making the amino acid residues on the surface of CD4/CD8 inaccessible for any interaction.
Interpretation & conclusions
The clinical observations and the in silico findings exhibited that propofol in comparison to isoflurane better regulated T cell immuno-inflammatory response in perioperative breast cancer patients.
Keywords
CD4+ T helper cells
CD8+ cytotoxic T cells
isoflurane
molecular docking
molecular dynamic simulations
propofol
Breast cancer is the most prevalent malignant form among women1,2 and by 2045, it is estimated that India may witness a rise of 448035 million new female breast cancers3. Despite the therapeutic advancements, primary ablative surgery is still the mainstay for breast cancer management.
Onco-anesthesiology prioritizes perioperative management for minimizing cancer recurrence and improving oncological outcomes4. The intravenous anesthetic propofol (2, 6-diisopropyl phenol)5, and the volatile anesthetic isoflurane (2-chloro-2-(difluoromethoxy)-1,1,1-trifluorethane)6 are commonly used during surgical resections with a favorable pharmacokinetic and pharmacodynamic profile7,8. Anesthetic agents have been indicated to deregulate immune and inflammatory responses either by regulating the stress response or impairing immune cells9. Studies have reported advantageous role of propofol over volatile anesthetics in terms of postoperative survival of patients10,11 and minimal immune suppression12. On the contrary, several recent clinical studies have observed no significant beneficial effect between propofol and other volatile anesthetics on cancer immunity and inflammatory responses13-16.
T lymphocytes play a vital role in the cell-mediated adaptive immune response. CD4+ helper T (Th) cells play a pivotal role in developing and sustaining effective anti-tumor immunity by stimulating other immune cells such as B cells, macrophages, and CD8+ cytotoxic T cells (Tc)17. The knowledge of the contradictory reports on the choice of anesthetic agent during surgery and the absence of any such reports from Indian background made it imperative to investigate the differential impact of propofol and isoflurane on the T cell immune responses, if any, among individuals of perioperative breast cancer patients.
Materials & Methods
Study setting
This prospective study recruited individuals with breast cancer presenting to the Department of Surgical Oncology, Chittaranjan National Cancer Institute, Kolkata, who were advised for surgery [modified radical mastectomy (MRM)/breast conserving surgery (BCS)] according to the considered inclusion and exclusion criteria18. Recruitment of patients in this study was administered through informed consent form. Patients were block-randomized in two arms- volatile anesthestic isoflurane (n=50) in one arm and intravenous anaesthestic propofol (n=50) in the other. The study was approved by the Institutional Ethics Committee. The study adhered to the Indian Council of Medical Research’s ethical guidelines for biomedical research on human participants (2017)19 and was registered at Clinical Trials Registry-India (CTRI/2020/11/02886 dated Nov 3, 2020).
Protocol for anesthetic management
The protocol for anesthetic management was same as reported before18. In the isoflurane group induction was achieved with thiopentone, 3-5 µg/kg and maintenance was done by using isoflurane 50 per cent in nitrous oxide to achieve minimum alveolar concentration of 1.0. For the propofol group induction was same but the maintenance was done using propofol with a target-controlled infusion pump, at an effect-site concentration of 2-3 µg/ml.
Collection of blood samples
Perioperative blood samples (preoperative, 1 day before surgery; intraoperative, 1 h after surgical incision and postoperative, 48 h after surgery) were collected from the study participants in both anesthetic arms18.
Typically, these anesthetic drugs are rapidly metabolized and eliminated from the body. The terminal elimination phase for propofol lasts for 1.5-31h20 whereas in case of isoflurane the elimination half-life of serum fluoride levels (metabolite of isoflurane) has been estimated to approximately 21 h21. Therefore, in this study, we have investigated the T cell immune responses for 1 h (intra) and 48 h (post) after incision.
Monitoring of anesthetic agents in serum samples
propofol was detected in serum samples using high-performance liquid chromatography (HPLC)22,23. Fluoride concentration during the pre, intra and postoperative periods after isoflurane anesthesia were measured directly from serum samples using fluoride ion selective electrode technique21 (Supplementary Material, Supplementary Fig. 1).
Flow cytometry for detection of lymphocyte subtypes
The immunophenotype of the lymphocytes was analyzed with flow cytometry24-26. Whole blood (100 µl) was incubated with a cocktail of fluorescent-tagged antibodies, in dark for 45 min at 4°C, suspended in PBS and was analyzed in a flow cytometer. The detailed protocol and gating strategy have been depicted in Supplementary Material (Supplementary Fig. 2 and 3).
Estimation of cytokines
ELISA kits [Ray Biotech Peachtree Corners, GA, USA] were used to measure serum concentrations of interleukin (IL)-2, IL-12, IL-10, tumor necrosis factor (TNF)-α and interferon (IFN)-γ at 450 nm in a microplate reader18.
In silico analyses
Grid-based Ligand Docking with Energetics (GLIDE, Schrödinger Release 2021-1: Maestro, Schrödinger, LLC, New York, NY, 2021) was used for docking analysis of isoflurane and propofol against CD4 and CD8 targeted proteins in extra precision mode. LigPlot+ ( https://www.ebi.ac.uk/thornton-srv/software/LigPlus/) and the ligand interactions module of Schrödinger were used to show the presence of intermolecular bonds between protein-drug complexes. Desmond program (Schrödinger Release 2021-1: Maestro, Schrödinger, LLC, New York, NY, 2022) was used for molecular dynamic simulations (MDS) of the Holo-1: CD4-isoflurane complex, Holo-2: CD4-propofol complex, Holo-3: CD8-isoflurane complex, and Holo-4: CD8-propofol complex, to understand the dynamic behaviour, mode of binding and inhibitor specificity for all the systems (details provided in Supplementary Material).
