Translate this page into:
Japanese encephalitis virus-induced neuropathology in mouse model infected through the conjunctival route
For correspondence: Dr G. Saikumar, Division of Pathology, ICAR-Indian Veterinary Research Institute, Bareilly 243 122, Uttar Pradesh, India e-mail: saikumarivri@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Mouse is a preferred animal model for studying pathogenesis of Japanese encephalitis virus (JEV) infections, and different routes of inoculation have been tried. Some neurotropic viruses can reach the brain following infection through ocular route. This study was undertaken to establish JEV-induced clinical disease in mouse model through conjunctival route and document the neuropathological effects.
Methods:
Ten two-week old Swiss albino mice were inoculated with 5 μl Vero cell cultured virus containing 104.7 TCID50 JEV through conjunctival route. Clinical signs of mice were observed twice daily. After necropsy examination, different organs including eyes and olfactory bulbs were collected for histopathological examination, quantification of viral copy number and antigen by real-time TaqMan assay and immunohistochemistry, respectively.
Results:
Infected mice showed characteristic clinical signs of JE by 4 days post-infection (dpi). Histopathological lesions in brain included perivascular cuffing by mononuclear cells, focal gliosis, necrosis of neurons and neuronophagia and astrocytosis in the cerebrum, cerebellum and the brainstem. JEV viral load was highest in the brain followed by intestine, heart, liver, spleen, lung and kidney. JEV antigen was detected in the bipolar and ganglion cells of the retina and in the mitral cells and periglomerular cells of olfactory bulb and other parts of the brain.
Interpretation & conclusions:
JEV infection in mice through conjunctival route produced characteristic clinical signs of the disease and neuropathological lesions. Demonstration of JEV antigen in association with neuropathological lesions in the central nervous system and neuronal cells of the eye showed that conjunctival route could be an effective alternate route for virus invasion into the brain. These findings have biosafety implications for researchers, veterinary practitioners and pig farmers.
Keywords
Biosafety
CNS
conjunctival route
inoculation
JEV
mouse model
neuropathology
The Japanese encephalitis (JE) virus of genus Flavivirus (Flaviviridae) has a positive sense RNA genome and presents an enzootic cycle involving mosquitoes1 and amplifying vertebrate hosts. It causes viral encephalitis which is primarily a disease of children. According to the World Health Organization (WHO), annually, there are about 68,000 global cases of JE, of whom 20-30 per cent are fatal, 30-50 per cent of survivors have significant neurological sequelae and three billion people are at the risk of infection in endemic countries including WHO South-East Asia and Western Pacific Regions2. The causative agent, JE virus (JEV) is vector borne with Culex mosquitoes as its main vectors and water birds such as egrets and herons as reservoirs34. Pigs serve as amplifying hosts in human epidemics5, and the virus causes reproductive disorder in pigs. Pathological and pathogenesis studies have been conducted on JEV infection in rabbit, guinea pig, monkey, hamster, rat and mouse models using different routes of infection, including intravenous (i.v.), intraperitoneal (i.p.), subcutaneous (s.c.), intracerebral (i.c.), intradermal and intranasal (i.n.) routes, but neuronal invasion has been confirmed in some of these models6. However, footpad inoculation closely mimics the conditions of human infection by natural route78. Rabbits and guinea pigs develop asymptomatic infection regardless of route of inoculation and hamsters reportedly die after i.c. or i.n. inoculation with asymptomatic viraemia after peripheral inoculation910. Mice represent the most extensively utilized animal models of flavivirus encephalitis for its high degree of susceptibility to most laboratory strains of flaviviral encephalitis, the similarity in disease presentation and virus tropism between rodents and humans along with availability of large numbers of animals for experimental purposes11. Besides, mice are also used to determine the efficacy and safety of vaccines and therapeutics of flavivirus. The disease severity varies depending on host age, dose of virus and route of inoculation12.
Neurotropic viruses may gain access to the central nervous system (CNS) through several routes including anterograde neuronal spread through sensory nerves13, across the blood-brain barrier as free virions14 or via entry of infected immune cells15. Experimental studies have demonstrated that ocular route of infection with rabies virus in rats16 and herpes simplex virus 1 in mice1718 results in virus transmission to the CNS.
