Translate this page into:
MALDI-TOF mass spectrometry proteomic based identification of clinical bacterial isolates
Reprint requests: Dr J.C. Samantaray, Department of Microbiology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110 029, India e-mail: drjcsamantaray@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Pathogenic bacteria often cause life threatening infections especially in immunocompromised individuals. Therefore, rapid and reliable species identification is essential for a successful treatment and disease management. We evaluated a rapid, proteomic based technique for identification of clinical bacterial isolates by protein profiling using matrix-assisted laser desorption-ionization time - of - flight mass spectrometry (MALDI-TOF MS).
Methods:
Freshly grown bacterial isolates were selected from culture plates. Ethanol/formic acid extraction procedure was carried out, followed by charging of MALDI target plate with the extract and overlaying with α-cyano-4 hydroxy-cinnamic acid matrix solution. Identification was performed using the MALDI BioTyper 1.1, software for microbial identification (Bruker Daltonik GmbH, Bremen, Germany).
Results:
A comparative analysis of 82 clinical bacterial isolates using MALDI -TOF MS and conventional techniques was carried out. Amongst the clinical isolates, the accuracy at the species level for clinical isolates was 98.78%. One out of 82 isolates was not in accordance with the conventional assays because MALDI-TOF MS established it as Streptococcus pneumoniae and conventional methods as Streptococcus viridans.
Interpretation & conclusions:
MALDI - TOF MS was found to be an accurate, rapid, cost-effective and robust system for identification of clinical bacterial isolates. This innovative approach holds promise for earlier therapeutic intervention leading to better patient care.
Keywords
Clinical bacterial isolates
identification
MALDI-TOF MS
Rapid and reliable species identification of pathogenic clinical bacterial isolates is conventionally dependent upon phenotypic (staining, culture, and serology) and genotypic (DNA and RNA) characteristics of the microorganisms. Routine characterization of these isolates is often a time taking process involving long incubation periods, battery of biochemical reactions and requires considerable expertise. For the treatment with appropriate antimicrobial agent a fast and reliable identification can be time saving. Commercially available enzymatic panels, for example, the VITEK ID YST System (bioMérieux, France) is widely used for this purpose, though these are occasionally disadvantageous due to limited database and misidentification has also been reported12.
As an alternative to various other identification methods, mass spectral analysis for identification of microorganisms has been recognized. Matrix-assisted laser desorption-ionization-time–of-flight mass spectrometry (MALDI-TOF MS) can be used for accurate and rapid identification of various microorganisms34, such as Gram - positive bacteria567891011, Enterobacteriaceae12, yeast131415, mold16, non-fermenting bacteria17181920 and mycobacteria2122232425262728. This technique is based upon the detection of highly abundant proteins in a mass range between 2 and 20 kDa by computing their mass (m) to charge (z), m/z values. Thus, a typical fingerprint is generated for each microorganism which is used for comparison with the stored reference spectra and thereby providing identification for the sample. The striking advantage of mass spectral approach over phenotypic and genotypic procedures is a simple straight forward sample preparation procedure and the short time required for analysis.
In this study, we evaluated the suitability of identification of clinical bacterial isolates by MALDI-TOF MS. The technology was used for the identification of 82 clinical samples with part of the study being under routine diagnostic conditions, this approach was validated by conventional cultural, morphological and biochemical criteria as well as the VITEK ID YST system for isolates with discrepant result.
Material & Methods
This study was conducted in the department of microbiology, All India Institute of Medical Sciences (AIIMS), New Delhi, India during August 2012 to December 2012. A total of 82 fresh bacterial clinical isolates (blood, urine, pus, biopsy, swab from any site of the body, cerebrospinal fluid, respiratory tract and wound specimens) analyzed in the present study, were collected from the routine clinical diagnostic laboratory of AIIMS. The following ATCC reference strains were used as controls in this study for MALDI-TOF MS analyses: Escherichia coli ATCC 25922; Pseudomonas aeruginosa ATCC 27853; Staphylococcus aureus ATCC 25923; Neisseria meningitidis ATCC 13077.