Statistical analysis
To compare the clinicopathological characteristics, Student’s t-test and Chi-square test were performed. The comparison of repeated measurement indicators at different observation time among the isoflurane and propofol group was performed using a linear mixed effect model. The model was used with patient as a random effect and with arm, timepoints and arm-by-timepoints as fixed effects. The arm-by-timepoints interaction indicates whether the change over time differed between the two anesthetic arms27. Post-hoc analyses were used with Bonferroni correction wherever applicable. The same linear mixed effect model was applied to investigate the effect of the anesthetic arm on Th cells with adjustment of confounding factors including age, height, weight, type of surgery, histopathology, grade, stage, molecular subtypes, ASA classification, duration of anesthesia and surgery, and pain score. Data were analyzed using Statistical Package for Social Sciences (IBM SPSS, ver. 25.0, Chicago, IL, USA). A value of P< 0.05 was considered significant.
Results
Participant clinicopathological characteristics
Participants were recruited from December, 2020 to July, 2023 and followed up till August 2024 (Fig. 1). The clinicopathological characteristics of the enrolled participants with breast cancer (Table I) showed no significant difference between the two anesthetic groups in terms of age, weight, height, American Society of Anesthesiologists (ASA) classification, histopathology, estrogen receptor (ER)/ progesterone receptor (PR) / human epidermal growth factor receptor 2 (Her2) nu status, grade, stage, type of surgery, duration of surgery, duration of anesthesia and the post-operative numerical pain score. We also monitored the presence of anesthetic agents in serum samples. propofol and fluoride were detected only in the intraoperative serum samples (Supplementary Fig. 1).
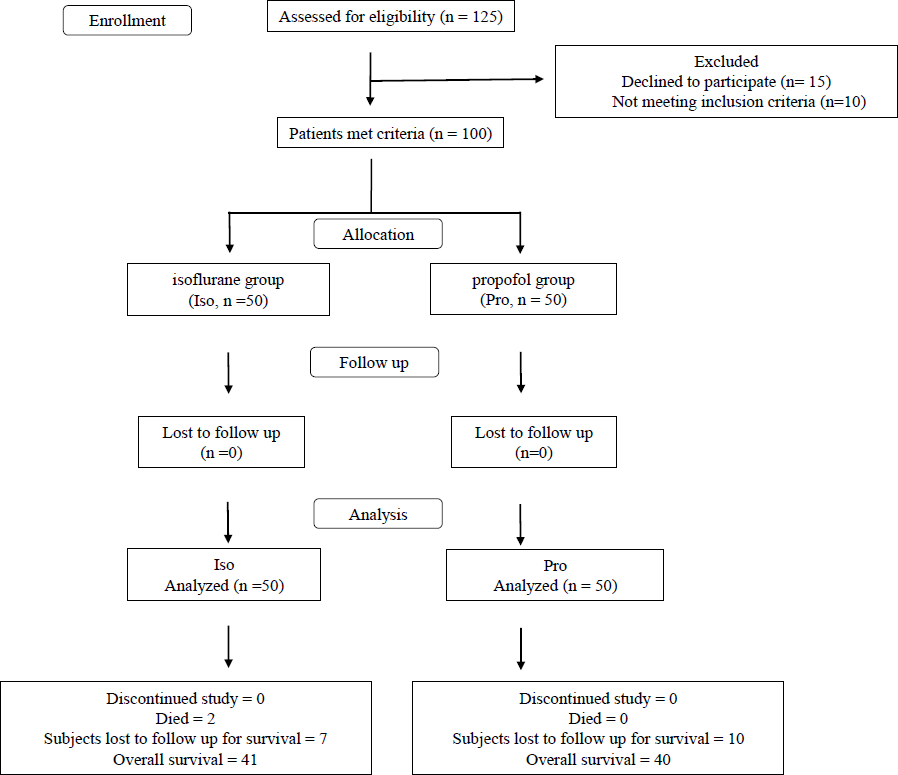
- CONSORT flow diagram for recruitment of breast cancer patients in the study.
