Translate this page into:
Laboratory evaluation of molluscicidal & mosquito larvicidal activities of leaves of Solanum nigrum L.
Reprint requests: Dr Goutam Chandra, Professor, Department of Zoology, Mosquito & Microbiology Research Units Parasitology Laboratory, The University of Burdwan, Golapbag, Burdwan 713 104, India e-mail: goutamchandra63@yahoo.co.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Indiscriminate use of synthetic pesticides has created serious problem for the aquatic flora and fauna, and also resulted in appearance of pesticide resistance in vector population. This study was designed to evaluate the biocontrol efficacy of aqueous and solvent extracts of mature leaves of Solanum nigrum L., against fresh water snail Lymnaea acuminata f. rufescens (Gray) (an intermediate host of parasites causing fasciolopsiasis) and larvae of Culex vishnui group (Reuben) (vector of Japanese encephalitis).
Methods:
Aqueous and solvent extracts of fresh, mature, green/shed dried leaves of S. nigrum were tested against adult L. acuminata and larvae of Cx. vishnui group. The lethal concentration was determined and the appropriate lethal concentration at 24 h of benzene extract was also studied on non target organisms such as Daphnia sp, Diplonychus annulatum and Chironomus circumdatus. A qualitative phytochemical analysis was carried out in search of active ingredient and the chemical nature of the active substance was also evaluated by infrared (IR) analysis.
Results:
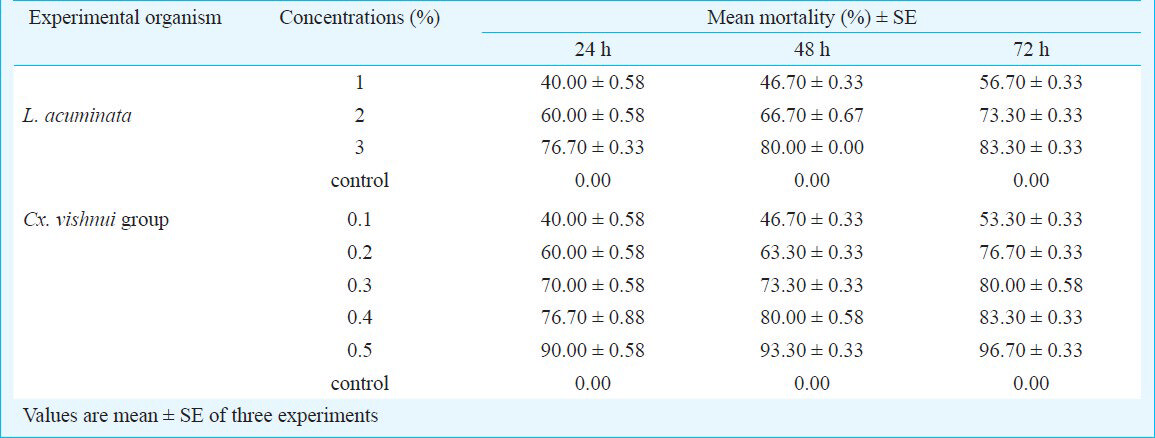
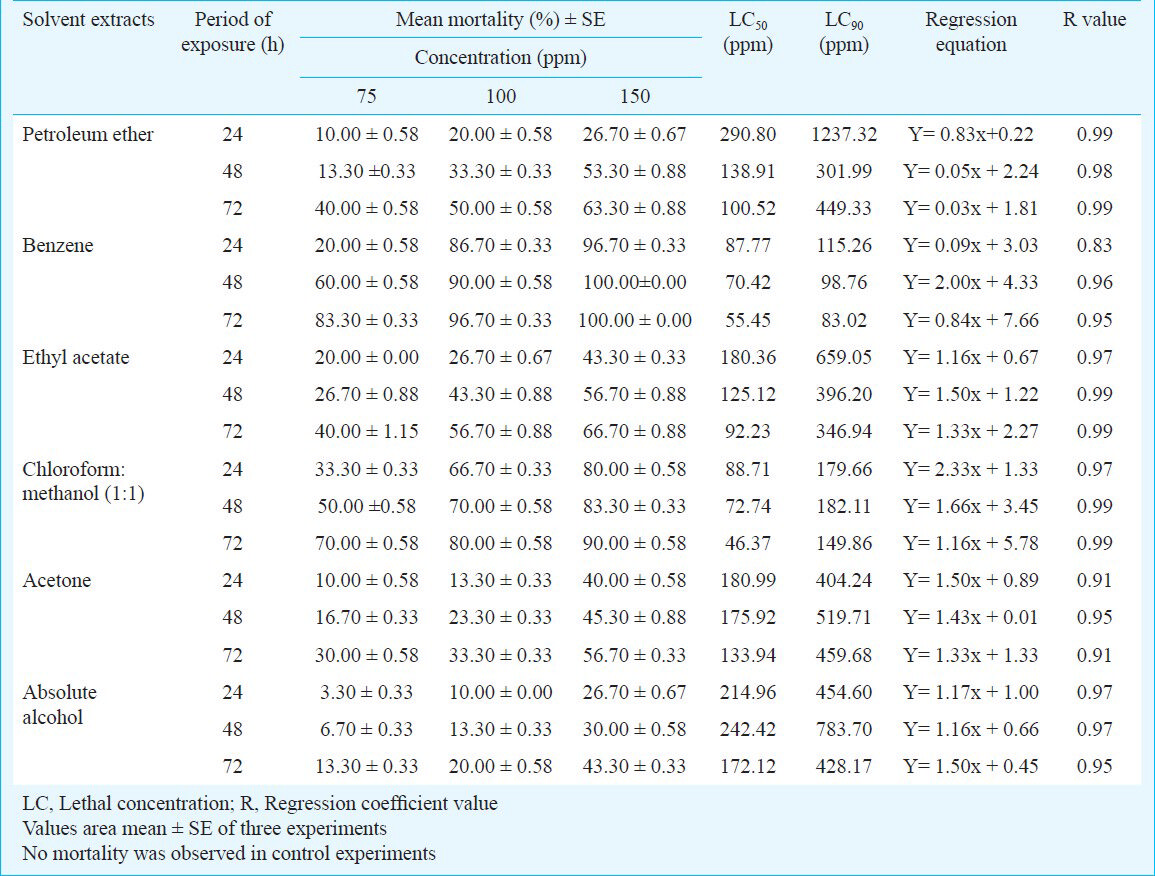
In a 72 h bioassay experiment with the aqueous extract, the highest mortality was recorded in 0.5 and 3 per cent extract against larvae of Cx. vishnui group and L. acuminata, respectivela. In the benzene solvent extract, the maximum mortality was recorded at a concentration of 150 ppm against L. acuminata and at 50 ppm against larvae of Cx. vishnui group with LC50 values of 55.45 and 11.59 ppm, respectively at 72 h. The log probit analysis (95% confidence level) recorded lowest value at 72 h of exposure. Qualitative phytochemical analysis reported the presence of some biochemical compounds, such as saponin, flavonoids, steroid and tannin. Among these, the toxic compound was detected by IR analysis having Rf = 0.87 (showed 66.70% and 76.70% mortality of L. acuminata and larvae of Cx. vishnui group, respectively). IR analysis provided preliminary information about the aliphatic amide nature of the active ingredient.
Interpretation & conclusions:
The study results provide considerable scope in exploiting local indigenous plant resources for molluscicidal and mosquito larvicidal activities.