In a study conducted under controlled conditions, it has been observed that JEV can be transmitted from infected pigs to in-contact pigs in the absence of arthropod vectors through oronasal secretions19. One study on JEV infection through ocular route in mouse model showed virus multiplication in the brain with definite titres20, but this report did not describe neuropathological lesions in the CNS and the presence of virus within the neuronal cells of eye. The present study was conducted to ascertain the neuropathological effects in mice inoculated with a swine isolate of JEV through conjunctival route.
Material & Methods
Swiss albino mice were obtained from the Laboratory Animal Resources Section, Indian Veterinary Research Institute (IVRI), Izatnagar, India. Vero cell cultured JEV isolate 395A/14/SW/IVRI was used for the experimental infection. The median lethal dose (LD50) challenge dose through i.c. route in mouse was determined to be 103 median tissue culture infectious dose (TCID50)/100 μl (unpublished data). Ten two-week old Swiss albino mice were inoculated with 5 μl Vero cell cultured virus having a titre of 1×107 TCID50/ml by conjunctival route. The final concentration of virus inoculated by ocular route was 104.7 TCID50/5 μl which was one log higher than the LD50 for i.c. route to compensate for loss of virus during transit to the brain.
Control Swiss albino mice were inoculated with Dulbecco's Modified Eagle's Medium (DMEM, Sigma-Aldrich, USA). The mice were observed twice daily for any signs and symptoms of JEV infection.
This study was approved by the Institute Animal Ethics Committee, IVRI, Izatnagar, India (Permit number: F.1-53/2012-13/JDR).
Histopathology: Detailed necropsy examination was conducted on euthanized mice, and gross findings were recorded. Tissue samples were collected from brain, eyes, olfactory bulb, lungs, heart, liver, spleen, kidneys, stomach and intestines in 10 per cent buffered neutral formalin as fixative and on ice for molecular examination. Fixed tissues were processed, for routine haematoxylin and eosin (H and E) examinations as per standard procedures21. The H and E stained sections were microscopically examined, and the histopathological alterations were recorded and digitally photomicrographed (Olympus BX41, Japan).
Real-time polymerase chain reaction (PCR) by TaqMan assay: RNA was extracted using the QIAamp Viral RNA Mini kit (QIAGEN, Germany) from tissues according to the manufacturer's instruction. TaqMan polymerase chain reaction (PCR) assay was performed using the Cepheid SmartCycler (USA) real-time PCR machine and commercial reagents. The assay used self-designed primers and probe; forward primer: JEVRTF 5'-TGGTCCATAGGGAATGGTTT-3', reverse primer: JEVRTR 5'-AAGAGCAACAACGGACTGTTT-3' and TaqMan probe: JEVRTP 5'Fam-TTTGAAGA GGCGCACGCCAC-3'Tamra targeted for E gene of JEV synthesized commercially (IDT, USA). The real-time PCR was carried in 25 μl volume in individual, special flat tubes with good interlocking cap, designed exclusively for the SmartCycler by Cepheid, USA. The reaction mixture contained the following ingredients: Kapa qPCR Probe Fast Buffer (12.5 μl), forward primer - 10 μM; 0.8 μl, reverse primer - 10 μM; 0.8 μl, Probe - (10 μM; 1 μl) and template cDNA (1 μg) and rest nuclease free water to prepare 25 μl reaction mixtures. In each run, appropriate positive control and no template control were included. The cycling condition was as follows: an initial denaturation step of 4 min at 95°C was followed by 35 cycles each comprising denaturation 15 sec at 95°C, annealing 20 sec at 52°C and extension 30 sec at 72°C. Data acquisition was done at extension step.
Absolute quantification of viral load was done by E gene-based TaqMan assay in tissues of different organs. A standard curve was generated using serial dilution of gel purified PCR product22 of JEV E gene with efficiency of 100.21 per cent, R2 (0.982), a slope of −3.317 and the copy number ranged from 2.1×101 to 2.1×108 copies/μl against the corresponding threshold cycles (Ct value). The viral load in different organs to be determined was run in triplicates by using 1 μl of cDNA from each sample to know the threshold cycles (Ct value). The equation obtained by linear regression of standard curve and threshold cycles (Ct value) of the organs was used for determination of copy number of the virus present in each tissue of the different organs.