| Characters | Character subtype | Anesthetic agent | P value | |
|---|---|---|---|---|
|
Isoflurane (n=50) |
Propofol (n=50) |
|||
| Age (yr) | 52.36±11.35 | 53.10±11.75 | 0.749 | |
| Height (cm) | 149.5±4.85 | 149.4±7.75 | 0.959 | |
| Weight (kg) | 54.05±10.48 | 53.35±9.42 | 0.728 | |
| ASA classification; n (%) | ASA I | 20 (40) | 21 (42) | 0.839 |
| ASA II | 30 (60) | 29 (58) | ||
| Histopathology; n (%) | Invasive carcinoma of NOS type | 14 (28) | 16 (32) | 0.531 |
| Invasive/ Infiltrating ductal carcinoma | 28 (56) | 30 (60) | ||
| Ductal carcinoma in situ | 6 (12) | 2 (4) | ||
| Mucinous carcinoma | 2 (4) | 2 (4) | ||
| Molecular subtypes; n (%) | ER+PR+HER2- | 18 (36) | 24 (48) | 0.615 |
| ER-PR-HER2+ | 8 (16) | 4 (8) | ||
| ER-PR-HER2- | 7 (14) | 7 (14) | ||
| ER+PR+HER2+ | 6 (12) | 7 (14) | ||
| ER+PR-HER2- | 6 (12) | 6 (12) | ||
| ER+PR-HER2+ | 5 (10) | 2 (4) | ||
| Type of surgery; n (%) | MRM | 35 (70) | 26 (52) | 0.065 |
| BCS | 15 (30) | 24 (48) | ||
| Duration of surgery (min) | 88.10±25.61 | 93.18±32.26 | 0.385 | |
| Duration of anesthesia (min) | 110.5±27.93 | 115.8±30.36 | 0.365 | |
| Pain score (Number) | 1.76±0.68 | 1.72±0.64 | 0.764 | |
| Grade; n (%) | Grade I | 2 (4) | 5 (10) | 0.212 |
| Grade II | 33 (66) | 25 (50) | ||
| Grade III | 15 (30) | 20 (40) | ||
| Stage; n (%) | Stage I | 5 (10) | 5 (10) | 0.893 |
| Stage II | 34 (68) | 32 (64) | ||
| Stage III | 11 (22) | 13 (26) | ||
Impact of anesthetic agents on Th and Tc cells
isoflurane significantly suppressed the frequency of Th (CD3+CD8-CD4+) cells during the intraoperative than the preoperative period which was evident from the ‘intra-group’ analysis (Fig. 2; panels A and B). On the other hand, Th cells were significantly increased in postoperative compared to pre and intraoperative periods of propofol group (Fig. 2A and B). ‘Inter-group’ analysis showed the intraoperative Th cell depletion by isoflurane was significant compared to propofol (β-coefficient: -8.75; 95% CI: -13.00 to -4.51; Fig. 2C). Further subgroup analysis showed that the isoflurane-induced suppression of Th cell frequency during intraoperative period was prominent in stage II breast cancer as observed from ‘intra-group’ comparisons (Fig. 2D) and ‘inter-group’ comparisons (β: -8.51; 95% CI: -13.96 to -3.06; Fig. 2E). The intraoperative suppressive effect of isoflurane on Th cell frequency was also evident in the molecular subtype ER+PR+HER2- during the ‘intra-group’ comparison between intraoperative and preoperative period (Fig. 2F) and in the molecular subtype ER+PR+HER2- (β: -8.29; 95% CI: -14.37 to -2.21) as well as in ER-PR-HER2- (β: -11.10; 95% CI: -25.10 to 2.90) during the ‘inter-group’ comparison between isoflurane and propofol (Fig. 2G). According to the type III fixed effects of linear mixed model analysis (Supplementary Table I) it was further confirmed that Th cell frequency was significantly impacted by the interaction of the anesthetic arm with the timepoints. However, the age of the individuals also showed significant effect on the Th cells but not as an interaction of age with arm. The other confounding factors did not have any significant effect on the Th cell frequency of the individuals with breast cancer.
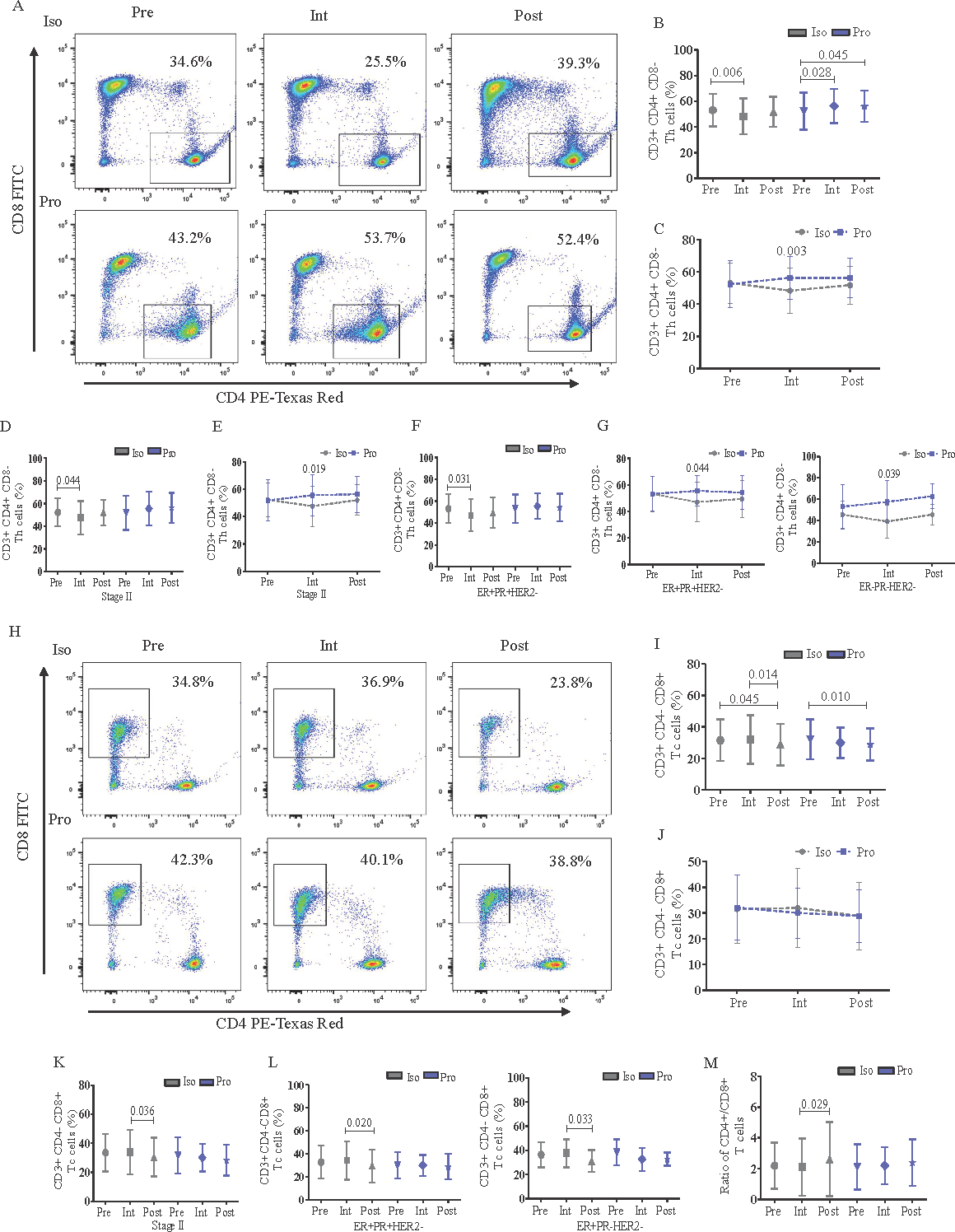
