Translate this page into:
M types & toxin gene profile of group A streptococci isolated from children in Dibrugarh district of Assam, India
* For correspondence: jmahanta@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Streptococcus pyogenes is one among the more than 100 known species of the genus Streptococcus1 and invasive group A streptococci (GAS) infection is associated with mortality and morbidity globally2. Streptococcal M protein, pyrogenic exotoxins (SPE), streptococcal mitogenic exotoxin Z (SMEZ) and streptococcal superantigen (SSA) are among the virulence determinants of GAS3. Invasiveness of the GAS has been associated with the presence of these virulence factors3. It is necessary to know the molecular epidemiology of a bacterial strain to plan any preventive strategy. The M protein is encoded by the emm gene. This is one of the candidate genes that have been studied for vaccine development though its success as a vaccine candidate has been questioned due to the variation of circulating strains in different geographical regions4. New emm types are being continuously added to the Centers for Disease Control and Prevention (CDC) database5. The production of the toxin enzymes has been shown to vary between different emm types6. Studies have documented geographical variation of the emm types as well as toxin gene prevalence among the isolates of streptococci7-11. A higher incidence of SPE A, B and F has been found among GAS isolates from rheumatic fever/rheumatic heart disease (RF/RHD) cases as compared to pharyngeal isolates from India8. In the present study we tried to evaluate the frequency of nine toxin genes namely spe A, B, C, F, G, H, J, streptococcal mitogenic exotoxin Z (smeZ) and streptococcal superantigen (ssa) as well as emm types among the GAS isolates from throats of healthy school children and from their skin lesions from the northeastern region of India.
The GAS isolates (n=14) obtained during a previous study12 were used. Isolates were stored in 15 per cent glycerol stock at -70°C. Identification was done by Gram stain, catalase test and bacitracin (0.04 units) susceptibility test as mentioned previously12. The isolates were Lancefield serogrouped using HiStrep Latex test kit (Hi-Media, Mumbai) according to the manufacturer's instructions12.
For the isolation of bacterial genomic DNA, a loopful of colony was inoculated into 1.5 ml Todd Hewitt broth (Hi-Media, Mumbai) with added yeast extract and incubated overnight at 37°C. Bacterial cells were harvested by centrifugation and DNA was recovered using Gene JET Genomic DNA Purification kit (Fermentas, Thermo Scientific, Lithuania) according to the manufacturer's instruction. DNA was stored at -20 °C for later use.
M (emm) typing was carried out as per protocol described by CDC13 with modification in the thermal profile for carrying out PCR. Emm primer 1: 5’-TATTCGCTTAGAAAATTAA-3’ and primer 2: 5’-GCAAGTTCTTCAGCTTGTTT-3’ available at CDC site (http://www.cdc.gov/streplab/protocol-emm-type.html) were taken for M typing. Ten pmol of each primer was used in 50 µl PCR reaction mixture (2x PCR master mix, Promega, USA) to amplify the emm gene. The PCR reaction was done in a thermal cycler (Veriti 96 well thermal cycler, Applied Biosystems-Life Technologies Corporation), and the thermal profile used for the PCR was as follows: initial denaturation at 94°C for 10 min, 30 cycles of denaturation at 94°C for one min, primer annealing at 50°C for one min, extension at 72°C for 90 sec and final extension for seven min at 72°C. PCR product (5 µl) was mixed with 1 µl of 6x gel loading dye (Promega, USA), and subjected to electrophoresis on 1.5 per cent agarose gel to confirm the PCR amplification. The amplified product was purified using Roche DNA Purification Kit (Roche Diagnostics GmbH Mannheim, Germany) following which commercial sequencing was done (Xcelris Labs Limited, Ahmadabad, India). Sequences obtained were assembled and aligned using CodonCode Aligner software (http://www.codoncode.com/aboutus.htm). The resultant emm gene sequence was initially searched for homology by BLAST (Basic Local Alignment Search Tool) search analysis (https://blast.ncbi.nlm.nih.gov/Blast) and sequences were finally submitted to CDC website (cdc.gov/ncidod/biotech/strep/strepblast.htm) for allotment of emm type.
Individual PCRs were performed to check the presence of streptococcal pyrogenic exotoxin A (speA), B(speB), C(speC), F (speF) G(speG), H(speH), J(speJ), smeZ and ssa in all the streptococcal isolates with the reported primers7. PCRs were performed 30 cycles each consisting of an initial denaturation at 96°C for five min, denaturation at 96°C for 50 sec, annealing at 44°C for 65 sec, elongation at 72°C for 70 sec and final elongation at 72°C for five min. PCR products were resolved on a 2 per cent (w/v) agarose gel. Staphylococcus aureus ATCC 29213 was taken as negative control.