Immunohistochemistry: The representative paraffin-embedded tissue sections of infected mice were dewaxed, rehydrated and subjected to antigen retrieval by Heat Induced Epitope Retrieval (HIER) method23 using citrate buffer (pH 6.0) for 8 min. After overnight incubation with primary rabbit polyclonal antibody to JEV (dilution 1:250, Abcam, USA) and secondary goat anti-rabbit antibody conjugated with horseradish peroxidase (HRP) (dilution 1:250, GeNei, Bengaluru) for one hour followed by AEC (3-amino-9-ethylcarbazole) substrate solution (Sigma-Aldrich, USA), the sections were counterstained with Mayer's haematoxylin. IHC slides were examined microscopically under high resolution microscope (Olympus BX41, Japan). Negative controls in the assay included tissue sections from control (uninfected) mice and sections without primary antibody application.
Results & Discussion
This study demonstrated that JEV infection through conjunctival route of inoculation in two-week old mice resulted in characteristic clinical disease with 100 per cent mortality with mean survival rate of five days. Initially, on 4 days post-infection (dpi), mice showed dullness, mask-like face, anorexia, weight loss, ruffled fur with hunchback posture. Later on, at 5 to 6 dpi, tail twitching, circling movements and paralysis of hind limbs were noticed (Fig. A). According to a previous study, the mortality rate and mean survival days were 100 per cent and 4.8 days in case of i.c. inoculated mice, 58 per cent and 13.4 days in mice inoculated through the i.p. route24. In the present study, JEV inoculated mice started showing clinical signs at 4 dpi which indicated that like i.c. route, the conjunctival route was also an effective route for virus invasion into the brain and to induce neuropathogenesis. These results were also consistent with a previous report of intrabulbar inoculation, which caused increase of JE virus titre in the brain three days after inoculation20. The i.c. route of inoculation differs from natural infection in which the virus is introduced peripherally through the bite of mosquitoes. In case of i.p. route of inoculation, the virus pathogenesis is comparatively reduced and the survival time of mice is prolonged as virus may be neutralized on its way to the brain20. The findings of the present study indicated that the virus pathogenesis and time taken for appearance of clinical signs were comparable to i.c. route of inoculation.
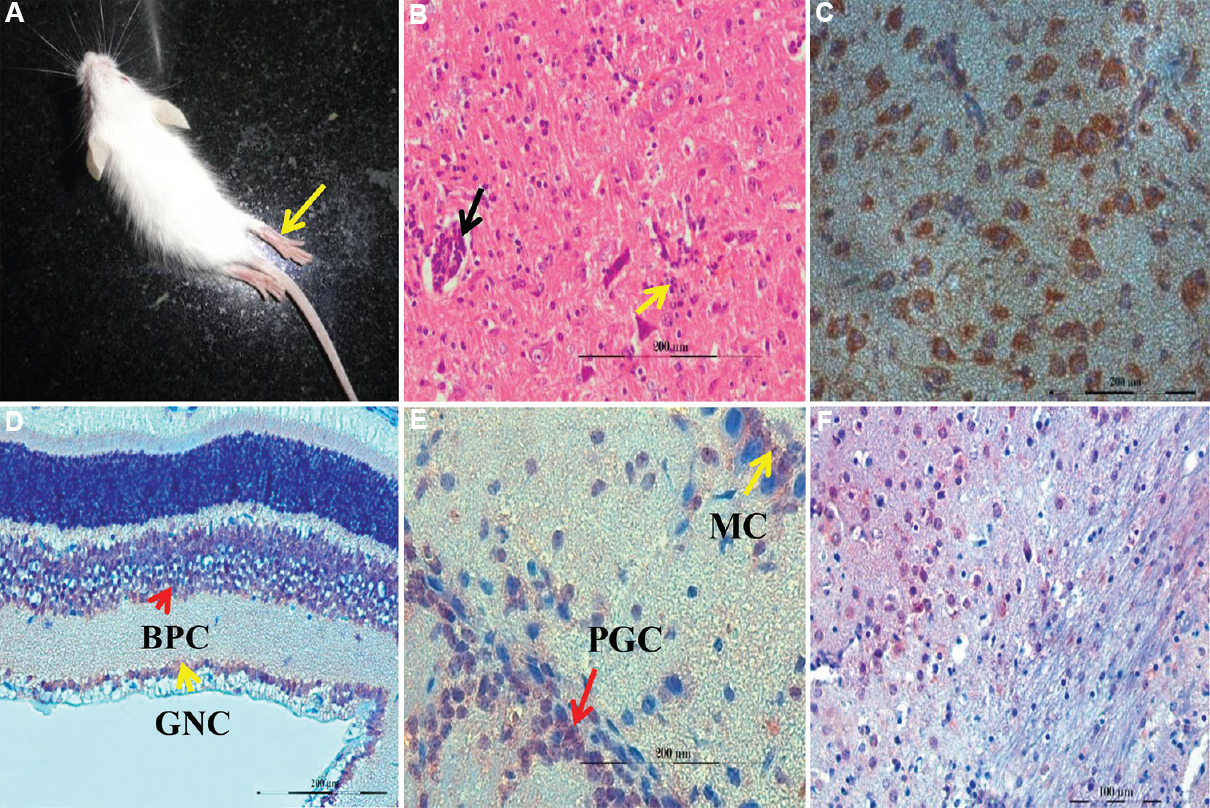
- (A) Japanese encephalitis virus (JEV) infected mice showing fur ruffling and hind limb paralysis. (B) perivascular cuffing (black arrow) and gliosis (yellow arrow) in brainstem (H and E, ×200). (C) JEV antigen (reddish brown) in the cytoplasm of neuronal cells of cerebrum (IHC, ×400). (D) JEV antigen in the bipolar cells (BPC) of inner nuclear layer and ganglion cells (GC) of retina (IHC, ×400). (E) JEV antigen in the mitral cells (MC) and periglomerular cells (PGC) of olfactory bulb (IHC, ×400). (F) JEV antigen (reddish brown) in the cytoplasm of neuronal cells in pons (IHC, ×400).