Keywords
Biocontrol efficacy
Culex vishnui group
Log probit analysis
Lymnaea acuminata
Solanum nigrum
Mosquitoes transmit several vector-borne diseases including malaria, dengue fever, Japanese encephalitis, chikungunya, filariasis, etc. Schistosomiasis and fasciolopsiasis are transmitted through aquatic snails. Many of these diseases can be prevented through vector control measures. The important aspect of mosquito control is to control them in their larval form due to their restricted habitat. Due to non judicious use chemical insecticides now have little effect on mosquitoes because of the developed resistance, and chemical insecticides also show adverse effects on aquatic ecosystem due to their non biodegradable nature and biological magnification property. The use of local plants as molluscicides and larvicides is beneficial in reducing burden of purchasing expensive synthetic molluscicides and larvicides used in snail as well as in mosquito larval control. These botanical pesticides are of economic importance especially in developing countries1234567. The search of herbal preparations that do not produce any adverse effects in the non-target organisms and are easily biodegradable remains a topic for research.
The black nightshade, common name of Solanum nigrum L., is a small, erect and delicate annual herb with soft and smooth stems and branches. The plant has alternate, egg-shaped, elliptic leaves; white, cream or violet flowers in clusters, purple or black fruit when ripe; and with blue or black seeds8. This plant is known for its anti-inflammatory, antioxidant, antinociceptive, anti-pyretic, anti-tumour, anti-ulcerogenic, cancer chemopreventive, hepatoprotective, and immunomodulatory effects910. Eltayeb et al11 demonstrated that the concentration of steroidal alkaloid solasodine was highest in the leaves, though some amounts are also present in stem, root and fruits. Hu et al12 isolated three anti-neoplastic steroidal glycosides; beta 12-solamargine, solamargin and degalactotigonin. Sun et al13 reported the variability of the concentration of organic acids between seedlings of S. nigrum and the mature plants. The molluscicidal and larvicidal activity of solvent extracts of fresh mature leaves of S. nigrum has been reported against adult Biomphalaria alexandrina1415 and larvae of Culex quinquefasciatus16.
The objective of the present study was to examine the molluscicidal (against Lymnaea acuminata) and larvicidal (against 3rd instar larvae of Culex vishnui group) activities of the matured leaves of S. nigrum and to gather preliminary information about the nature of the toxic compound which might be responsible for this toxicity.
