Translate this page into:
Investigation into a community outbreak of Salmonella Typhi in Bengaluru, India
Reprint requests: Dr. Vasan K. Sambandamurthy, Mazumdar Shaw Centre for Translational Research, Narayana Health City, A-Block, 8th Floor #258/A, Bengaluru 560 099, Karnataka, India e-mail: vasan.sambandamurthy@ms-mf.org
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Outbreaks of infection due to Salmonella enterica servovar Typhi (S. Typhi) are a great threat to public health. A rapid molecular typing method to characterize strains implicated in an outbreak is critical in implementing appropriate control measures. This study was done to demonstrate the power of a PCR-based method to provide rapid insights into the genetic relatedness amongst the Salmonella isolates implicated in a suspected typhoid fever outbreak.
Methods:
Forty two S. Typhi isolates originating from three geographically distinct areas, with one area suspected to have a single-source outbreak were included in the study. The genetic fingerprint of all isolates was generated using enterobacterial repetitive intergenic consensus sequence based-PCR (ERIC-PCR). The antimicrobial susceptibility profiles were also evaluated.
Results:
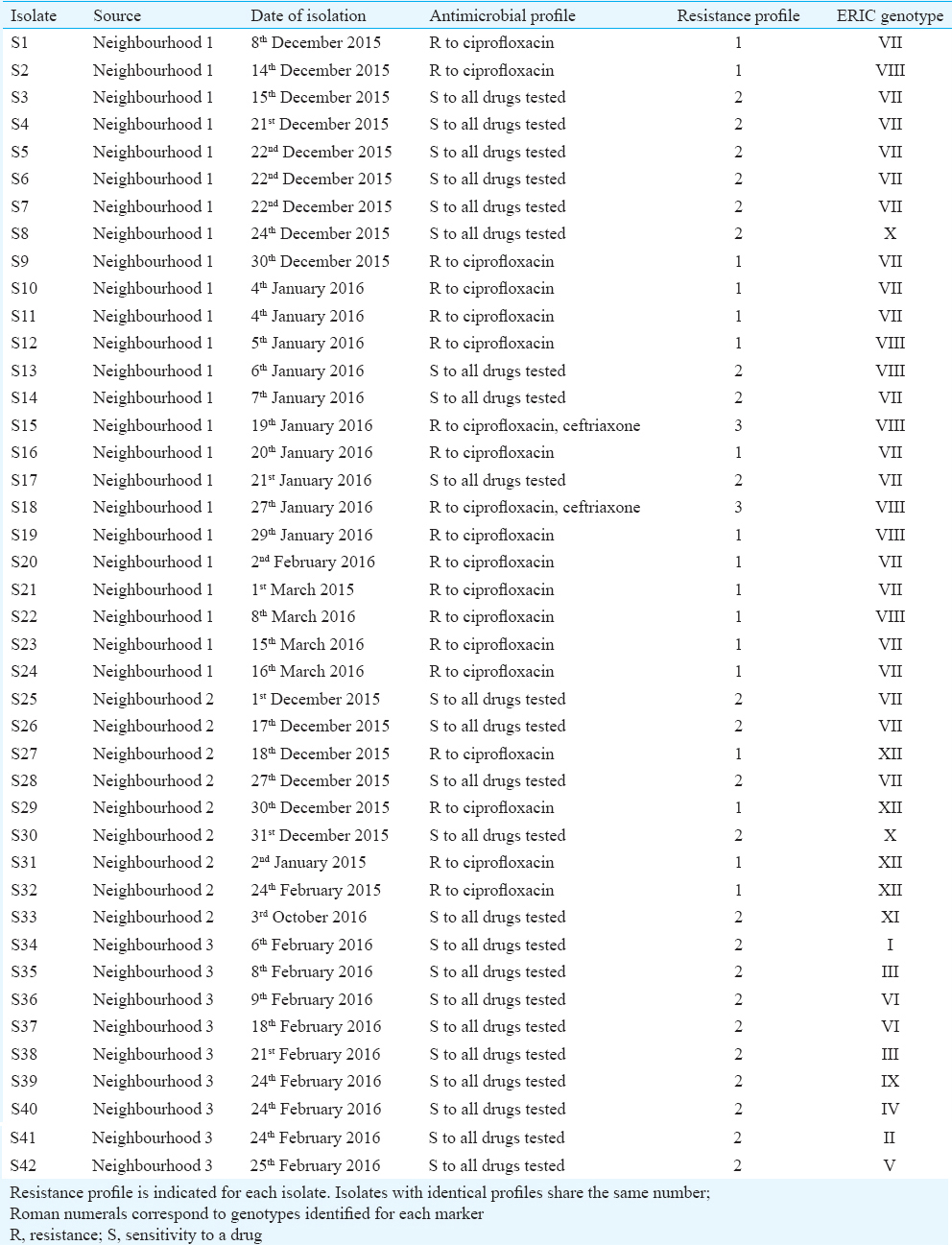
ERIC-PCR was found to be rapid and reproducible with a discriminatory index of 0.766. The dendrogram constructed based on ERIC-PCR fingerprinting revealed the existence of 12 distinct genotypes. The location suspected to have an outbreak displayed two genotypes amongst the 24 isolates. The other two locations (18 isolates) displayed genetic heterogeneity. The clonality of the outbreak isolates from the time-matched control isolates was established. The observed antimicrobial susceptibility profiles did not have any discriminatory power to subtype the isolates compared to the genetic fingerprints.
Interpretation & conclusions:
Our study demonstrated the discriminatory power and value of ERIC-PCR in the typing of S. Typhi isolates and providing valuable epidemiological insights.
Keywords
Clonality
enterobacterial repetitive intergenic consensus-polymerase chain reaction
outbreak investigation
Salmonella Typhi
Salmonella enterica serovar Typhi (S. Typhi) is the leading cause of community-acquired bloodstream infection resulting in prolonged fever and in some cases leading to an asymptomatic carrier state1. Typhoid fever is widely prevalent in many regions of the world with poor sanitation. The scenario is one of either endemicity due to multiple sources of infection or occasionally a single source outbreak2.