Microscopic examination of the CNS of the JEV-infected mice showed perivascular cuffing (PVC) by mononuclear cells and haemorrhages in cerebral cortex and brainstem (Fig. B). Variable inflammatory cell infiltration with formation of occasional microglial nodules, rarely oedema, focal necrosis of neurons and astrocytosis in the parenchyma of cerebrum, cerebellum and brainstem were also noticed. Meninges showed mild to moderate haemorrhages and infiltration. In all other organs examined, no microscopic abnormality was observed except for focal haemorrhages. In a previous study24, it was noticed that mice peripherally inoculated with JE virus showed marked inflammatory changes with leucocytic infiltration but mice infected intracerebrally showed little inflammatory changes with mild generalized glial cell proliferation and mononuclear cell infiltration. The findings of this study established that conjunctival route of JEV led to severe and fatal neuropathological disease with symptoms resembling naturally occurring JEV in fatal human cases, and it was more severe than lesions reported following i.c. inoculation although time duration of disease onset was the same. Histopathological findings of PVC and formation of glial nodules in the brain of experimentally infected mice corroborated with other reports2526. Histopathological sections of eye in the present study showed haemorrhages in the layers of retina as well as in the sclerochoroidal region in some cases. In mouse infected with zika virus, a related flavivirus, retinal ganglia cell necrosis and vitreitis with cellular infiltrate in vitreous humour has been reported27. Histopathological examination of olfactory bulb showed increased proliferation of glial cells and infiltration of a few mononuclear cells in the periglomerular areas along with a few focal haemorrhages. Neuronal cell necrosis with glial nodule formation in the olfactory bulb has been found in piglets inoculated intranasally with JEV28. In all other visceral and lymphoid organs, no significant histopathological lesions were found except congestion and mild infiltration.
The quantification of viral RNA in different organs by real-time TaqMan PCR assay indicated highest copy number of JEV in brain, followed by intestine, heart, liver, spleen, lung and kidney (4.8×107, 3×105, 2.8×105, 2.35×105, 1.57×105, 1.15×105, 2×104 copies/μl cDNA, respectively). The relative virus amount in brain was up to 109 RNA copies/ml and in case of spleen up to 104 RNA copies/ml in different dpi in i.p. route of infection of JEV of different species of mice29. In mice infected with JEV through i.v. route, the log C gene copies/μg RNA of brain, spleen, lymph nodes and liver were 9, 5, 4.5 and 4, respectively26. This suggests that no matter which route is used for inoculation of mice, the neurotropic virus reaches brain and multiplies to high copy number and induces neuropathological lesions.
