Translate this page into:
Intervention strategy for Rapid Grower Mycobacteria outbreak among post-laparoscopic surgical site infection patients in a tertiary care hospital
For correspondence: Dr Nupur Pal, Department of Microbiology, Institute of Post Graduate Medical Education & Research, Kolkata 700 020, West Bengal, India e-mail: docnupur80@gmail.com
-
Received: ,
Accepted: ,
Abstract
Background & objectives
The emergence of Rapid Grower Mycobacteria (RGM) infections recently produced a great challenge among surgeons following laparoscopic surgical site infection. Infections caused by atypical mycobacteria may be overlooked due to limitations of proper diagnostic infrastructure and unawareness in the resource-limited set-up. In this study, we proposed an intervention strategy for RGM infection among patients having post-laparoscopic surgical site infections in our hospital.
Methods
Five hundred sixty-five samples were collected within one year (April 2018- March 2019) from the surgery outpatient department (OPD), suspecting atypical mycobacteria infection following surgery. Samples were processed in the microbiology department by conventional staining and culture. RGM was diagnosed up to the species level by both conventional and molecular methods [line probe assay (LPA)]. The antibiogram was performed by the microbroth dilution method on the RAPMYCOI kit as per Clinical and Laboratory Standard Institute (CLSI) guidelines. Simultaneous source identification was carried out.
Results
A biofilm-producing Mycobacterium abscessus strain was detected from the plastic disinfection tray of the surgical operation theatre (OT), which may be the continuous source of iatrogenic post-surgical infection. RGM prevalence among suspected patients was 19.47 per cent, and around 92 per cent of them were from laparoscopic surgery. Antibiotic sensitivity, as per CLSI guidelines, showed most of them (88.8%) were resistant to commonly given antibiotic clarithromycin. Most sensitivity was to antibiotics amikacin, tobramycin, moxifloxacin, and doxycycline. The game-changing intervention related to this outbreak scenario was the introduction of gas-plasma sterilization and maintaining strict asepsis in surgical operation theatre.
Interpretation & conclusions
Based on the analysed data, we proposed an intervention strategy in our hospital for treating and preventing RGM infection. Such an approach will help arrest the RGM-outbreaks in future.
Keywords
Biofilm
drug resistance
intervention strategy
laparoscopic surgery
outbreak investigation
rapid grower mycobacteria
surgical site infection
In the 1990s, laparoscopic surgery brought a new era in modern surgery. By limiting invasiveness, it reduced morbidity such as postoperative pain, ileus, and postoperative infections, as well as hastened recovery, decreased hospital stay, and improved cosmesis. Port site infections (PSI), though a rare complication following laparoscopic surgery, the emergence of Rapid Grower Mycobacterial (RGM) infections in recent times have posed challenges among surgeons1,2. It soon abolishes the benefits of laparoscopic surgery (LS); the patient becomes anxious with this indolent, distressing treatment refractory infection and loses faith in the operating surgeon. The cosmetic purpose of laparoscopic surgery and the quality of life of the patient are seriously affected. Rapid Grower Mycobacteria (Runyon group IV of atypical mycobacteria) are a large, diverse group of environmental organisms ubiquitous in water and soil and known to produce soft tissue infections following surgical procedures as secondary colonizers3. Clinical procedures, especially laparoscopic surgery using heat-sensitive non-autoclavable instruments, are associated with RGM infections. Infections caused by atypical mycobacteria may be overlooked due to limitations of proper diagnostic infrastructure and unawareness. Previous studies report an incidence of nontuberculous mycobacteria (NTM) ranging from 3.4 per cent to 24.7 per cent in India4. Due to its chronicity, diagnostic dilemma, unresponsiveness to conventional antibiotics, and lack of standard treatment guidelines, it becomes a major problem for both doctors and patients in developing countries like India. In this study, we proposed an intervention strategy for RGM infections based on the data analysed among patients with post-laparoscopic surgical site infections in our hospital.
Materials & Methods
The study was conducted at the department of Microbiology, Institute of Post Graduate Medical Education and Research (IPGMER), Kolkata, West Bengal from April 2018 to March 2019 after obtaining ethical approval from the Institute Ethics Committee.
Study samples
As per clinical criteria for suspecting non-tuberculous mycobacteria (NTM) infections5,6, samples were collected from General Surgery OPD, IPGMER, Kolkata, from individuals who had undergone laparoscopic surgical procedures and presented with abscess or chronic inflammation that was not responding to usual antibiotic treatment.