In a community outbreak of typhoid fever, early detection of the source of infection and rapid typing of isolates is critical for the prevention of further transmission3. Pulse-field gel electrophoresis (PFGE) has been used as the molecular typing technique to study clonality amongst isolates4. This technique is expensive, laborious and has limited interlaboratory reproducibility5. Several rapid, PCR-based typing methods to delineate genetic relatedness of bacterial strains have been reported6.
One such technique, based on the enterobacterial repetitive intergenic consensus (ERIC) relies on the PCR-based amplification of short target sequences that are highly conserved. The variability in the position of repeat elements between the genomes of bacteria has been exploited as a genetic marker to characterize isolates within a bacterial species7. An earlier study established ERIC-PCR to be the most reliable method for differentiation of Salmonella serovars based on a comparison of four molecular techniques8. In addition, molecular genotyping using ERIC-PCR is faster and cheaper than PFGE for establishing genetic relatedness amongst Salmonella serovars910. Therefore, repetitive DNA elements remain as a valid analytical tool for epidemiological investigation of infection outbreaks in countries with limited public health resources. This study was undertaken to demonstrate the resolution of ERIC-PCR-based method to rapidly determine the genetic relatedness of S. Typhi isolates implicated in a typhoid outbreak from a single neighbourhood within a defined period of time.
Material & Methods
This study was carried out in the Infection Control Laboratory at Narayana Health City, Bengaluru, India. S. Typhi isolates (24 isolates) were collected consecutively from blood cultures from patients who presented to Narayana Health, City Bengaluru, over a period of four months (December 2015-March 2016) during the outbreak, as opposed to a low number of isolates (<5) usually seen over a quarter of a year. A retrospective analysis in terms of their age, sex and geographical location was carried out to evaluate the epidemiological context of all 24 patients who were diagnosed with typhoid fever. As matching controls, 18 isolates (time-matched) that were randomly collected from two different geographical locations (Bommasandra and Ulsoor in Bengaluru) were evaluated. Most of the patients presented with continuous fever and abdominal pain with or without diarrhoea. The study was approved by the Institutional Ethics Committee, and all participants gave written informed consent.