Culture conditions and sampling (conventional technique): The isolates were recovered after aerobic and microaerophilic incubation at 35 - 37°C on 5 per cent sheep blood agar, chocolate agar, cysteine-lactose electrolyte-deficient (CLED) agar, Macconkey's agar and Thayer Martin agar (Hi-media Laboratories, Mumbai, India). A single colony of a (sub) culture was further processed and deposited in duplicate on a MALDI-TOF plate (Bruker Daltonik GmbH, Bremen, Germany). Simultaneously, biochemical identification was performed on the isolates after Gram staining29. Briefly, catalase test, oxidase test, indole test, urease production test, citrate utilization test, nitrate reduction test, sugar fermentation test, phenylpyruvic acid production test, oxidative fermentation test, amino acid decarboxylation test and triple sugar iron (TSI) media for Gram-negative isolates and catalase test, coagulase test and bile esculin production test for Gram-positive isolates were used. Reagents for these biochemical tests were supplied by Hi-media Laboratories, Mumbai, India. Individuals performing one method of identification were unaware of the results obtained from the other method.
Sample preparation for MALDI-TOF MS: Ethanol/formic acid extraction procedure was followed according to the manufacture's instruction (Bruker Daltonik GmbH, Bremen, Germany). Briefly, 2-3 isolated colonies were transferred into the 1.5 ml screw cap extraction tube with an inoculation loop or pipette tip and mixed thoroughly in 300 μl of double distilled water. Absolute ethanol (0.9 ml) was added, the contents of the tube were carefully mixed, and the tubes were then centrifuged at 13,000 × g for 2 min; the supernatant was discarded and the pellet was air dried. Approximately 10 μl of the pellet was mixed thoroughly with 50 μl of formic acid (70%), before addition of an equivalent volume of acetonitrile. The mixture was centrifuged at 13,000 × g for 2 min and 1 μl of the supernatant was placed onto a ground steel MALDI target plate and allowed to dry at room temperature. Subsequently, each sample was overlaid with 1 μl of matrix solution, which consisted of a saturated solution of α-cyano-4 hydroxy-cinnamic acid (HCCA) in 50 per cent acetonitrile and 2.5 per cent trifluoroacetic acid (final concentration: 10 mg HCCA/ml) and air dried at room temperature. The MALDI-TOF MS target was subsequently introduced into the MALDI-TOF mass spectrometer (Brucker Daltonik GmbH, Bremen, Germany) for automated measurement and data interpretation. For identification, all the samples were blinded and run in duplicates.
The samples were analyzed using a microflex LT MALDI-TOF MS instrument (Bruker Daltonik GmbH, Bremen, Germany). The spectra were recorded in the linear positive mode at a laser frequency of 20 Hz within a mass range from 2,000 to 20,000 Da. Parameter settings for microflex instrument were ion source 1 at 20 kV, ion source 2 at 18.5 kV, lens at 8.5 kV, pulsed ion extraction of 250 ns and no gating.
Freshly prepared Bruker Bacterial Test Standard Escherichia coli (#255343) (Bruker Daltonik GmbH, Bremen, Germany) was used as the reference strain for the calibration and instrument parameter optimization. MALDI Biotyper Calibration Procedure was followed according to manufacturer's instruction. Briefly, right flexControl method was used and one sum spectrum was measured at the desorption level of the laser energy using inbuilt AutoX method. The software selects the calibration peaks in the range of ± 300 ppm automatically and performs calibration. Once the calibration was achieved, the calibration values were saved and were further applied to the measurement of clinical experimental strains. The mass accuracy after external calibration using Bruker Bacterial Test Standard Escherichia coli (#255343) protein signals was about 200 ppm.
Data processing: Initial manual/visual estimation of the mass spectra was performed using the FlexAnalysis 2.4 software (Bruker Daltonik GmbH, Germany). For automated data analysis, raw spectra were processed using the MALDI BioTyper 1.1 software (Bruker Daltonik GmbH, Germany) with default settings. The smoothing, normalization, baseline subtraction and peak picking was carried out by the software, thereby creating a list of the most significant peaks of a spectrum (m/z values with a given intensity). The generated peak lists derived from the bacterial MALDI-TOF profile mass spectra were compared with each entry of the MALDI Biotyper database, which currently contains 3287 references, using the standard parameters of the pattern-matching algorithms. These algorithms have different mathematical approaches, which have already been described303132. The results of the pattern-matching process were expressed as log (score) values, computed by comparison of the peak list for an unknown isolate with the reference main spectral pattern (MSP) in the database. The log (score) value ranged from 0 to 3, a log (score) value ≥1.7 is indicative of a close relationship (i.e., at the genus level) and a log (score) value ≥2.0 is the set threshold for a match at the species level. The highest log (score) of a match against the score in the database was used for species identification.