Study procedure
In the department of Microbiology, IPGMER, Kolkata, West Bengal, samples were processed for Gram stain, acid-fast bacillus (AFB) stain, aerobic culture and sensitivity (C/S), and AFB culture on Lowenstein-Jensen (LJ) media. AFB growth on LJ media with or without a positive AFB smear was processed for identification. RGM was diagnosed by observing growth within seven days of incubation on LJ media and a negative TBMPT64 antigen test. Speciation was done using conventional biochemical tests like the nitrate reduction test, growth on MacConkey agar with 5%NaCl and arylsulfatase test7. All members of the Mycobacterium chelonae, M. abscessus group, and M. fortuitum group show strong arylsulfatase activity at three days, whereas the M. smegmatis group does not. M. fortuitum reduces nitrate to nitrites, whereas M. abcessus grows on 5%NaCl. An antibiotic sensitivity test was performed by the micro broth dilution method of Rapid Grower Mycobacteria with RAPMYCOI kit (Thermo Fisher Scientific, MN, USA) as per CLSI guidelines8. The following antibiotics were used for the drug sensitivity study: Sxt-cotrimoxazole, LZD-linezolid, CIP-ciprofloxacin, IMI-imipenem, MXF-moxifloxacin, FEP-cefepime, FOX-cefoxitin, AUG-coamoxiclav, AMI-amikacin, AXO-ceftriaxone, DOX-doxycycline, MIN-minocycline, TGC-tigecycline, CLA-clarithromycin, and TOB-tobramycin RAPMYCOI plates contain freeze-dried antibiotics in a range of concentrations (μg/ml). Simultaneously, an outbreak investigation was carried out in surgical operation theatre (OT). Samples were collected from laparoscopic instruments, sterile gauze, suture material, mouth of the water-tap, tap water, disinfectant tray, OT trolley, etc. Growth was identified by conventional and molecular methods. Line probe assay (LPA) was done using genotype mycobacterium CM/AS (Hain Lifescience, GnbH, Germany). In vitro colony biofilm study was performed for environmental isolate on polycarbonate membrane filter and surface topography was studied outside by atomic force microscopy to demonstrate biofilm structure.
Results
Five hundred sixty-five samples were collected within one year (April 2018-March 2019) from surgery OPD suspecting atypical mycobacteria infection. Of them, 110 (19.47%) were found to be positive for Rapid Grower Mycobacteria infection: 88 per cent of them were from laparoscopic cholecystectomy, 14 per cent from laparoscopic herniotomy, six per cent from open surgery, and two per cent from fallopian tube ligation. M. abscessus prevalence was highest (51.28%), followed by M. fortutum (24.36%) and M. chelonae (15.38%). The remaining were slow-grower mycobacteria (3.84%), unidentified (2.56%) and smear-positive culture negative (2.56%). Infections were identified more in female (55.46%) than in male (44.54%) patients. The antibiotic sensitivity profile (Figure) showed that moxifloxacin (MOX), amikacin (AMI), tobramycin (TOB) and doxycycline (DOX), were 100 per cent sensitive, followed by ciprofloxacin (CIP), cotrimoxazole (SXT), and minocycline (MIN) >80 per cent sensitive. The least sensitive drugs were linezolid (LZD), cefoxitin (FOX), coamoxiclav (AUG), ceftriaxone (AXO), tigecycline (TGC), and clarithromycin (CLA). Among them, cefepime (FEP) and imipenem (IMI) showed full resistance.
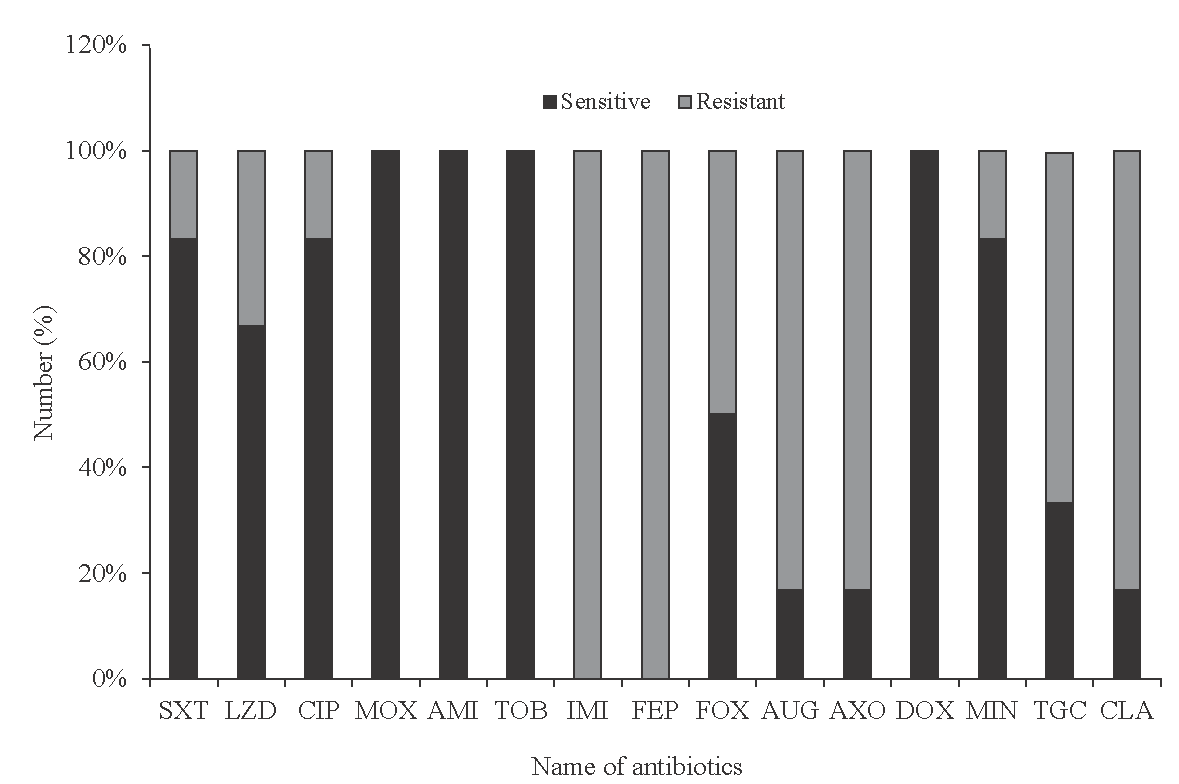
- Antibiotic sensitivity and resistance pattern chart of isolated Rapid Grower Mycobacteria against different antibiotics. SXT, cotrimoxazole; LZD, linezolid; CIP, ciprofloxacin; IMI, imipenem; MXF, moxifloxacin; FEP, cefepime; FOX, cefoxitin; AUG, coamoxiclav; AMI, amikacin; AXO, ceftriaxone; DOX, doxycycline; MIN, minocycline; TGC, tigecycline; CLA, clarithromycin; TOB, tobramycin.