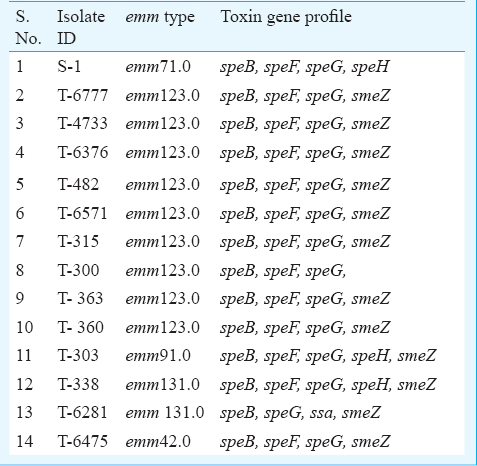
Table I shows the emm types of GAS isolates. A total of five different emm types were identified among the 14 isolates. The most common emm type was 123.0. Five different toxin gene profiles were observed among the 14 isolates. The genes speB and G, were detected in all the isolates, speF in 13, smeZ in 12, speH in three, ssa in one whereas speA, speC, speJ were detected in none of the isolates. All isolates belonging to emm 123.0 except one had carried speB, speF, speG, smeZ genes. A similar pattern of toxin gene profile was observed among isolates belonging to both emm 131.0 (one isolate) and emm 91.0 while isolate belonging to emm 42.0 had a similar pattern with that of emm 123.0. The skin isolate belonged to 71.0 emm type and its toxin gene profile was different from the throat isolates.
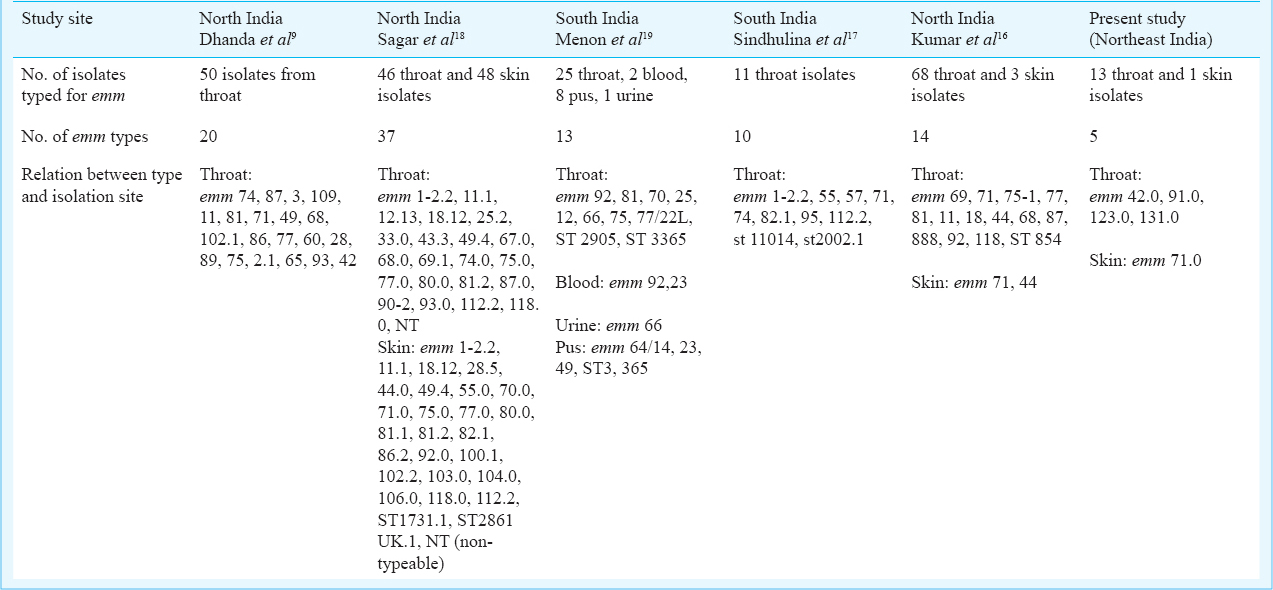
A large scale study done in India which included ten centres including Dibrugarh, reported the prevalence of rheumatic heart disease to vary from 0.13 to 1.5/1000 (overall 0.9/1000) in the 5 to 14 yr age group14. The M protein has been implicated in the pathogenesis of rheumatc fever/ rheumatic heart disease, a post-streptoccal sequelae, due to its molecular mimicry with host proteins2. There seems to be variation of emm type distribution and toxin gene profile within the country891011151617181920. Much of the existing data are from northern and southern part of India and no such data are available from northeastern part of India. The present study though includes small number of isolates, provides baseline data for future reference.