Criteria for identification and discrepant analysis: When routine conventional biochemical identification and MALDI-TOF MS had exactly the same identification to the species level, the identification was considered final. In the case of discrepant results, the results from the external laboratory [Jai Prakash Narayan Apex Trauma Center (JPNATC), AIIMS, New Delhi, India] performed using VITEK ID YST system (bioMerieux, France) was considered as final identification. Inaccurate identification at genus level was considered a major error.
Results
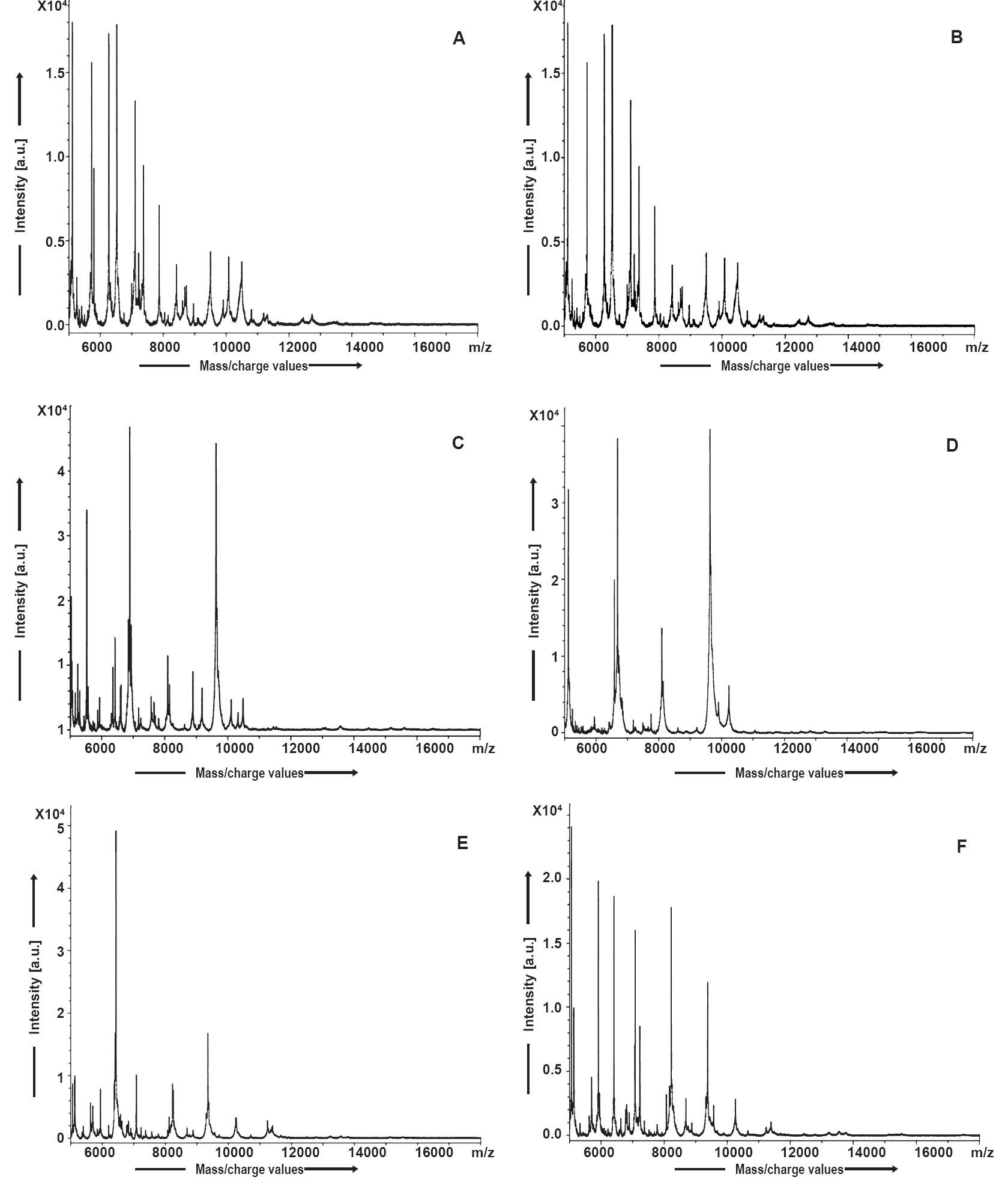
In this study, a total of 82 clinical bacterial culture isolates and 12 ATCC controls were analyzed by MALDI-TOF MS. It was found that all the 12 ATCC reference strains were correctly identified by MALDI TOF MS with a log (score) values of >2.30 which indicated ‘highly probable species identification’. Among the total 82 bacterial isolates, 23 isolates were identified as Escherichia coli, 13 isolates were identified as Staphylococcus aureus, 10 as S. epidermidis, four were identified as Neisseria gonorrhoeae, eight isolates were identified as Acinetobacter baumannii, seven were identified as Pseudomonas aeruginosa, two were identified as Salmonella sp., six isolates were identified as Klebsiella pneumoniae, three as Stenotrophomonas maltophilia, two as Enterococcus faecium, two Streptococcus pneumoniae, one isolate each as Delftia acidovorans and N. meningitidis.