- Flowcytometric analysis and frequency of CD3+CD8-CD4+ (Th) and CD3+CD8+CD4- (Tc) cells (%) in peripheral blood of perioperative breast cancer patients administered with anesthetic agents, Iso or Pro. (A) Th cell frequency in representative female anesthetized with Iso/Pro; (B) the effect of Iso/Pro on Th cells at different time points with intra-group analysis; (C) with inter-group analysis; (D) the effect of Iso/Pro on Th cells according to tumor stage (II) with intra-group analysis; and (E) with inter-group analysis; (F) the effect of Iso/Pro on Th cells according to breast cancer molecular subtype (ER+PR+HER-) with intra-group analysis; and (G) with inter-group analysis (ER+PR+HER- and ER-PR-HER2-); (H) Tc cell frequency in representative female anesthetized with Iso/Pro; (I) the effect of Iso/Pro on Tc cells at different timepoints with intra-group analysis; and (J) with inter-group analysis; (K) the effect of Iso/Pro on Th cells according to tumor stage (II); and (L) molecular subtypes (ER+PR+HER-, ER+PR-HER-); (M) ratio of CD4+/CD8+ T cells in perioperative breast cancer patients. The graphs were plotted based on the mean±SD. Iso: isoflurane; Pro: propofol; Pre: preoperative; Intra: intraoperative; Post: postoperative.
Tc cells (CD3+CD4-CD8+) were also significantly reduced in count during the postoperative than preoperative period by both isoflurane and propofol (Fig. 2H and I) and the ‘inter-group’ comparison showed no differential effect of the anesthetics (Fig. 2J). However, subgroup analysis showed that isoflurane inflicted significant reduction in Tc cell frequency during the postoperative period compared to intraoperative period particularly in stage II cases but the same was not evident with propofol (Fig. 2K). Similarly, the decrease in Tc cells during postoperative period compared to intraoperative period by isoflurane was also observed across the molecular subtypes ER+PR+HER2- and ER+PR-HER2- whereas propofol did not induce any such changes (Fig. 2L). Additionally, isoflurane significantly increased the ratio of CD4+/CD8+ T cells postoperatively than intraoperative period, while the same ratio was maintained with propofol (Fig. 2M).
Effect of isoflurane/propofol on T cell activity markers, B cells and inflammatory cytokines
In order to activate a Tc or a Th cell to proliferate and differentiate into an effector cell, an antigen presenting cell (APC) provides two signals – (i) through foreign peptide bound to major histocompatibility complex (MHC) on the surface of APC which signals through T cell receptor (TCR); and (ii) through co-stimulatory molecules - CD80 and CD86, which are identified by the co-receptor protein CD28 on the surface of the T cell28. Therefore, we checked the effect of isoflurane/propofol on the TCR and CD28 activity and did not observe any significant difference between the anesthetic groups (Supplementary Fig. 4).
The Th cells inflict a direct anti-tumor response and help B lymphocytes to produce antibodies29. Therefore, we investigated the impact of the anesthetic agents on B cells. The ‘intra-group’ analysis revealed that the increase in B cell (CD3-CD19+) frequency was significant in the postoperative as compared to the intraoperative period in the isoflurane group whereas the same was higher in the postoperative than the preoperative period in the propofol group (Fig. 3A, B). However, ‘inter-group’ analysis showed significant decrease of B cell frequency by isoflurane compared to propofol during the intraoperative period (β: -7.51; 95% CI: -15.46 to 0.44; Fig. 3C). Subgroup analysis exhibited that isoflurane-induced increase in B cells during the postoperative period compared to pre and intraoperative period was significant only in breast cancer stage II cases (Fig. 3D). However, comparison between isoflurane and propofol showed that B cells were significantly reduced by isoflurane than propofol during the intraoperative period (β: -7.14; 95% CI: -16.47 to 2.18; Fig. 3E). The increment of B cell frequency by propofol during the postoperative than preoperative period was significant only in the ER-PR-HER2- breast cancer molecular subtype (Fig. 3F).