All the isolates were identified from blood culture (20 ml blood collected during the febrile phase) using BD Phoenix Automated system (B D Diagnostic Systems, Sparks, MD, USA) following the manufacturer’s guidelines.
Antimicrobial susceptibility profiles: Antimicrobial susceptibility profiles of the clinical isolates were determined using the Phoenix™ (B D Diagnostic Systems) as recommended by the manufacturer. The minimal inhibitory concentration (MIC) values obtained by the above-mentioned methods were categorized according to Clinical and Laboratory Standards Institute breakpoints, as susceptible (S), intermediate (I) or resistant (R) to various drugs11. The following antibiotics were tested ampicillin, amoxicillin/clavulanic acid, ceftriaxone, chloramphenicol, ciprofloxacin and co-trimoxazole.
Molecular fingerprinting using enterobacterial repetitive intergenic consensus-polymerase chain reaction (ERIC-PCR): A single colony from a pure culture of each isolate was resuspended in sterile water and boiled for 10 min12. One microlitre of the boiled extract was used in a 25 µl PCR mixture to amplify the target genes using specific primers as described. The PCR amplification was performed by adding a mixture of 18 µl of sterile distilled water, 2.5 µl of 10X PCR buffer, 1 µl of 10 mM dNTPs, 1 µl of each primer, ERIC 1R: 5’-ATG TAA GCT CCT GGG GAT TCA-3’ and ERIC 2: 5’-AAG TAA GTG ACT GGG GTG AGC G-3’, 0.5 µl of Taq polymerase and 1 µl of the template DNA to the reaction mixture7. The cycling parameters were as follows: initial denaturation for three minutes at 94°C and thirty cycles of one minute at 94°C, three minutes at 55°C and two minutes at 72°C followed by a final amplification of 10 min at 72°C.
The amplified products were separated by electrophoresis on 1.5 per cent agarose gel and photographed under an ultraviolet transilluminator. A 100 kb DNA ladder was used as the molecular size marker.
Molecular fingerprint analysis: Isolates were categorized according to their PCR banding pattern, as identical, similar or unrelated. A similarity index was calculated using the Dice coefficient, and cluster analysis of the matrices was generated using the unweighted pair group method using arithmetic averages (UPGMA). Dendrograms of isolates were generated with the tree option using the PAST programme13. The discriminatory (D) index was calculated according to the method of Hunter and Gaston14. The index of discrimination represents the probability that two randomly chosen isolates, sampled consecutively, would be distinguished by the test and range from D=0 to D=1.
Results
A total of 42 patients, (19 female and 23 male) were included in the study. The mean age of the patients was 32±15 yr, ranging from 7 to 61 yr. Retrospective analysis revealed that 24 of these patients were all residents of the same locality (HSR layout in Bengaluru).
All isolates recovered from patients at three hospitals in geographically different locations within Bengaluru city were typed using ERIC-PCR. Being a community outbreak, we were unable to obtain the food or water consumed by the patients during the epicentre of this outbreak.
Molecular fingerprinting using ERIC-PCR: All 42 S. Typhi isolates were fingerprinted using ERIC-PCR to assess an epidemiological link amongst the isolates. Using specific primers for ERIC-PCR, five to eight bands were detected ranging from sizes 0.2 to 2 kb. Four bands at approximately 188, 252, 489 and 522 bp were more frequently observed in 68 per cent (29/42) of the isolates (Fig. 1). With a diversity index of 0.766 and polymorphic loci generated by this marker, 12 distinct genotypes (I-XII) were identified (≥90% similarity level). The most prevalent ERIC-PCR types were Type VII found in 45 per cent (19/42) of the isolates and Type VIII found in 17 per cent (7/42) of the isolates. The remaining genotypes contained 1-3 isolates each (Table).