Conventional tests were also performed simultaneously on the same 82 clinical bacterial culture isolates, and 23 isolates were identified as E. coli, 15 isolates were identified as S. aureus, 8 as S. epidermidis, four as N. gonorrhoeae, eight as A. baumannii, eight as P. aeruginosa, two isolates were identified as Salmonella sp., six as K. pneumoniae, three as Stenotrophomonas maltophilia, two isolates were identified as E. faecium, one each as N. meningitidis, S. pneumoniae and Streptococcus viridans were identified.
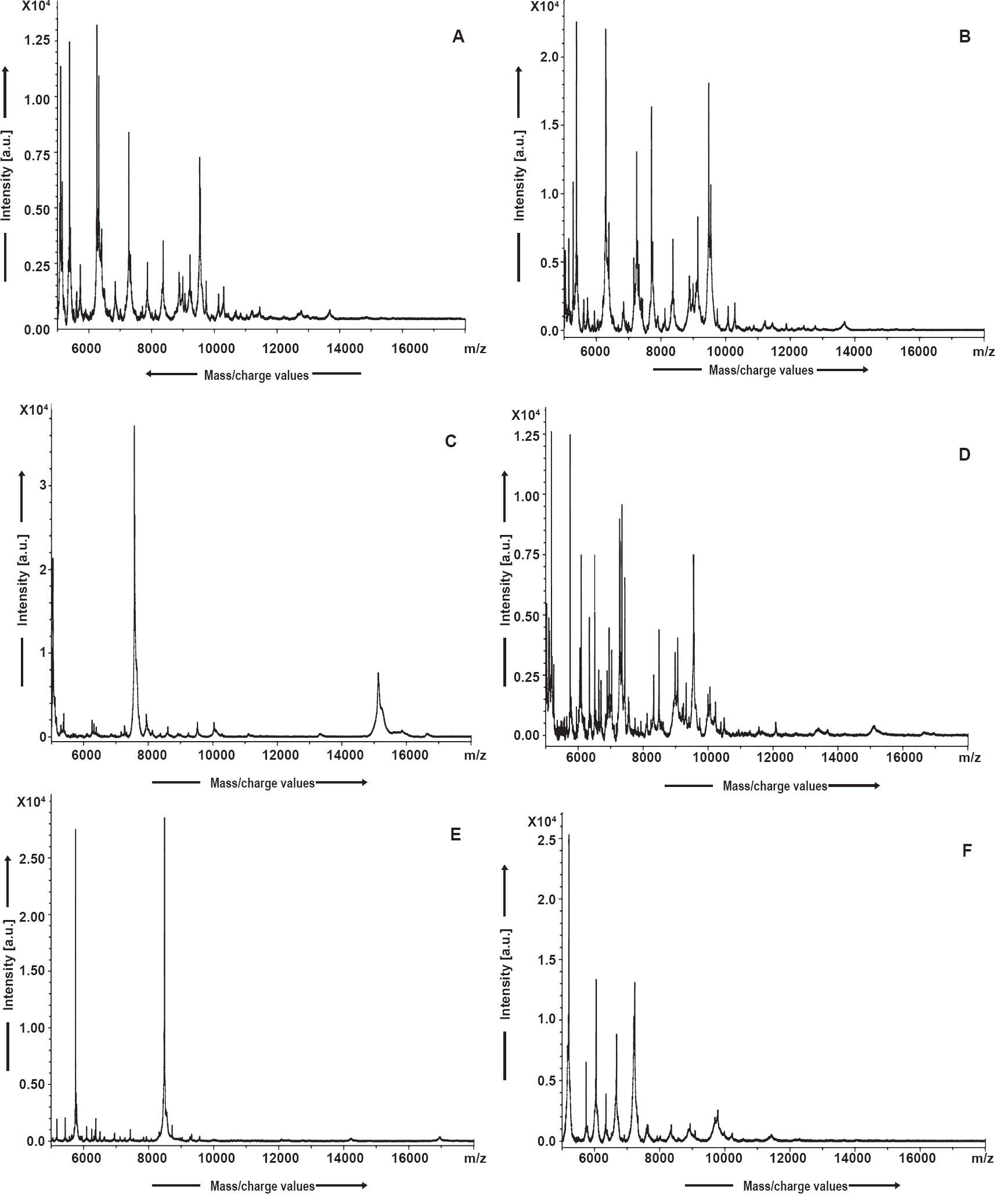
On comparing and analyzing the results obtained by MALDI-TOF MS with those from conventional techniques, it was noticed that four samples showed discrepant results (Table I). The discrepant results were reanalyzed by an external laboratory and three were found to be in agreement with MALDI-TOF MS result (Table II). The remaining one isolate was an inherent limitation of the BioTyper software which fails to distinguish Streptococcus viridans from Streptococcus pneumoniae. Therefore, the accuracy at the species level for clinical isolates was 98.78% (81/82). The spectral processing results have shown signature peaks for a particular species (Fig. 1 and 2).
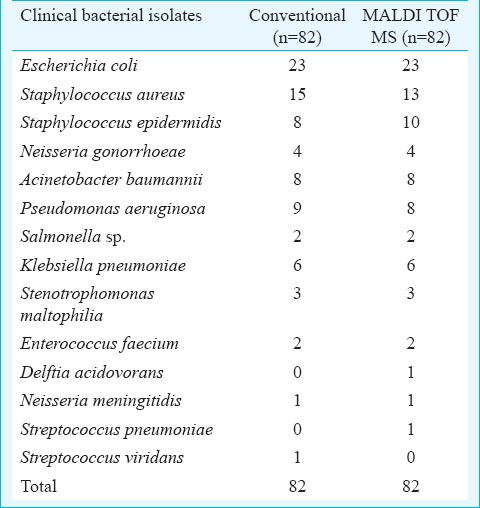

- MALDI-TOF MS mass spectra of Escherichia coli (A), Klebsiella pneumoniae (B), Salmonella sp. (C), Enterococcus faecium (D), Acenitobacter baumannii (E), Pseudomonas aeruginosa (F).
- MALDI-TOF MS mass spectra of Stenotrophomonas maltophilia (A), Delftia acidovorans (B), Staphylococcus aureus (C), Staphylococcus epidermidis (D), Neisseria meningitidis (E), Neisseria gonorrhoeae (F).
Discussion