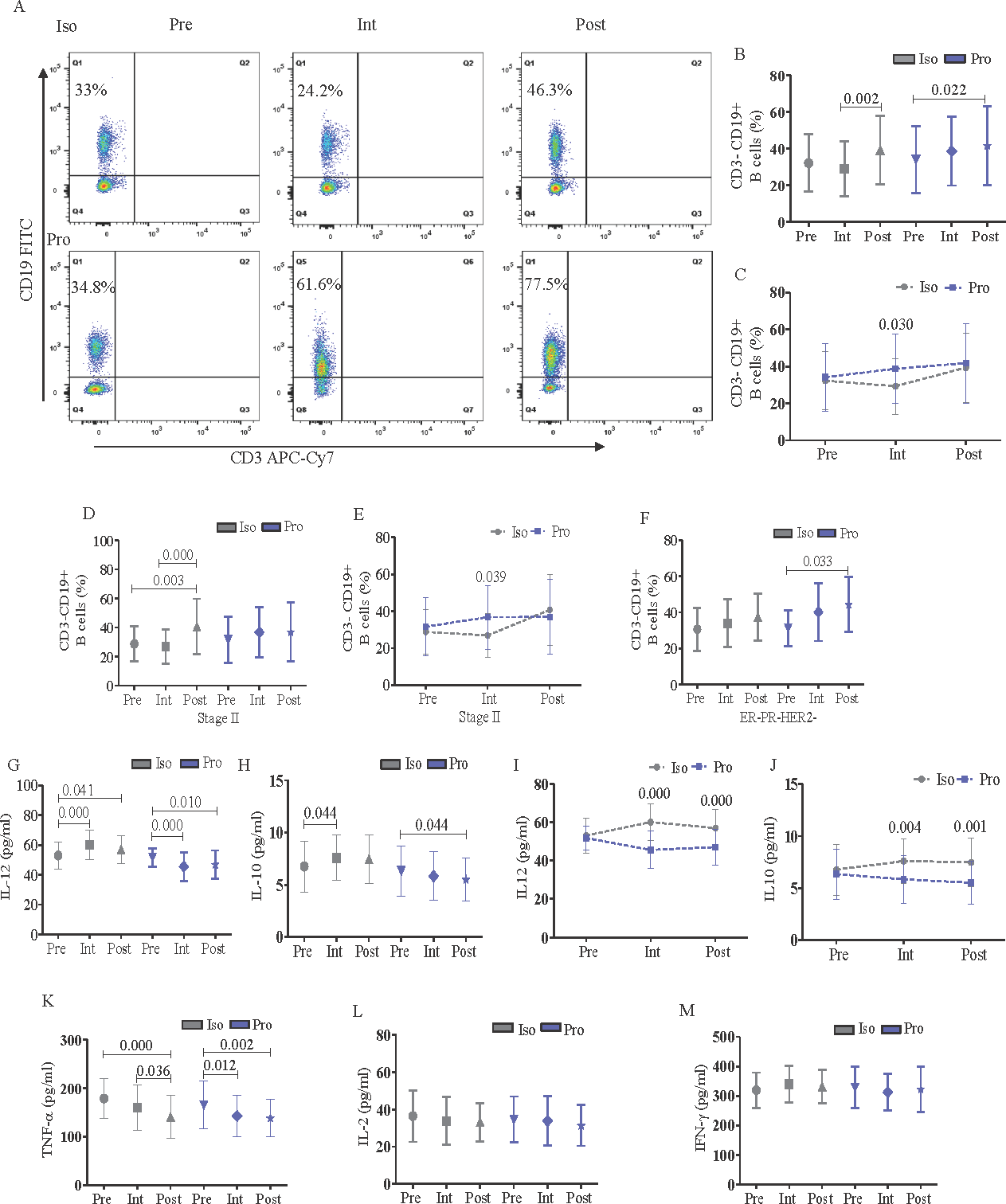
- Flowcytometric analysis of CD3-CD19+ B cells and expression of serum inflammatory markers in peripheral blood of perioperative breast cancer patients administered with anesthetic agents, Iso or Pro. (A) B cell frequency in representative female anesthetized with Iso/Pro; (B) comparative effect of Iso/Pro on B cells at different timepoints with intra-group analysis; and (C) with inter-group analysis; (D) the effect of Iso/Pro on B cells according to tumor stage (II) with intra-group analysis; and (E) with inter-group analysis; (F) the effect of Iso/Pro on B cells according to breast cancer molecular subtype (ER-PR-HER2-); (G and H) comparative effect of Iso /Pro on serum inflammatory markers such as IL-12 and IL-10 according to timepoint with intra-group analysis respectively; (I and J) IL-12 and IL-10 with inter-group analysis respectively; (K) TNF-α; (
L) IL-2; and (M) IFN-γ in perioperative breast cancer patients. The graphs were plotted based on the mean± SD.
A panel of cytokines including IL-12, IL-10, TNF-α, IL-2 and IFN-γ involved in innate and adaptive immune responses mediated through T and B cells were investigated in isoflurane/propofol groups at different timepoints. Both IL-12 (Fig. 3G) and IL-10 (Fig. 3I) were increased with isoflurane in the intra than preoperative periods and reduced with propofol in the post than preoperative period. However, inter-group analysis revealed significant increase in both IL-12 and IL-10 levels during intra (IL-12 β: 13.16; 95% CI: 8.73 to 17.60; IL-10 β: 1.33; 95% CI: 0.39 to 2.27) and postoperative periods (IL-12 β: 8.70; 95% CI: 4.26 to 13.13; IL-10 β: 1.53; 95% CI: 0.59 to 2.47) in the isoflurane group than propofol (Fig. 3I and J). TNF-α was significantly inhibited postoperatively in both the anesthetic groups (Fig. 3K) whereas IL-2 (Fig. 3L) and IFN-γ (Fig. 3M) did not show any significant alteration across isoflurane/propofol groups.