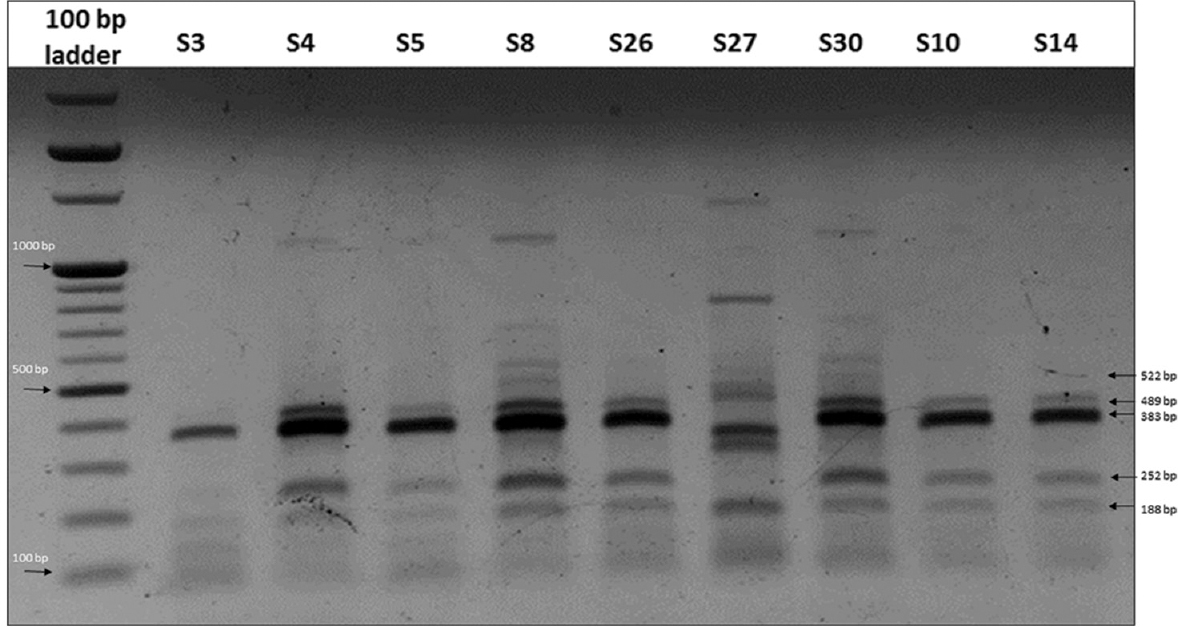
- A representative 1.5 per cent agarose gel stained with ethidium bromide showing the amplification pattern obtained for Salmonella Typhi isolates using enterobacterial repetitive intergenic consensus-polymerase chain reaction (ERIC-PCR). Lanes 1-10, 100 bp ladder, S3, S4, S5, S8, S26, S27, S30, S10, S14.
The dendrogram showed that distinct fingerprint types were observed for isolates from different geographical regions. Fig. 2 shows all isolates from neighbourhood 1 to display three distinct profiles, with one isolate (S8) quite distinct from the predominant profile from that location (ERIC-PCR Type VII and VIII). Of the 18 time-matched control isolates (S25-S42), 11 distinct ERIC-PCR fingerprints were observed. All isolates from neighbourhood 3 (S34-S42), displayed unique ERIC-PCR profiles, with the exception of isolates S36 and S37. Isolates from neighbourhood 2 (S25-S33) showed lesser variability and revealed three distinct ERIC-PCR profiles. In contrast to the above finding, geographically distant isolates occasionally shared the same ERIC-PCR profile. This was seen in isolates S25, S26, S28 (Type I), S8 and S30 (Type X). Overall, cluster analysis revealed greatest variability in isolates from neighbourhood 3 and least variability amongst isolates from neighbourhood 1.