Material & Methods
Fresh, mature, green leaves of S. nigrum were randomly harvested during March 2010 to March 2011 from plants growing at the outskirts of Burdwan. Adult L. acuminata (2.25 ± 0.2 cm in length) were collected locally from small and big ponds and low lying submerged fields, located adjacent to the Burdwan University campus, Burdwan, West Bengal, India. Ten experimental animals were kept in a glass aquaria containing 3 liter of dechlorinated tap water at 22 to 24° C. The pH of the water was 7.1-7.3 and dissolved oxygen, free carbon dioxide and bicarbonate alkalinity were 6.5- 7.2, 5.2-6.3 and 102.0-105.0 mg/l, respectively. The snails were kept in the laboratory at 21° C for one month before being used in experiments. For the identification of freshwater snails checklist and method available in literature were followed171819.
Larvae of Cx. vishnui group were collected from rice fields. They were kept separately in different plastic trays and fed with artificial food, i.e. mixture of dog biscuits and dried yeast powder at the ratio of 3:1. Larvae of Cx. vishnui were a mixed population of larvae of all JE vectors (Cx. vishnui, pseudovishnui and tritaeniorhynchus). Larvae were reared to adult stage and identified following the key of Chandra20 which was prepared basing on Christiphar21 and Barraud22. Culture was kept free from exposure to pathogen, insecticides or repellents.
Preparation of aqueous extracts: Fresh mature leaves of S. nigrum were collected from outskirts of Burdwan, West Bengal, India. The samples were initially rinsed with tap water and then distilled water and soaked on paper towel. Leaves were chopped into small pieces of approximately 1 cm size by sharp razor and crushed with a mechanical blender and the juice was filtered by Whatman no.1 filter paper. The filtrate was used as stock solution (100% concentration) for further bioassay experiment and required concentrations i.e. from 0.1 to 0.5 per cent and 1-3 per cent were prepared through mixing up of stock solution with variable amount of distilled water.
Preparation of solvent extracts: Fresh mature leaves of S. nigrum (100 g) were harvested, rinsed with distilled water, soaked on paper towel and dried for 7-8 days in a shed. The dried leaves were put in a Soxhlet apparatus and the plant extracts were prepared using petroleum ether, benzene, ethyl acetate, chloroform: methanol (1:1, v/v), acetone and absolute alcohol (extraction period 72 h for each solvent and the temperature was < 40°C). The extract was collected separately. The extract was evaporated in a rotary evaporator at 40°C to 100 ml. The solid residues were weighed and dissolved in a suitable amount of sterilized distilled water for the formulation of graded concentrations. The total yields of solvent extracts were noted.
Toxicity experiment
Molluscicidal evaluation of the plant extracts were performed according to WHO guidelines23. Groups of 10 uninfected snails were placed in glass tanks (containers) with some sand, snail food and 250 ml of deionized and dechlorinated tap water bubbled with atmospheric air. Tests were carried out at room temperature (25-27°C). In each set up, the snails were prevented from crawling out of the glass container by means of a fine stainless steel mesh placed above the water surface. The test snails were challenged with various doses of the aqueous plant extracts (1, 2 and 3%) and solvent extracts (75, 100 and 150 ppm). After 72 h of exposure to the aqueous and solvent extracts, the snails were transferred to fresh dechlorinated and deionized water and maintained there for another 24 h. Death of the snails was determined by lack of reaction to irritation of the foot with a blunt wooden probe to elicit typical withdrawal movements. Control setups were also made with deionized and dechlorinated tap water without the test sample on three different days.
Mosquito larvicidal bioassay followed the World Health Organization standard protocols24 with slight modifications. Aqueous extract (0.1 to 0.5%) was transferred into sterile glass Petri dishes (9 cm diameter/150 ml capacity). Ten 3rd instar larvae of Cx. vishnui group were separately introduced into different Petri dishes containing appropriate graded concentrations and the mortalities were recorded after 24, 48 and 72 h of exposure periods. Similar types of bioassay were conducted with solvent extracts (concentrations of 15, 30 and 50 ppm) on third instar larval form. Control setups were also made with deionized and dechlorinated tap water without the test sample on three different days.
Phytochemical analysis of the aqueous extract: Aqueous extracts of the mature leaves were subjected to qualitative phytochemical analysis252627. The phytochemicals included under study were saponins, terpenoids, alkaloid, steroids, tannin, flavonoids and cardiac glycosides.
Alkaloids: The extract (0.55) was diluted with 10 ml of acid alcohol, then boiled and filtered by Whatman filter paper. To 5 ml of this filtrate, 2 ml of dilute ammonia was added. After this, 5 ml chloroform was added and shaken gently to extract the alkaloidal base. The chloroform layer was extracted with 10 ml of acetic acid. The resultant extract was divided into two part. Mayer's reagent was added to one portion and Draggendoff's reagent to the other. The formation of a cream (with Mayer's reagent) or reddish brown precipitate (with Draggendoff's reagent) was regarded as positive for the presence of alkaloids.
Saponins: To 0.5 g of extract, 5 ml of distilled water was added in a test tube. The solution was shaken vigorously through hand and observed for the presence of a stable persistent froth. The resultant frothing was mixed with three drops of olive oil and shaken vigorously. The appearance of creamy mass of small bubbles indicated the presence of saponins.