Molecular docking and trajectory analysis of isoflurane and propofol with CD4 and CD8
The binding of the anesthetics with CD4 and CD8 can modulate their activity, altering immune responses. CD4 and CD8 co-receptors play crucial roles in T cell activation by binding to MHC and recruiting lymphocyte-specific protein tyrosine kinases (lck), which are essential for T cell signaling30. Any alteration in these interactions due to anesthetics can impact T cell function and the overall immune response. Therefore, next we investigated molecular docking and MDS of the anesthetics, isoflurane and propofol with CD4 and CD8. Molecular docking revealed that propofol elicited better binding energy against CD4/CD8 with higher binding scores than isoflurane (Table II). Propofol showed better binding affinity and higher scores compared to isoflurane against the said targets (Fig. 4; panels A and F). The 100 nanoseconds (ns) MDS trajectory revealed a stable root mean square deviation (RMSD) from 0 to 75 ns in Holo-1 but later exhibited higher deviation, which may be due to conformational changes. Holo-2 maintained a stable trajectory throughout the time frame. Holo-3 showed consistent deviations after 20 ns compared to Holo-4. Overall, propofol’s binding appeared to better stabilize CD4 and CD8 than isoflurane, as shown by lower RMSD values (Fig. 4B and G). Root mean square fluctuation (RMSF) analysis further elucidated the impact of ligand binding on residue mobility. The Apo state showed greater fluctuations, while specific residues in Holo states (notably in Holo-2 and Holo-4) exhibited reduced mobility, indicating constrained motions due to ligand interactions (Fig. 4C and H). Radius of gyration (rGyr) measurements indicated Holo-2 with more compactness compared to Holo-1. Similarly, Holo-3 and Holo-4 showed varying degrees of compactness, with Holo-4 being notably stable. These observations aligned with the RMSF results, highlighting the influence of ligand binding on the structural integrity of the proteins (Fig. 4D and I). Solvent accessible surface area (SASA) analysis during later simulation stages demonstrated that many residues transitioned from accessible to buried states upon ligand binding. In SASA, Holo-2 and Holo-4 depicted decreased values which signified its shift towards a buried state compared to Holo-1 and Holo-3 (Fig. 4E and J). Collectively, these results affirmed that propofol bonded more effectively to CD4 and CD8, contributing to their stability through reduced residue mobility, enhanced compactness, and significant changes in protein surface orientation. Additionally, post-MDS conferred that conventional H-bonds were broken down between CD4/CD8 and isoflurane but were retained with propofol (details provided in the Supplementary Material, Supplementary Fig. 5).
| S. No. | Target | PubChem CID | Drug | Binding energy (kcal/mol) | No. of H-bonds | H-Bond forming residues | Average distance of H-bonds (Å) |
|---|---|---|---|---|---|---|---|
| 1 | CD4 | 3763 | Isoflurane | -3.80 | 1 | Thr228 | ∼2.556 |
| 2 | CD4 | 4943 | Propofol | -4.85 | 1 | Thr233 | ∼1.715 |
| 3 | CD8 | 3763 | Isoflurane | -2.74 | 1 | Ser55 | ∼2.504 |
| 4 | CD8 | 4943 | Propofol | -3.70 | 1 | Ser116 | ∼1.985 |
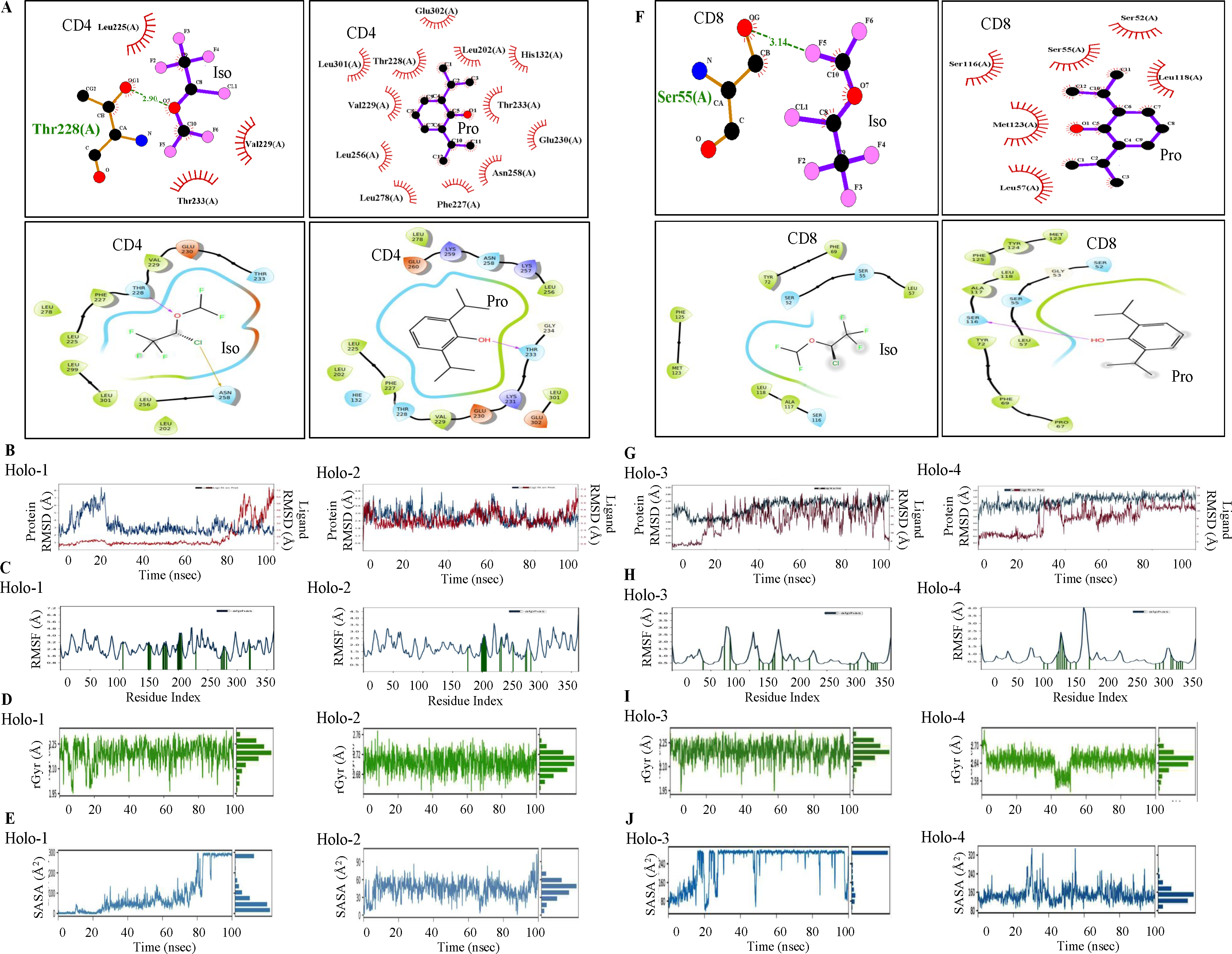
- Molecular docking and molecular dynamic (MD) simulations of Iso and Pro with CD4 and CD8. Intermolecular hydrogen bonding, electrostatic and hydrophobic interactions formed between (A) CD4-Iso/ Pro; (F) CD8-Iso/ Pro complex; MD simulation of Iso and Pro with CD4 and CD8. Conformational stability of Apo (blue) and Holo (red) states of CD4 and CD8 proteins throughout 100 nanoseconds (ns) time period of MD simulations as evidenced by Holo-1, -2, -3, and -4 profile of MDS backbone- (B and G) RMSD; (C and H) Cα-RMSF (protein residues that may interact with the ligand were marked with green-colored vertical bars); (D and I) rGyr; and (E and J) SASA analysis. Iso: isoflurane; Pro: propofol; Holo1, CD4-isoflurane complex; Holo-2, CD4-propofol complex; Holo-3, CD8-isoflurane complex; Holo-4, CD8- propofol complex; rGyr: radius of gyration, SASA: solvent accessible surface analysis.