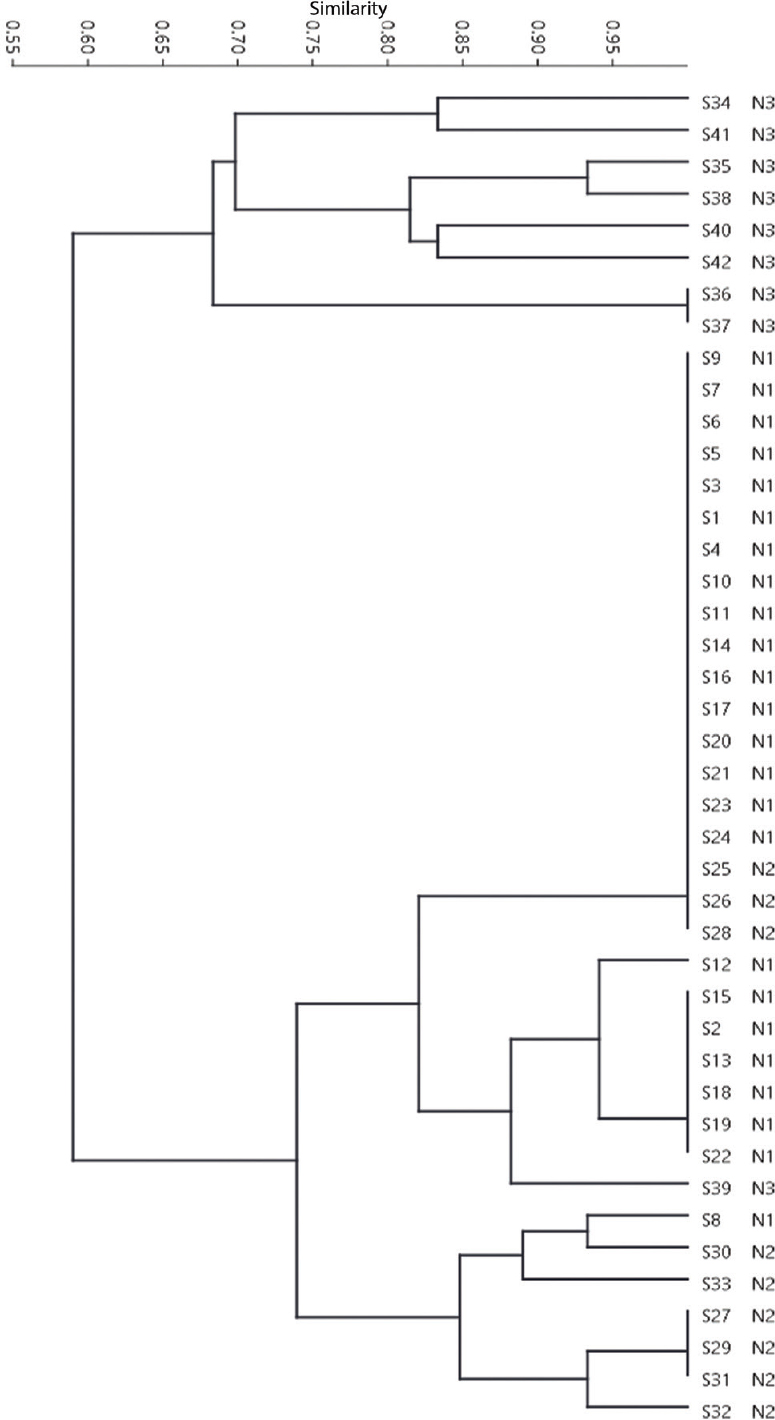
- Unweighted pair group method using arithmetic averages dendrograms at 90 per cent similarity level of the studied Salmonella serotype Typhi isolates derived from amplification patterns using enterobacterial repetitive intergenic consensus-polymerase chain reaction (ERIC-PCR). S and N numbers indicate isolate and neighbourhood numbers, respectively.