MALDI-TOF MS has successfully been used for the identification of a wide array of bacterial species1213141516171819202122232425262728. However, only a few studies have assessed the performance of MALDI-TOF MS based identification under routine laboratory conditions. In our study, MALDI-TOF MS could accurately identify all the ATCC controls deposited in duplicate. The results obtained from our study suggested that identification of different bacterial species and the different strains within, was possible using MALDI-TOF MS. Since our study was a blinded one with the samples being processed and run in duplicates, the reproducibility of the instrument was tested and was subsequently found to be consistent for all the samples. The MALDI MS mass spectral patterns were also reproducible for the isolates belonging to the same genus and species.
In the present study, identification by MALDI-TOF MS yielded a valid score for 78 of 82 (87.80%) isolates when compared to results obtained by conventional methods. On carrying out an extensive analysis of the conventionally verified isolates, four discordant results were noticed. The discrepant results were reanalyzed by an external laboratory and three were found to be in agreement with MALDI-TOF MS result. Therefore, the accuracy at the species level for clinical isolates identified by MALDI TOF MS was 98.78 per cent. The remaining one isolate which was considered discordant in the study could be ascribed to an inherent limitation of the BioTyper software which failed to distinguish Streptococcus viridans from Streptococcus pneumoniae, due to inaccurate taxonomic assignment of the spectra in the MALDI-TOF MS database. The taxonomical discordances can be easily corrected by an update of the database.