Tannins: About 0.5 g of the shed-dried powdered sample was boiled in 20 ml of water in a test tube and then filtered by Whatman filter paper. A few drops of 0.1 per cent ferric chloride was added and observed for the appearance of brownish green or a blue-black coloration, indicating the presence of tannins.
Flavonoids: About 0.5 g of the plant extracts was dissolved in diluted sodium hydroxide and hydrochloric acid was added to the solution. A yellow solution that later turns colourless indicated the presence of flavonoids.
Steroids: The 2 ml acetic anhydride was added to 0.5 g of the extract along with two ml sulphuric acid. The color changed from violet to blue or green in some samples indicating the presence of steroids.
Terpenoids (Salkowski method)26: In 0.5 g of the extract two ml of chloroform was added in a test tube. Concentrated sulphuric acid (3 ml) was carefully added to the same test tube to form a layer. A reddish brown colouration of the interface indicated the presence of terpenoids.
Cardiac glycosides (Keller-Killiani test)26: About 0.5 g of extract was diluted to 5 ml in distilled water and 2 ml of glacial acetic acid containing one drop of ferric chloride solution was added in it. This was underplayed with 1 ml of concentrated sulphuric acid. A brown ring at the interface indicated the presence of deoxysugar characteristic of cardiac glycosides.
Preparation of samples for active principle and further bioassay experiment: The benzene extract was evaporated upto 1/4th of its original volume in rotary evaporator and then chromatographed using silica gel ‘G’ TLC plates. The plates (50 in number and thickness 0.5 mm) were prepared with silica gel G (Sigma, USA) and a thin-layer coating apparatus (Unoplan-Shandon, London). The mobile phase was benzene. The preparative chromatogram reported a single spot (having Rf=0.87) as identified in the Iodine chamber. The compounds along with the silica gel were scrapped of the particular Rf value region of each plate, combined in a conical flask and dissolved in 10 ml absolute alcohol. The alcohol was evaporated and the solid mass present at the bottom of the conical flask was scrapped and weighed. A part of the scrapped fraction was dissolved in distilled water to prepare different concentrations and treated against third instar larvae of Cx. vishnui group and L. acuminata, and death was recorded after 24, 48, and 72 h. Control setups were also made with deionized and dechlorinated tap water without the test sample in three different days.