Antimicrobial susceptibility profile: To evaluate if there was an association between the antimicrobial susceptibility pattern and ERIC-PCR profile, the MICs of all 42 S. Typhi isolates were determined. Based on the susceptibility profile to six antimicrobials, the 42 isolates were found to group into three distinct profiles. More than half of the isolates (23/42) were susceptible to all of the drugs tested. Of the 45 percent of isolates (19/42) that were resistant to ciprofloxacin, 12 percent (5/42) displayed resistance to ceftriaxone also (Table).
Despite sharing a similar antibiogram, a few outbreak isolates were genetically distinct from the control isolates (Table). This observation was also noted in isolates within a neighbourhood (N3). In some instances, the outbreak isolates that shared the same ERIC-PCR profile, differed in sensitivity to ciprofloxacin. Overall, there was no association between the genotype and susceptibility profile for the isolates investigated in this study.
Discussion
Salmonella serovars are known to cause salmonellosis through the consumption of contaminated food such as raw meat, milk and poultry products2. A rapid molecular fingerprinting method is required in an epidemiological surveillance during an outbreak. The present work evaluated the discriminatory power of ERIC-PCR genetic relatedness between S. Typhi isolates from a suspected outbreak. PFGE analysis of S. Typhi isolates from various typhoid endemic regions have shown the outbreak isolates to be homogenous and clonal in nature, whereas sporadic isolates to display a high degree of diversity15. Similar observation was made in the present study, wherein the outbreak isolates (neighbourhood 1) displayed very little genetic heterogeneity in comparison to isolates from the other two neighbourhoods (neighbourhood 2 and 3) where S. Typhi infections are known to be endemic. Within the outbreak isolates, there were two major clones, suggesting the likelihood of two concurrent infections caused by genetically distinct strains within the same neighbourhood. This result highlights the resolution power of ERIC-PCR method and demonstrates the value in identifying the type of strain involved in an outbreak where most of the patients have a similar clinical presentation. In addition, this method also had the power to differentiate endemic from outbreak isolates. The observed discriminatory potential (0.766) of ERIC-PCR in this study was in concordance with the report of Kumao et al16, who showed the intraserotype discriminatory power of this technique. In another report, an analysis of S. Typhi strains from patients estimated a discriminatory index value of 0.993, reiterating the potential of ERIC-PCR17. In agreement with a previous report, we found low molecular weight bands in random amplified polymorphic DNA analysis to be irreproducible and faint, thereby introducing a high degree of subjectivity (data not shown)17.
There were three categories of sensitivity patterns observed in the isolates. Isolates from the same outbreak did not share an identical antimicrobial profile possibly due to antimicrobial usage and patient compliance. The observed difference in resistance to antimicrobials within the isolates from different neighbourhood reflects likely variances in therapeutic measures, in the type of drugs and the frequency of their use. The susceptibility patterns herein reported show similarities relative to those reported for the serotype Typhi in other countries41819. Consistent with earlier published reports, low level of resistance to ampicillin, chloramphenicol and co-trimoxazole was observed in all isolates420. In agreement with previous findings, low-level resistance (5%) to the third-generation cephalosporin, ceftriaxone, was also observed in the present study212223. It is well documented that drug resistance in S. Typhi arises due to either a chromosomal mutation or the acquisition of a plasmid or transposon24. However, in non-typhoidal Salmonella species, increased ceftriaxone resistance is linked to the expression of plasmid-mediated ampC or ESBL genes blaCTX-M and blaCMY-22526. Given that fluoroquinolones remain an important therapeutic option for typhoid fever, it was worrisome to note that 45 per cent of the isolates were resistant to ciprofloxacin. Our results were in concordance with an earlier report on an increase in quinolone resistance amongst strains in India27. The emergence of drug-resistant S. Typhi isolates emphasizes the necessity to implement stringent sanitation practices to prevent community level outbreaks.