In previous studies, investigators have demonstrated the applicability of MALDI-TOF MS for identification of various bacterial species, but only a fraction has concentrated on clinical isolates3456789101112171819202122232425262728. In the present study, the prime focus was on clinical bacterial isolates at a tertiary care hospital. Since a majority of the protein signals occurred between 2 to 13 KDa, it could imply that a significant portion of the signals was derived from ribosomal proteins which range from 2000 to 20,000 Daltons33. Within the observed mass range, a few unique signals were conserved among the isolate of same species. Such conclusions were reached by other researchers as well192021.
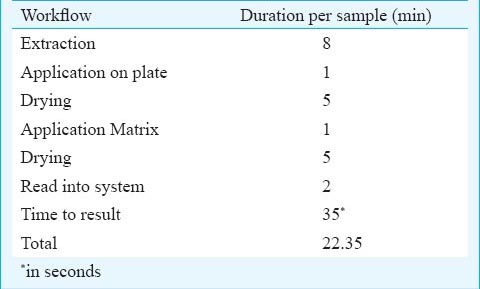
The identification using MALDI -TOF MS method could analyse samples rapidly within minutes after culture positivity. Typically, the extraction procedure for a single sample takes calculated 23 min (Table III), but per sample processing time is reduced by batch processing (approximately 3 h for 82 samples) and thereby facilitates high throughput outcome. However, identification by conventional biochemical methods is a time consuming process requiring 24-48 h after culture positivity, thereby delaying therapeutic interventions. In addition to this, the simple extraction procedure, low running cost and the non requirement of high technical expertise provide MALDI-TOF MS an edge over the other methods for identification. But the application must be carefully performed, as the quality of the result reduces by the application of too much of material. Furthermore, care should be taken when the sample is being overlaid with the matrix solution to not to induce a liquid smear between spots, which may result in cross -contamination.
In conclusion, our study demonstrated that owing to its rapid and accurate nature, MADLI-TOF MS could be a possible alternate diagnostic tool for identification and differentiation of routine isolates in the clinical microbiology laboratory setting. The high throughput potential saves considerable time for earlier therapeutic intervention and better patient care. It may further be validated with larger sample numbers in a multicentric study.
Acknowledgment
Authors acknowledge Shriyut Rajesh Vashisht, Bala Sankar Thangasamy at Bruker Daltonik Pvt. Ltd., Chennai for their technical assistance; Dr Purva Mathur at Jai Prakash Narayan Apex Trauma Center (JPNATC), New Delhi, India, for her invaluable guidance and support. Authors thank the All India Institute of Medical Sciences (AIIMS), New Delhi, India, for providing financial support for this study.
References
- Resolution of discrepant results for Candida species identification by using DNA probes. J Clin Microbiol. 2004;42:858-61.
- [Google Scholar]
- Comparison of VITEK 2 with ITS2-fragment length polymorphism analysis for identification of yeast species. J Clin Microbiol. 2004;42:2209-11.
- [Google Scholar]
- Matrix-assisted laser desorption ionization time-of-flight mass spectrometry, a revolution in clinical microbial identification. Clin Microbiol Infect. 2010;16:1614-9.
- [Google Scholar]
- Matrix-assisted laser desorption ionization-time of flight mass spectrometry for the identification of bacteria and yeasts in a clinical microbiological laboratory: A review. Acta Clin Belg. 2011;66:267-73.
- [Google Scholar]
- Rapid identification and typing of Listeria species by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl Environ Microbiol. 2008;74:5402-7.
- [Google Scholar]
- Identification and discrimination of Staphylococcus aureus strains using matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Proteomics. 2002;2:747-53.
- [Google Scholar]
- Rapid identification of viridans streptococci by mass spectrometric discrimination. J Clin Microbiol. 2007;45:2392-7.
- [Google Scholar]
- Challenging the problem of clostridial identification with matrix-assisted laser desorption and ionization-time-of-flight mass spectrometry (MALDI-TOF MS) Anaerobe. 2008;14:242-9.