Infrared analysis of the active ingredient: Another part of previously scrapped fraction (having Rf=0.87) was subjected to infrared (FT-IR) spectroscopy. The IR spectroscopy analysis of the active spot was performed using potassium bromide (KBr) plates (JASCO FT-IR Model-420, Japan). All solvents and reagents used were of analytical grade and purchased from E. Merck, India.
Effect on non target organisms: The effect of the benzene extracts was tested against non-target organisms like Daphnia sp., Diplonychus annulatum (predatory water-bug) and Chironomus circumdatus larvae (insect) as they share the common habitats of target mosquito larvae and some of them were natural predators of mosquito larvae. The predators were exposed to appropriate lethal concentration of benzene extracts at 24 h to observe the mortality and other abnormalities such as sluggishness and reduced swimming activity up to 72 h of exposure.
Statistical analysis: The percentage mortality observed (%M) was corrected using Abbott's formula38. Statistical analysis of the experimental data was performed using the computer software Statplus 2007 (Trial version: http://statplus.en.softonic.com) and MS EXCEL 2003 to find the LC50, regression equations (Y = mortality; X = concentrations) and regression coefficient values.
Results
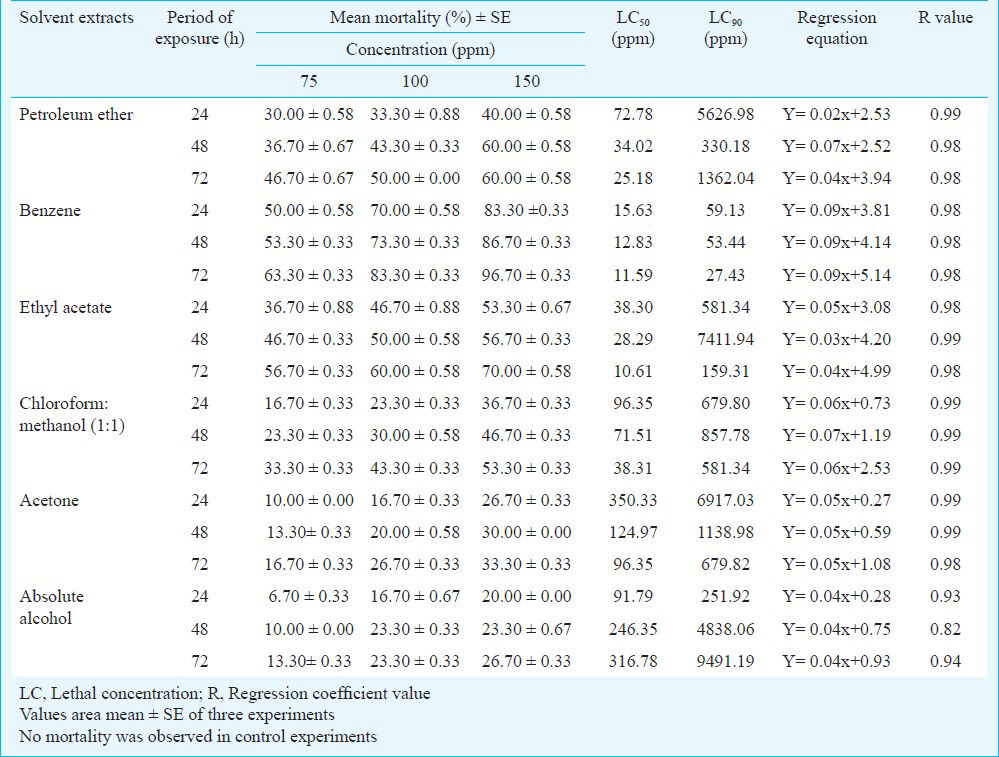
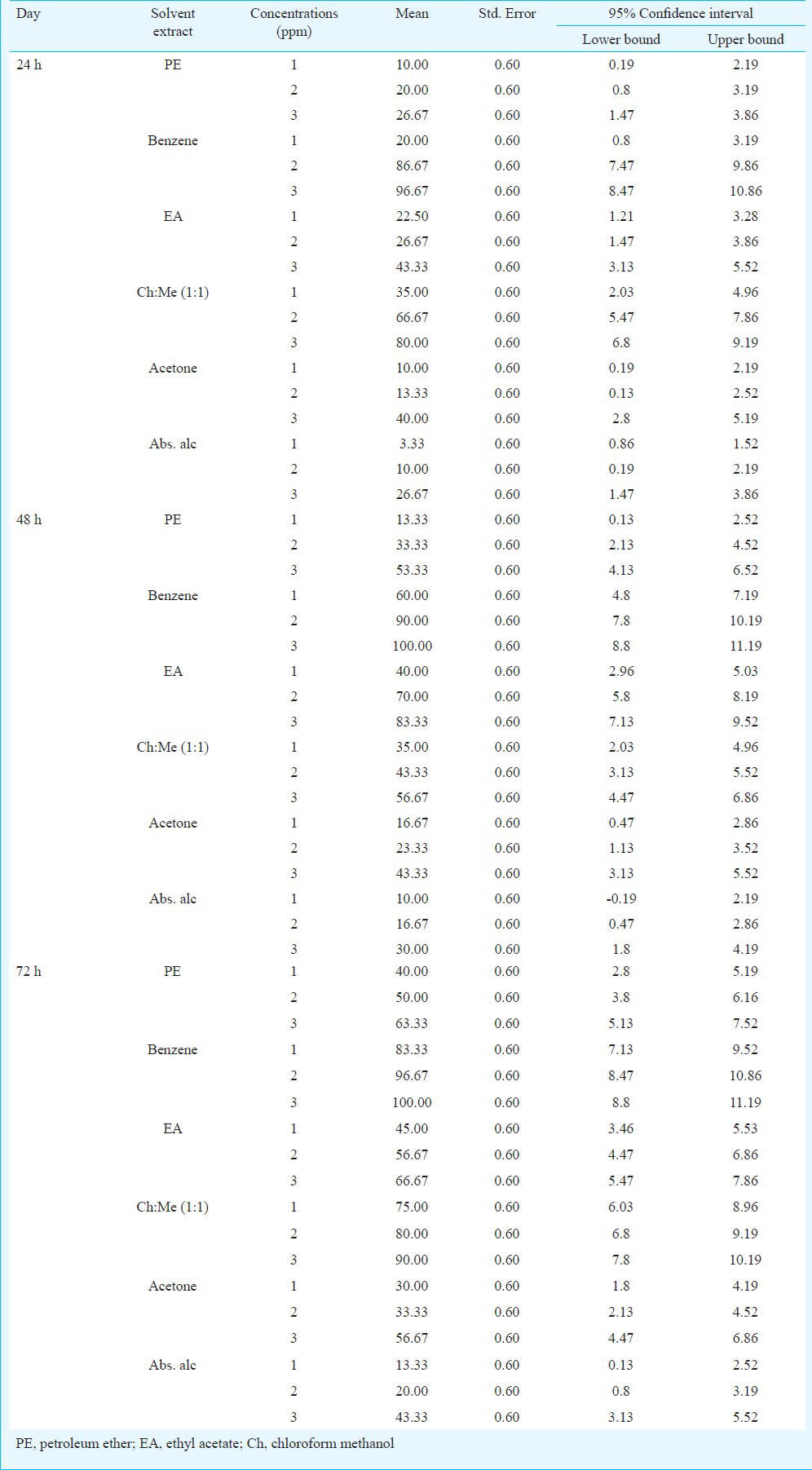
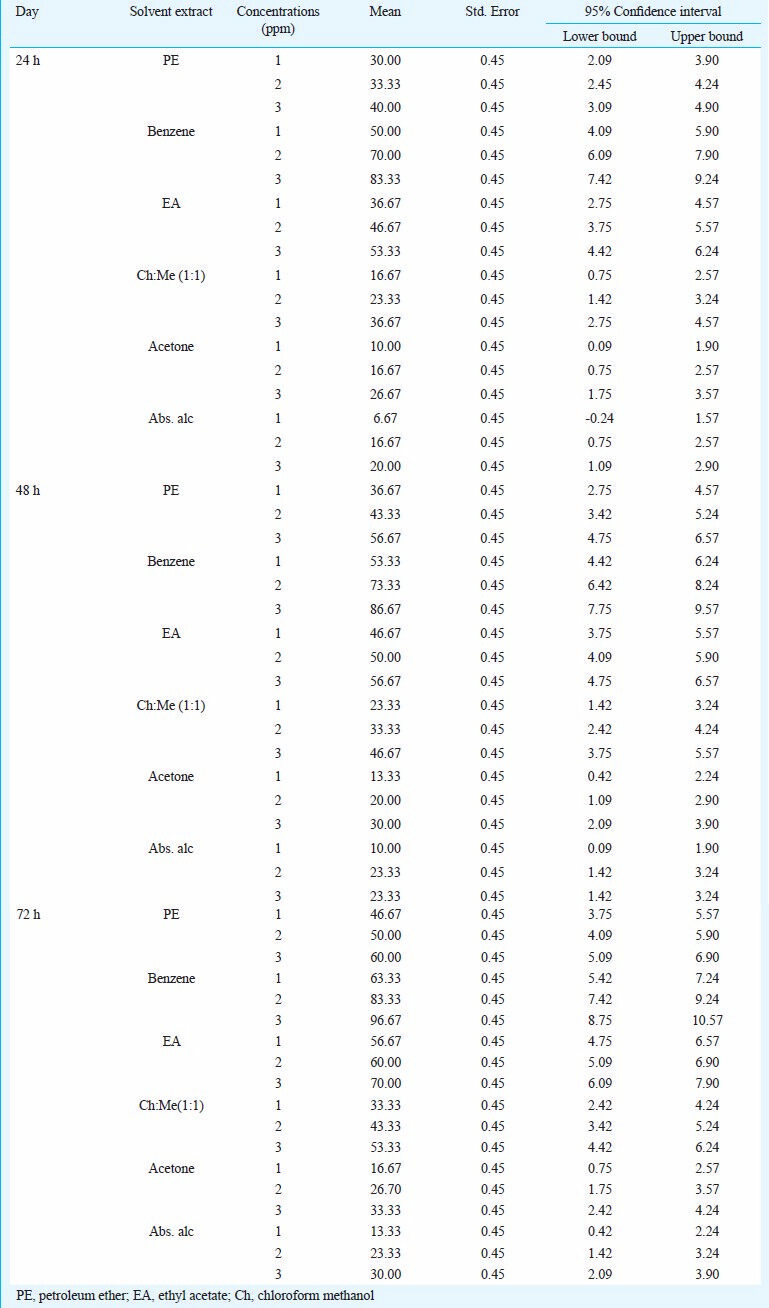
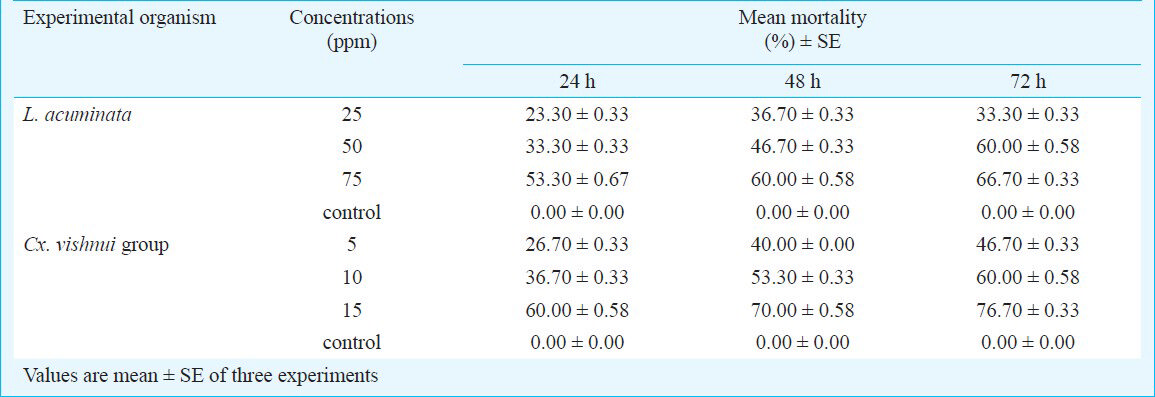
The activity of aqueous extract against L. acuminata and third instar larvae of Cx. vishnui group are presented in Table I. The 3 per cent of aqueous extract showed the highest mortality against L. acuminata and 0.5 per cent of aqueous extract showed the highest mortality against 3rd instar larvae of Cx. vishnui group. The mortality rate was highest at 72 h compared to 24 and 48 h. The mortality of solvent extracts against L. acuminata and third instar larval form of Cx. vishnui group were presented in Tables II and III, respectively. Benzene extract showed the highest mortality against both the vectors. The LC50 values of benzene extract against L. acuminata were 87.77, 70.42 and 55.45 ppm after 24, 48 and 72 h, respectively (Table II) and the LC50 values of benzene extract against third instar larvae of Cx. vishnui group were 15.63, 12.83, and 11.59 ppm after 24, 48 and 72 h, respectively (Table III). Comparison of mean percentage mortality, standard error and their upper and lower bound at 95% confidence level of all