Antimicrobial profiles alone were found to be of limited value in differentiating closely related strains. However, ERIC-PCR was able to differentiate amongst isolates that were indistinguishable based on their drug susceptibility profile. No relation between the antimicrobial susceptibility pattern and ERIC profile was observed for most isolates. We observed a lack of association between the antimicrobial profile and the molecular fingerprint. For instance, isolates sharing ERIC-PCR profile VII have isolates that are susceptible as well as resistant to ciprofloxacin. It is difficult to explain why a particular cluster has isolates that display different antibiogram profiles. Lack of association between the genotype and the antimicrobial susceptibility profile has already been documented for S. enterica serotypes92829. This interpretation can be explained by the fact that repetitive genetic elements are widely dispersed throughout the bacterial chromosome, whereas the genetic resistance determinants are usually clustered in defined genetic regions on the bacterial chromosome or mediated through extrachromosomal mechanisms involving plasmids or transposons. In addition, detection of isolates with different antibiotic resistance profile within a cluster suggests the likelihood of ongoing selection pressure amongst the strains belonging to a clone, making them either sensitive or resistant to a given drug. The existence of some isolates that did not cluster with any of the major clones suggest the existence and dissemination of several different haplotypes of serotype Typhi within the community resulting in independent transmission events1530. The selective pressure induced by the use of antimicrobials act as a driving force to trigger the emergence of resistance. In most instances, these mechanisms are genetically encoded and can be readily transmitted horizontally across bacterial species24. Given the global emergence of drug-resistant strains of Salmonella, it is imperative to restrict the usage of extended-spectrum cephalosporins and rely on continuous surveillance for resistant strains in the environment.
The present study also documents the limitation of not collecting environmental samples during an outbreak, a limitation that hinders the design to infection prevention plans in a timely manner. This would also have provided key information into identifying the source of the outbreak.
In conclusion, our results suggested that two genetically related clones of S. Typhi were responsible for an outbreak in a neighbourhood within a short span of time. This further reinforces the power of molecular genetic markers to effectively discriminate isolates associated with outbreaks. As demonstrated in the present study, ERIC-PCR can be used for routine molecular typing of S. Typhi to provide information for designing and implementing effective prevention and control programmes.
Acknowledgment
The authors thank Dr Devi Shetty for his valuable guidance throughout the course of this study. Authors also thank Drs Karthik S.M., Anirudh Shetty, Reshu Aggarwal, Avinash S, Abhinav Malhotra and Vinutha Vinutha J.O. for their clinical support. Authors thank Dr Nikhil Moorchung for providing us with control isolates (neighbourhood 3) in this study.
Conflicts of Interest: None.
References
- Salmonella enterica serovar typhi and the pathogenesis of typhoid fever. Annu Rev Microbiol. 2014;68:317-36.
- [Google Scholar]
- Genetic typing methods applied to the differentiation of clonal lines among Salmonella enterica serogroup G strains causing human salmonellosis. FEMS Immunol Med Microbiol. 1997;19:215-21.
- [Google Scholar]
- Antimicrobial resistance trends in blood culture positive Salmonella typhi isolates from Pondicherry, India 2005-2009. Clin Microbiol Infect. 2012;18:239-45.
- [Google Scholar]
- Pulsed field gel electrophoresis: A review of application and interpretation in the molecular epidemiology of infectious disease. Infect Genet Evol. 2010;10:866-75.
- [Google Scholar]
- Comparison of random amplified polymorphic DNA analysis and enterobacterial repetitive intergenic consensus-PCR for epidemiological studies of Salmonella. FEMS Immunol Med Microbiol. 1996;14:129-34.
- [Google Scholar]
- Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823-31.
- [Google Scholar]
- Comparison of four molecular typing methods for the differentiation of Salmonella spp. Int J Food Microbiol. 2005;105:411-8.
- [Google Scholar]
- Epidemiology and antibiotic resistance of Salmonella enterica serovar Kentucky isolates from Tunisia: The new emergent multi-drug resistant serotype. Food Res Int. 2012;45:925-30.