Further, viral antigens were detected in the cytoplasm of neurons of the cerebral cortex (Fig. C), the brainstem and rarely in the granule cells and Purkinje cells of the cerebellum. Mononuclear cells surrounding the blood vessels in the brain parenchyma also showed signals for JEV antigen in some cases. Control mice did not show antigenic signals for JEV. The highest density of viral antigens was located in the neuronal bodies and processes in cerebral cortex, thalamus, brainstem, caudate nucleus and putamen as in case of footpad-inoculated two-week and five-week old mice model8. Necrotic neurons of cerebral cortex showed strongly positive signals for JEV as has been reported earlier2530. Viral antigens were also detected in mononuclear cells and endothelial cells of blood vessels in the brain parenchyma suggesting viral replication in these cells also31.
JEV antigenic signals were found in bipolar cells and ganglion cells of inner nuclear layer of retina (Fig. D). Axon fibres of ganglion cells form optic nerve to reach the mid brain by forming optic chiasm and some optic nerve fibres enter into the brainstem region. In case of herpes simplex virus 1(HSV-1), virus transmission through the optic nerve to brain32 and virus transmission from experimentally injected eye to contralateral infected eye has been reported through optic nerve33. So JE virus transmission to the brain may possibly occur through the optic nerve route.
In the present study, JEV antigen was found prominently in mitral cells, periglomerular cells and occasionally within the granule cells of the granular layer of the olfactory bulb (Fig. E) and in the neurons of pons (Fig. F). In case of mice infected with HSV-1 through the ocular route, it was demonstrated that virus transmission occurred to the olfactory bulb through the trigeminal nerve branch18. Possibly similar pathway was followed by the JEV to reach the olfactory bulb from eye.
In the light of above observations, the potential risk of contracting JEV infection through conjunctival route by pig handlers and researchers needs to be better appreciated. Aerosol transmission of West Nile virus, a closely related neurotropic flavivirus, has been reported in monkeys, hamsters and mice34, and under controlled conditions, vector-free transmission of JEV to in-contact pigs has also been demonstrated19.
In conclusion, inoculation of JEV into mouse model via conjunctival route produced characteristic clinical signs and histopathological lesion of encephalitis. Transmission and spread of virus to neuronal cells in the eye and subsequently to olfactory bulb and other target tissues in the brain was confirmed by detection of JEV-specific nucleic acids by real-time TaqMan assay and viral antigen by immunohistochemistry. Absolute quantification of viral load in different organs showed highest virus load in brain followed by intestine, heart, liver, spleen, lung and kidney. These observations suggested that conjunctival route of infection in mice could be an alternative and convenient model for study of JEV neuropathogenesis. These findings also have biosafety implications for researchers, veterinary practitioners and pig handlers.
Financial support & sponsorship: Authors acknowledge Indian Council of Agricultural Research and University Grant Commission, Government of India, for providing financial support.
Conflicts of Interest: None.
References
- European Aedes albopictus and Culex pipiens are competent vectors for Japanese encephalitis virus. PLoS Negl Trop Dis. 2017;11:e0005294.
- [Google Scholar]
- Estimated global incidence of Japanese encephalitis: A systematic review. Bull World Health Organ. 2011;89:766.
- [Google Scholar]
- Ecologic studies of Japanese encephalitis virus in Japan VI Swine infection. Am J Trop Med Hyg. 1959;8:698-706.
- [Google Scholar]
- Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol. 2009;54:17-35.
- [Google Scholar]
- Arbovirus infections in Sarawak: The role of the domestic pig. Trans R Soc Trop Med Hyg. 1976;70:66-72.
- [Google Scholar]
- Flavivirus encephalitis: Pathological aspects of mouse and other animal models. Vet Pathol. 2010;47:806-18.
- [Google Scholar]
- Pivotal role of antibody and subsidiary contribution of CD8+ T cells to recovery from infection in a murine model of Japanese encephalitis. J Virol. 2011;85:5446-55.
- [Google Scholar]
- Pathological findings in a mouse model of Japanese encephalitis infected via the footpad. Neurol Asia. 2015;20:349-54.
- [Google Scholar]
- Vaccines and animal models for arboviral encephalitides. Antiviral Res. 2003;60:153-74.