M types 1, 3, 5, 6, 14, 18, 19, 24, 27, and 29 are considered rheumatogenic while 1, 2, 4, 12, 15, 18, 25, 42, 49, 55, 56, 57, 59, 60, 61 are known to have nephritogenic potential3. The emm profile of the study isolates was found to be different from that reported from other parts of the country (Table II). In the present study, one of the isolates belonged to emm type 42.0 which is known to be nephritogenic.
The toxin gene profile among the GAS isolates in the present study also differed to some extent from that reported from other parts of the country. In contrast to an Indian study10, speA and speC were not detected among our isolates. SpeB which is a highly conserved gene, was detected in 100 per cent of the study isolates and similar finding was also observed in other geographic region21. SpeB antigen of Streptococcus has been proposed to have a causal role in glomerulonephritis. From different geographical regions of the country this gene was reported in 33.39 to 97 per cent820 of the GAS isolates. In the present study speG was present in all the isolates while it was found in 86 per cent of the invasive isolates from north and south India11. In our study speF was found in 93 per cent isolates while in another Indian study its prevalence was 88 per cent8; speJ was not detected in any of our isolates which was in conformity with an Indian study15. Haggar et al11 reported detection of speJ in 28 per cent of invasive isolates.
Thus, there exist different M types in this part of the country with a variation in the toxin gene profile. Continuous surveillance for monitoring of prevalence of M types would be helpful for early identification of emergence of new strains.
Acknowledgment
The authors acknowledge the financial support from Indian Council of Medical Research (ICMR), New Delhi.
Conflicts of Interest: None.
References
- List of Prokaryotic names with standing in nomenclature-genus Streptococcus. Available from: http://www.bacterio.net
- [Google Scholar]
- Streptococcus pyogenes and re-emergence of scarlet fever as a public health problem. Emerg Microbes Infect. 2012;1:e2.
- [Google Scholar]
- Centers for Disease Control and Prevention. Streptococcus pyogenes emm sequence database. Available from: http://www.cdc.gov/ncidod/biotech/strep/types_emm103-124.htm
- [Google Scholar]
- Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470-511.
- [Google Scholar]
- Toxin -gene profile heterogeneity among endemic invasive European group A streptococcal isolates. J Infect Dis. 2003;188:1578-86.
- [Google Scholar]
- Association of pyrogenic exotoxin genes with pharyngitis and rheumatic fever/rheumatic heart disease among Indian isolates of Streptococcus pyogenes. Lett Appl Microbiol. 2002;35:237-41.
- [Google Scholar]
- Group A Streptococcus virulence factors genes in north India & their association with emm type in pharyngitis. Indian J Med Res. 2011;133:110-5.
- [Google Scholar]
- speA and speC toxin genes among group A Streptococcus isolates from schoolchildren in Chennai, India. J Med Microbiol. 2007;56:1574-5.
- [Google Scholar]
- Clinical and microbiologic characteristics of invasive Streptococcus pyogenes infections in north and south India. J Clin Microbiol. 2012;50:1626-31.
- [Google Scholar]
- The prevalence and antimicrobial susceptibility patterns of beta-hemolytic streptococci colonizing the throats of school children in Assam, India. J Infect Dev Ctries. 2011;5:804-8.
- [Google Scholar]
- Centers for Disease Control and Prevention. Streptococcus Laboratory, Protocol for emm typing. http://www.cdc.gov/streplab/protocol-emm-type.html
- [Google Scholar]
- Rheumatic fever and rheumatic heart disease: The last 50 years. Indian J Med Res. 2013;137:643-58.
- [Google Scholar]
- Superantigen profiles of emm and emm-like typeable and nontypeable pharyngeal streptococcal isolates of south India. Ann Clin Microbiol Antimicrob. 2012;11:3.
- [Google Scholar]
- Epidemiology of group A streptococcal pharyngitis & impetigo: a cross-sectional & follow up study in a rural community of northern India. Indian J Med Res. 2009;130:765-71.
- [Google Scholar]
- Bacteriological and molecular studies of group A streptococcal pharyngitis in a south Indian hospital. Indian J Med Microbiol. 2008;26:197-8.
- [Google Scholar]
- Comparative analysis of emm type pattern of group A Streptococcus throat and skin isolates from India and their association with closely related SIC, a streptococcal virulent factor. BMC Microbiol. 2008;8:150.
- [Google Scholar]
- EMM types of Streptococcus pyogenes in Chennai. Indian J Med Microbiol. 2001;19:161-2.
- [Google Scholar]
- Clinical and molecular epidemiology of beta-hemolytic streptococcal infections in India. J Infect Dev Ctries. 2014;8:297-303.
- [Google Scholar]
- Evaluation of emm gene types, toxin gene profiles and clonal relatedness of group A streptococci. Bosn J Basic Med Sci. 2013;13:163-9.
- [Google Scholar]