the tested extracts and concentration for both L. acuminata and 3rd instars larvae of Cx. vishnui group are presented in Tables IV and V, respectively. The results of regression analysis of solvent extracts revealed that the mortality rate (Y) was positively correlated with the concentration of exposure (X) having a regression coefficient (R) close to 1 in each case (Tables II, III). Qualitative phytochemical analysis reported the presence of some biochemical compounds, such as saponin, flavonoids, steroid and tannin. The fraction showing mortality gave an Rf value of 0.87. Result of the bioassay with this fraction of bioactive compound against 3rd larvae of Cx. vishnui group and snail are presented in the Table VI. Mortality rate of 3rd instars larvae of Cx. vishnui group was higher (P<0.05) at 15 ppm concentration than 10 and 5 ppm. Mortality rate of L. acuminata at 75 ppm was higher (P<0.05) than 50 and 25 ppm. IR analysis of the compound and their respective functional groups revealed the N-H stretching, C-H stretching, C=O stretching and C-N stretching vibrations of amide group. No change in the survival rate and swimming activity of the non target organisms were observed within 72 h of post exposure to the plant extract with the concentration equals to their respective LC50 values at 24 h. The total yield of each extract in the Soxhlet extraction from 250 g of leaves was as follows: petroleum ether extract, 5.77 g; benzene extract, 2.38 g; ethyl acetate extract, 4.30 g; chloroform: methanol (1:1, v/v) extract, 6.33 g; acetone extract, 3.00 g; and absolute alcohol extract, 2.36 g.
Discussion
High cost and toxicity of synthetic pesticides have led to an interest in plant based molluscicidal and larvicidal compounds which are lethal to only target organism having no effect on non target organism. Plants contain several active compounds that can be more effective than the synthetic chemical molluscicides29. The use of plant molluscicides is attractive due to the economic advantage of cultivating the plants locally instead of importing synthetic compounds30. The present study showed the biocontrol potentiality of aqueous and solvent extracts of mature leaves of S. nigrum against L. acuminiata and larvae of Cx. vishnui group. The larvae of tested mosquito species showed highest mortality (95%) against 0.5 per cent aqueous extract, and benzene extract showed highest mortality at 50 ppm dose. L. acuminata showed 70 per cent mortality at 3 per cent aqueous extract and 100per cent