- [Google Scholar]
- Flaviviruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, eds. Fields virology (5th ed). Philadelphia, PA: Lippincott Williams & Wilkins; 2007. p. :1153-252.
- [Google Scholar]
- The contribution of rodent models to the pathological assessment of flaviviral infections of the central nervous system. Arch Virol. 2012;157:1423-40.
- [Google Scholar]
- The effect of host age, virus dose and route of inoculation on inapparent infection in mice with Japanese encephalitis virus. Proc Soc Exp Biol Med. 1966;123:118-24.
- [Google Scholar]
- Reciprocal transmission of herpes simplex virus type 1 (HSV-1) between corneal epithelium and trigeminal neurites in an embryonic chick organ culture. FASEB J. 2002;16:878-80.
- [Google Scholar]
- The blood-brain barrier in the cerebrum is the initial site for the Japanese encephalitis virus entering the central nervous system. J Neurovirol. 2008;14:514-21.
- [Google Scholar]
- West Nile virus-induced cell adhesion molecules on human brain microvascular endothelial cells regulate leukocyte adhesion and modulate permeability of the in vitro blood-brain barrier model. PLoS One. 2014;9:E102598.
- [Google Scholar]
- Pathways of the early propagation of virulent and avirulent rabies strains from the eye to the brain. J Virol. 1985;55:158-62.
- [Google Scholar]
- Neural spread of herpes simplex virus after anterior chamber inoculation. Invest Ophthalmol Vis Sci. 1991;32:2462-72.
- [Google Scholar]
- Herpes simplex virus-1 infects the olfactory bulb shortly following ocular infection and exhibits a long-term inflammatory profile in the form of effector and HSV-1-specific T cells. J Neuroinflammation. 2017;14:124.
- [Google Scholar]
- Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nat Commun. 2016;7:10832.
- [Google Scholar]
- Intrabulbar inoculation of Japanese encephalitis virus of mice. Kurume Med J. 1968;15:43-50.
- [Google Scholar]
- Manual of histological staining methods of the Armed forces institute of pathology (3rd ed). New York: McGraw Hill Book Co; 1968.
- Improved synthesis of full-length RNA probe at reduced incubation temperatures. Nucleic Acids Res. 1990;18:6463.
- [Google Scholar]
- Neuropathogenesis of Japanese encephalitis in a primate model. PLoS Negl Trop Dis. 2014;8:e2980.
- [Google Scholar]
- Comparative study of mouse brains infected with Japanese encephalitis virus by intracerebral or intraperitoneal inoculation. Int J Exp Pathol. 1990;71:857-69.
- [Google Scholar]
- A preliminary neuropathological study of Japanese encephalitis in humans and a mouse model. Trans R Soc Trop Med Hyg. 2006;100:1135-45.
- [Google Scholar]
- Viral infection of the central nervous system and neuroinflammation precede blood-brain barrier disruption during Japanese encephalitis virus infection. J Virol. 2015;89:5602-14.
- [Google Scholar]
- Tissue tropisms, infection kinetics, histologic lesions, and antibody response of the MR766 strain of zika virus in a murine model. Virol J. 2017;14:82.
- [Google Scholar]
- Brain lesions induced by experimental intranasal infection of Japanese encephalitis virus in piglets. J Comp Pathol. 2009;141:156-62.
- [Google Scholar]
- Mice with different susceptibility to Japanese encephalitis virus infection show selective neutralizing antibody response and myeloid cell infectivity. PLoS One. 2011;6:e24744.
- [Google Scholar]
- The pathogenesis of 3 neurotropic Flaviviruses in a mouse model depends on the route of neuroinvasion after viremia. J Neuropathol Exp Neurol. 2015;74:250-60.
- [Google Scholar]
- Japanese encephalitis: Immunocytochemical studies of viral antigen and inflammatory cells in fatal cases. Ann Neurol. 1985;18:567-73.
- [Google Scholar]
- Immunohistochemical proof of intraneural localization of herpes simplex virus in experimental retinitis. Jpn J Ophthalmol. 1995;39:143-51.
- [Google Scholar]
- Optic nerve involvement in viral spread in herpes simplex virus type 1 retinitis. Invest Ophthalmol Vis Sci. 1990;31:1683-9.
- [Google Scholar]