- [Google Scholar]
- MALDI-TOF mass spectrometry as a tool for differentiation of invasive and noninvasive Streptococcus pyogenes isolates. FEMS Immunol Med Microbiol. 2008;53:333-42.
- [Google Scholar]
- Rapid characterization of spores of Bacillus cereus group bacteria by matrix-assisted laser desorption-ionization time-of-flight mass spectrometry. Appl Environ Microbiol. 2000;66:3828-34.
- [Google Scholar]
- Identification of Gram-positive cocci using MALDI-TOF MS: comparison of different preparation methods and implementation of a practical algorithm for routine diagnostics. J Clin Microbiol. 2013;51:1834-40.
- [Google Scholar]
- Phyloproteomics: secies identification of Enterobacteriaceae using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Mol Microbiol Biotechnol. 2001;3:103-12.
- [Google Scholar]
- Matrix-assisted laser desorption ionization-time of flight mass spectrometry for fast and reliable identification of clinical yeast isolates. J Clin Microbiol. 2009;47:2912-7.
- [Google Scholar]
- MALDI-TOF mass signatures for differentiation of yeast species, strain grouping and monitoring of morphogenesis markers. Anal Bioanal Chem. 2008;392:439-49.
- [Google Scholar]
- Differentiation of species of the genus Saccharomyces using biomolecular fingerprinting methods. Appl Microbiol Biotechnol. 2013;97:4597-606.
- [Google Scholar]
- Development of a clinically comprehensive database and a simple procedure for identification of molds from solid media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2013;51:828-34.
- [Google Scholar]
- Matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of nonfermenting gram-negative bacilli isolated from cystic fibrosis patients. J Clin Microbiol. 2008;46:3361-7.
- [Google Scholar]
- High interlaboratory reproducibility of matrix-assisted laser desorption ionization-time of flight mass spectrometry-based species identification of nonfermenting bacteria. J Clin Microbiol. 2009;47:3732-4.
- [Google Scholar]
- Evaluation of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry in comparison to 16S rRNA gene sequencing for species identification of nonfermenting bacteria. J Clin Microbiol. 2008;46:1946-54.
- [Google Scholar]
- Rapid identification of Burkholderia cepacia complex species including strains of the novel Taxon K, recovered from cystic fibrosis patients by intact cell MALDI-ToF mass spectrometry. Analyst. 2009;134:1138-48.
- [Google Scholar]
- Novel mass spectrometry-based tool for genotypic identification of mycobacteria. J Clin Microbiol. 2004;42:339-46.
- [Google Scholar]
- Identification of mycobacteria by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry. J Clin Microbiol. 2006;44:1963-70.
- [Google Scholar]
- Discrimination of intact mycobacteria at the strain level: A combined MALDI-TOF MS and biostatistical analysis. Proteomics. 2006;6:6416-25.
- [Google Scholar]
- Mass spectrometry based methods for the discrimination and typing of mycobacteria. Infect Genet Evol. 2012;12:838-45.
- [Google Scholar]
- Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry identification of mycobacteria in routine clinical practice. PLoS One. 2011;6:e24720.
- [Google Scholar]
- Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49:1790-4.
- [Google Scholar]
- Rapid identification of mycobacterial whole cells in solid and liquid culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2010;48:4481-6.
- [Google Scholar]
- Rapid identification of clinical mycobacterial isolates by protein profiling using matrix assisted laser desorption ionization-time of flight mass spectrometry. Indian J Med Microbiol. 2013;31:117-22.
- [Google Scholar]
- Guidelines for the collection, transport, processing, analysis and reporting of cultures from specific specimen sources. In: Washington CW, ed. Koneman's color atlas and textbook of diagnostic microbiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. p. :68-111.
- [Google Scholar]
- Fingerprint matching of E. coli strains with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of whole cells using a modified correlation approach. Rapid Commun Mass Spectrom. 1998;12:630-6.
- [Google Scholar]
- An algorithm for automated bacterial identification using matrix-assisted laser desorption/ionization mass spectrometry. Anal Chem. 2000;72:1217-23.
- [Google Scholar]
- Testing the significance of microorganism identification by mass spectrometry and proteome database search. Anal Chem. 2000;72:3739-44.
- [Google Scholar]
- Fast and reliable MALDI-TOF MS-based microorganism identification. Chem Today. 2007;25:68-71.
- [Google Scholar]