mortality at 150 ppm benzene extract after 72 h. Mortality caused by the leaf extracts showed a positive association with doses. The order of 24 h toxicity against L. acuminata and Cx. vishnui group were benzene extract > chloroform: methanol (1:1, v/v) extract > ethyl acetate extract > petroleum ether extract, acetone > absolute alcohol. The IR spectra of the bioactive compounds during the present study indicated that any aliphatic amide compound might be responsible for the mortality of mosquito larvae and mollusk under study. As no mortality occured in the non-target organisms it could be assumed that the tested plant extracts were safe to use in the aquatic ecosystem. El-Sherbini et al14 reported the maximum mortality at a concentration of 90 ppm of ethanol extract of S. nigrum against Biomphlaria alexandrina snails, which are an intermediate host of parasites causing human schistosomiasis. Ahmed and Ramzy15 studied the molluscicidal activity of S. nigrum against adult Biomphalaria alexandrina snails and determined whether plants collected at various seasons would have different degrees of toxicity. Leaves and fruits of three S. nigrum varieties were collected from Faiyoum and / or Giza during the four seasons. Leaves collected in autumn had the highest effect followed by spring, summer and winter. Rawani et al16 reported the biocontrol potentiality of crude extracts of S. nigrum against Cx. quinquefasciatus. The highest mortality was recorded in 0.5 per cent crude extract of S. nigrum and 50 ppm concentration of ethyl acetate extract. Larhsini et al31 reported that n-butanol extract of roots of S. laeagnifolium had molluscicidal activity with LC50 of 12.19 ppm. The glycoalkaloids extract of S. elaeagnifolium fruits was fractioned by chromatography on silica gel and three active fractions were isolated with LC50 respective of 4, 6 and 10 ppm.
Plants are rich sources of bioactive organic chemicals and offer an advantage over synthetic pesticides as these are less toxic, less prone to development of resistance and easily biodegradable. A wide selection of trees and shrubs has been found to contain phytochemicals that may be of use in parasites control. After further purification, these partially purified extracts may become more effective at much lower doses. Further studies are required to identify the particular compound(s) and the specific mechanism of action of the bioactive principle present in the leaves of S. nigrum responsible for insecticidal and molluscicidal activities.