- [Google Scholar]
- Comparison of pulsed field gel electrophoresis and repetitive sequence polymerase chain reaction as genotyping methods for detection of genetic diversity and inferring transmission of Salmonella. Vet Microbiol. 2004;100:205-17.
- [Google Scholar]
- Performance Standards for Antimicrobial Susceptibility Testing;Twenty-Fourth Informational Supplement M100-S24. Wayne, PA: CLSI; 2014.
- A simple and rapid method for extracting bacterial DNA from intestinal microflora for ERIC-PCR detection. World J Gastroenterol. 2008;14:2872-6.
- [Google Scholar]
- PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electron. 2001;4:1-9.
- [Google Scholar]
- Numerical index of the discriminatory ability of typing systems: An application of Simpson's index of diversity. J Clin Microbiol. 1988;26:2465-6.
- [Google Scholar]
- Analysis of Salmonella typhi isolates from Southeast Asia by pulsed-field gel electrophoresis. J Clin Microbiol. 1995;33:1938-41.
- [Google Scholar]
- Molecular subtyping methods for detection of Salmonella enterica serovar Oranienburg outbreaks. J Clin Microbiol. 2002;40:2057-61.
- [Google Scholar]
- ERIC PCR and RAPD based fingerprinting of Salmonella typhi strains isolated over a period of two decades. Infect Genet Evol. 2010;10:530-6.
- [Google Scholar]
- Multi-drug resistance and reduced susceptibility to ciprofloxacin among Salmonella enterica serovar typhi isolates from the Middle East and Central Asia. New Microbes New Infect. 2014;2:88-92.
- [Google Scholar]
- What after ciprofloxacin and ceftriaxone in treatment of Salmonella typhi. Pak J Med Sci. 2006;22:51-4.
- [Google Scholar]
- Trends in antimicrobial susceptibility of Salmonella typhi from North India (2001-2012) Indian J Med Microbiol. 2014;32:149-52.
- [Google Scholar]
- Quinolone and cephalosporin resistance in enteric fever. J Glob Infect Dis. 2010;2:258-62.
- [Google Scholar]
- A highly ceftriaxone-resistant Salmonella typhi in Bangladesh. Pediatr Infect Dis J. 1999;18:387.
- [Google Scholar]
- Extended-spectrum-beta-lactamase production in a Salmonella enterica serotype typhi strain from the Philippines. J Clin Microbiol. 2008;46:2794-5.
- [Google Scholar]
- Hugo and Russell's pharmaceutical microbiology (8th ed). New Delhi: Wiley Blackwell Publishing House; 2011.
- Increasing ceftriaxone resistance in Salmonella isolates from a university hospital in Taiwan. J Antimicrob Chemother. 2005;55:846-52.
- [Google Scholar]
- Ceftriaxone resistance of nontyphoidal Salmonella enterica isolates in Northern Taiwan attributable to production of CTX-M-14 and CMY-2 beta-lactamases. J Clin Microbiol. 2005;43:3237-43.
- [Google Scholar]
- Current trend of antibiotic sensitivity of Salmonella typhi and other Salmonellae in Mumbai: A 5 years study. Indian J Med Microbiol. 2016;34:115-6.
- [Google Scholar]
- Phenotypic and genetic characterization of Salmonella enterica subsp. enterica serovar Typhimurium isolated from pigs in Rio Grande do Sul, Brazil. Res Vet Sci. 2007;83:302-10.
- [Google Scholar]
- A comparison of subtyping methods for differentiating Salmonella enterica serovar enteritidis isolates obtained from food and human sources. Osong Public Health Res Perspect. 2013;4:27-33.
- [Google Scholar]
- Epidemiologic analysis of sporadic Salmonella typhi isolates and those from outbreaks by pulsed-field gel electrophoresis. J Clin Microbiol. 1994;32:1135-41.
- [Google Scholar]