Discussion
The choice of intravenous anesthetics over volatile anesthetics has always been controversial regarding the perturbation of immune response during the perioperative period and consequential cancer recurrence12,14,31. The non-compliance of the beneficial11 or the non-beneficial32,33 role of propofol might have been due to variations in cancer type, grade, stage, hormone status, race, geographical location, and other socioeconomic factors. Therefore, in this study, we have considered only two specific types of surgery (MRM/BCS) with similar perioperative treatment regimens for both the groups. isoflurane in comparison to propofol induced a suppressive effect on Th and Tc cell frequency during the intra- and postoperative period respectively and this was prominent in stage II and some of the molecular subtypes of breast cancer. Small sample size in the stage and molecular subtype subgroups of breast cancer may have restricted their statistical significance.
The Th cells inflict a direct anti-tumor response and help B lymphocytes to produce antibodies as a part of humoral immunity17. In the current investigation, we observed that parallel to Th cells, B cell frequency decreased significantly with isoflurane than propofol during the intra-operative period. It might be corroborated that the reduction in the Th cell frequency by isoflurane might be one of the factors responsible for the depletion of the B cells in these patients.
Interestingly, during the intra- and postoperative period, IL-10 was predominantly upregulated by isoflurane than propofol. The reduction in the frequency of Th cells might have been partially regulated by the elevated levels of IL-10 during the intraoperative period of the isoflurane group. This finding conformed with a report where IL-10 secretion by Th2 cells inhibited Th cell differentiation and survival in an in vivo model34. In this study, T cell activity did not significantly differ between propofol and isoflurane as conferred by the expression of TCR, CD28, and cytokines IL-2, TNF-α, and IFN-γ. In concurrence with our findings another study reported that both propofol and desflurane triggered a similar beneficial immune response in terms of preservation of IL-2/IL-4 during the perioperative period of breast surgery13.
Studies have shown that the interaction of CD4 and CD8 co-receptors with p56lck may initiate tyrosine phosphorylation cascade leading to T-cell activation. The CD4- and CD8-p56lck complexes regulate several events in T cells including activation of transcription factors for gene expression, activation of integrin and intracellular calcium mobilization which are of prime importance in T-cell immunity related studies35. It has been reported that binding of glycoprotein 120 of human immunodeficiency virus (HIV) to CD4 on the T cells plays an important role in the induction of apoptosis36. The interaction of CD4 coreceptor with p56lck in its cytoplasmic tail is crucial in accelerating the HIV-induced apoptosis of CD4+ T cells37. Therefore, we were interested to check whether these coreceptors CD4 and CD8 have any interaction with the anesthetic drugs using in silico studies. Molecular docking exhibited that propofol strongly bonded with CD4 and CD8 as compared to isoflurane. MDS propagated that propofol formed a more compact and stabilized structure with CD4/CD8 than isoflurane. SASA analysis further portrayed that the binding of propofol with CD4/CD8 altered their surface chemistry and buried their accessible amino acid residues which might have prevented them from undergoing any form of interaction. In comparison, the binding of isoflurane kept the amino acid residues of CD4/CD8 more accessible for interaction. These interactions might play important role in anesthetic-induced T cell regulation including proliferation and apoptosis. However, these need further experimental validations.
The study had some limitations. Firstly, the effect of the anesthetic agents on the subtypes of Th and Tc cells and the interactions between the immune cells and surrounding factors were not studies. Secondly, the experimental validation of the functional molecular cues involved in the suppression of Th cells is yet to be deciphered.
Several studies have shown that these anesthetics have a significant impact on long term cancer outcome and perioperative immunoinflammatory profile. Immunomodulation during the intraoperative period may have a significant effect on the cancer metastasis and recurrence. Therefore, an anesthetic drug with minimal immunosuppressive effect during the intraoperative period may be beneficial for clinical practice12. In this study, propofol which minimally perturbed the T cell immune response and better controlled inflammatory mediators during intra or postoperative period might be indicated as a better anesthetic choice over isoflurane during surgical resection of breast cancer.
Acknowledgment
Authors acknowledge Dr. Sushant Kumar Singh, Director, Centre for Artificial Intelligence and Environmental Sustainability (CAIES) Foundation, Patna for his statistical inputs.
Financial support & sponsorship
This study received institutional intramural financial support from Chittaranjan National Cancer Institute. SK acknowledges the Department of Biotechnology, Govt. of India for the BIC project grant (BT/PR40161/BTIS/137/32/2021).