Acknowledgment
The authors acknowledge Dr Ambarish Mukherjee, Professor of Botany, The University of Burdwan, for identification of the plant. The financial support provided by the DST, New Delhi, India (D.O. NO.SR/SO/HS/84/2007 dated 08/02/08) is acknowledged.
References
- Molluscicidal activity of some Indian medicinal plants against the snail Lymnaea acuminata and in the control of Fascioliasis. J Herbal Med Toxicol. 2010;4:109-12.
- [Google Scholar]
- Molluscicidal activity of butanol fraction of Meryta denhamii flower against Lymnaea natalensis and Biomphalaria alexandrina. Glob Veterinar. 2010;4:15-21.
- [Google Scholar]
- Mosquito larvicidal and antimicrobial activity of protein of Solanum villosum leaves. BMC Comp Alternat Med. 2008;8:62.
- [Google Scholar]
- Efficacy of Limonia acidissima L. (Rutaceae) leaf extract on larval immatures of Culex quinquefasciatus Say 1823. Asian Pac J Trop Med. 2011;4:711-6.
- [Google Scholar]
- Efficacy of Solanum villosum Mill. (Solanaceae: Solanales) as biocontrol agent against fourth instar larvae of Culex quinquefasciatus Say. Turkish J Zool. 2007;31:365-70.
- [Google Scholar]
- Larvicidal activities of three plants against filarial vector Culex quinquefasciatus Say (Diptera: Culicidae) Parasitol Res. 2009;105:1411-7.
- [Google Scholar]
- Blake night shades Solanum nigrum L. and related species. Rome, Italy: International Plant Genetic Resources Institute; 1997.
- [Google Scholar]
- Antiinflammatory constituents from Solanum nigrum. Bull Korean Chem Soc. 2010;31:199-201.
- [Google Scholar]
- Changes in the steroidal alkaloid solasodine during development of Solanum nigrum and Solanum incanum. Phytochem. 1997;46:489-94.
- [Google Scholar]
- Anntineoplastic agents. III: steroidal glycosides from Solanum nigrum. Plant Med. 1999;65:35-8.
- [Google Scholar]
- Relationship between cadmium accumulation and organic acids in leaves of Solanum nigrum L. as a cadmium- hyperaccumulator. Plant Soil. 2006;285:125-34.
- [Google Scholar]
- Molluscicidal activity of some Solanum species extracts against the snail Biomphalaria alexandrina. J Parasitol Res. 2009;2009:1-5.
- [Google Scholar]
- Seasonal variation in molluscicidal activity of Solanum nigrum L. J Egypt Soc Parasitol. 1998;28:621-9.
- [Google Scholar]
- Mosquito larvicidal activities of Solanum nigrum L. leaf extract against Culex quinquefasciatus Say. Parasitol Res. 2010;107:1235-40.
- [Google Scholar]
- A checklist of land and freshwater mollusca of Maharashtra state. Zoos’ Print J. 2005;20:1912-3.
- [Google Scholar]
- The fauna of British India including Ceylon and Burma. London: Taylor and Francis Publisher; 1915.
- [Google Scholar]
- Ecological studies on freshwater gastropods (snails) of Indus river and its canals at Kotri barrage Sindh, Pakistan. Sindh Univ Res J. 2008;40:37-40.
- [Google Scholar]
- The fauna of British India, including Ceylon and Burma. Diptera, vol. iv Family Culicidae. In: Tribes Anophelini. London: Taylor and Francis; 1933. p. :371.
- [Google Scholar]
- The fauna of British India, including Ceylon and Burma. Diptera vol. V. Family Culicidae. In: Tribes Megarhinini and Culicini. London: Taylor and Francis; 1934. p. :463.
- [Google Scholar]
- Snail control in prevention of Bihariasis monograph, Ser. No. 50, 1965. Geneva, Switzerland: WHO; 1965. p. :124-38.
- [Google Scholar]
- Instructions for determining the susceptibility or resistance of mosquito larvae to insecticides, WHO/VBC/81.807. Geneva: WHO; 1981.
- [Google Scholar]
- Phytochemical methods. A guide to modern techniques of plant analysis. London: Chapman and Hall; 1984. p. :49-188.
- [Google Scholar]
- Pharmacognosy: A physicians guide to herbal medicine. (13th ed). London: Baillieri Tindal; 1989.
- [Google Scholar]
- Medicinal plants and traditional medicines in Africa. Ibaban: Harborne Spectrum Books Ltd; 1993. p. :55-71.
- [Google Scholar]
- A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265-6.
- [Google Scholar]
- Application of some aqueous plant extracts as molluscicidal agents on Bulinus truncatus snails in Sudan. J Basic Appl Sci Res. 2011;1:108-17.
- [Google Scholar]
- Transmission and control of schistosomiasis in irrigation schemes around Khartoum State, Sudan. Ph.D. thesis Sudan: Kharoum University; 2000.
- [Google Scholar]
- Screening of some Moroccan plant extracts for molluscicidal activity. Asia J Exp Biol Sci. 2010;1:964-7.
- [Google Scholar]