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-63.
- [CrossRef] [PubMed] [Google Scholar]
- Breast cancer in India: Present scenario and the challenges ahead. World J Clin Oncol. 2022;13:209-218.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cancer Tomorrow. Available from: https://gco.iarc.fr/tomorrow/en, accessed on August 29, 2023.
- Comparisons of different general anesthetic techniques on immune function in patients undergoing flap reconstruction for oral cancer. Medicine (Baltimore). 2024;103:e38653.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Clinical pharmacokinetics and pharmacodynamics of propofol. Clin Pharmacokinet. 2018;57:1539-1558.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pharmacokinetics of isoflurane in human blood. Pharmacology. 2008;81:344-9.
- [CrossRef] [PubMed] [Google Scholar]
- Anti-inflammatory properties of anesthetic agents. Crit Care. 2017;21:67.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Survival benefits of propofol-based versus inhalational anesthesia in non-metastatic breast cancer patients: A comprehensive meta-analysis. Sci Rep. 2024;14:16354.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Survival after primary breast cancer surgery following propofol or sevoflurane general anesthesia-A retrospective, multicenter, database analysis of 6305 Swedish patients. Acta Anaesthesiol Scand. 2020;64:1048-1054.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med. 2018;16:8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of propofol and desflurane on immune cell populations in breast cancer patients: A randomized trial. J Korean Med Sci. 2015;30:1503-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of equipotent doses of propofol versus sevoflurane anesthesia on regulatory T cells after breast cancer surgery. Anesthesiology. 2018;129:921-931.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of propofol and sevoflurane on cancer cell, natural killer cell, and cytotoxic T lymphocyte function in patients undergoing breast cancer surgery: An in vitro analysis. BMC Cancer. 2018;18:159.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Expression profiles of immune cells after propofol or sevoflurane anesthesia for colorectal cancer surgery: A Prospective double-blind randomized trial. Anesthesiology. 2022;136:448-458.
- [CrossRef] [PubMed] [Google Scholar]
- Revisiting the role of CD4+ T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther. 2021;28:5-17.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Effect of propofol and isoflurane on NK cells: A pilot study on perioperative breast cancer patients from Eastern India. Int J Sci Res Arch. 2024;11:1744-54.
- [Google Scholar]
- National ethical guidelines for biomedical and health research involving human participants. Available from: https://ethics.ncdirindia.org/asset/pdf/ICMR_National_Ethical_Guidelines.pdf, accessed on August 29, 2023
- PubChem Compound Summary for CID 4943, Propofol. Available from: https://pubchem.ncbi.nlm.nih.gov/compund/Propofol, accessed on March 6, 2024.
- Sevoflurane versus isoflurane for maintenance of anesthesia: Are serum inorganic fluoride ion concentrations of concern? Anesth Analg. 1996;82:1268-72.
- [CrossRef] [PubMed] [Google Scholar]
- Determination of propofol using high performance liquid chromatography in whole blood with fluorescence detection. J Chromatogr Sci. 2012;50:162-6.
- [CrossRef] [PubMed] [Google Scholar]
- Microanalysis of propofol in human serum by semi-microcolumn high-performance liquid chromatography with UV detection and solid-phase extraction. J Clin Pharm Ther. 2001;26:381-5.
- [CrossRef] [PubMed] [Google Scholar]
- Differential effects of propofol and isoflurane on the activation of T-helper cells in lung cancer patients. Anaesthesia. 2010;65:478-82.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of propofol and sevoflurane on T-cell immune function and Th cell differentiation in children with SMPP undergoing fibreoptic bronchoscopy. Ann Med. 2022;54:2574-80.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Comparative analysis of CD4+ and CD8+ T cells in tumor tissues, lymph nodes and the peripheral blood from patients with breast cancer. Iran Biomed J. 2015;19:35-44.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The effects of perioperative anesthesia and analgesia on immune function in patients undergoing breast cancer resection: A prospective randomized study. Int J Med Sci. 2017;14:970-976.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Molecular biology of the cell (4th ed). New York: Garland Science; 2002.
- Revisiting the role of CD4+ T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther. 2021;28:5-17.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- CD4 and CD8 binding to MHC molecules primarily acts to enhance Lck delivery. Proc Natl Acad Sci U S A. 2010;107:16916-21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Impact of anesthetics on oncogenic signaling network: A review on propofol and isoflurane. Fundam Clin Pharmacol. 2022;36:49-71.
- [CrossRef] [PubMed] [Google Scholar]
- The role of regional anaesthesia in the emerging subspecialty of onco-anaesthesia: A state-of-the-art review. Anaesthesia. 2021;76(Suppl 1):148-159.
- [CrossRef] [PubMed] [Google Scholar]
- Total intravenous anesthesia versus inhalation anesthesia for breast cancer surgery: A retrospective cohort study. Anesthesiology. 2019;130:31-40.
- [CrossRef] [PubMed] [Google Scholar]
- CD4+ Th2 cells are directly regulated by IL-10 during allergic airway inflammation. Mucosal Immunol. 2017;10:150-161.
- [CrossRef] [PubMed] [Google Scholar]
- How the discovery of the CD4/CD8-p56lck complexes changed immunology and immunotherapy. Front Cell Dev Biol. 2021;9:626095.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- CD4 +T cell depletion in human immunodeficiency virus (HIV) infection: Role of apoptosis. Viruses. 2011;3:586-612.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- HIV-induced apoptosis requires the CD4 receptor cytoplasmic tail and is accelerated by interaction of CD4 with p56lck. J Exp Med. 1996;183:39-48.
- [CrossRef] [PubMed] [Google Scholar]