In this outbreak investigation, the source of infection was identified. M. abscessus was found in a plastic disinfectant tray present in surgical OT in which laparoscopic instruments were sterilized by immersing them overnight in a 2% glutaraldehyde solution. An atomic force microscopy study of the in vitro colony biofilm method suggested that it was a biofilm producer strain. After source identification, control measures were taken immediately to stop the outbreak situation. The plastic tray used for disinfectant was replaced with a metal tray. Instructions were given to mechanically scrub surgical instruments before autoclaving or disinfecting at regular intervals, replace the disinfectant tray at regular intervals, avoid tap water, and follow the strict asepsis method. Strict sterilisation procedures were followed by introducing gas plasma in surgery OT. Subsequently, post-surgery site infection (PSI) cases were reduced after the intervention measures. Presently, only one to two cases of surgical site infections are encountered per month in our hospital.
Discussion
Though M. tuberculosis attracts more concern worldwide, NTM has emerged globally in both immunocompromised as well as immunocompetent patients. A rising incidence and prevalence of NTM diseases have become a major health problem in recent years9. RGM is posing a significant threat to infection control, leading to chronic, nagging, treatment-refractory hospital-acquired postsurgical infections. The clinical presentation of M. tuberculosis complex (MTBC) and NTM may or may not be the same, but the treatment regimen is always different for both infections10. NTMs are often resistant to anti-tubercular drugs (ATD) used for treating MTBC, which helps to misclassify the patients as multidrug-resistant tuberculosis (MDRTB) and to make mistakes in treatment with a battery of second-generation ATD as treatment of ‘MDRTB.’ Identification of NTM, in this context, is essential because positive microscopy cannot differentiate MTBC from NTM infection, causing diagnostic and clinical dilemmas in developing countries. Therefore, culture isolation, differentiation, and species identification that distinguishes MTBC from NTM are necessary prerequisites for suitable management of patients with mycobacterial infections. There has been much controversy surrounding the proper line of treatment for atypical mycobacteria. There is no consensus on treatment, and there are no prior well-controlled trials to guide the treatment of atypical mycobacteria5. Therefore, the need for antimicrobial susceptibility testing of NTMs is emphasized for the comprehensive management of NTM infection, especially in hospital settings.
This study showed that the Rapid Grower Mycobacteria group (19.47%) may be one of the most common hospital-acquired infections (HAI) unless proper surveillance and strict infection control measures are followed. One Indian study reported higher NTM rates (10.9%), and another Brazilian study confirmed NTM in 144 out of 1051 (13.7%) suspected surgical mycobacterial infections11,12. Variable NTM rates might be due to different geographical areas, study populations, types of samples, methods of detection, and the hospital’s aseptic measure policy. Most of the previously published reports on NTM were among pulmonary or extra-pulmonary infections other than SSI (surgical site infection), and limited publications were available on NTM among SSI13,14. RGM infections must be considered in post-surgical wounds that show nonhealing and do not respond to usual antibiotics. Although ZN smear is a simple and cost-effective method, it has low-sensitivity15. This was supported by the ZN sensitivity of our study (53.6%), which was quite close to other studies (63.6%)15.
In our study, we used the immunochromatography TB MPT64 antigen assay to differentiate NTM from M. tuberculosis16. M. abscessus was the predominant organism isolated from patients and hospital environments in our investigation. This could be due to improper sterilisation methods such as improper cleaning, cleaning without mechanical scrubbing, shorter holding time of disinfection of laparoscopic instruments in between surgeries, continuous use of the same disinfectant tray, use of tap water, and not following strict aseptic techniques. In our study, the disinfection tray was the main source of infection, where we found a biofilm-producing M. abcessus strain. Biofilm-producing isolates might be the source of continuous outbreaks, as they are resistant to standard disinfectants or sterilisation17. Other than this, patients can acquire the pathogens through medical or health-related procedures, whereby tap water or other contaminated products are directly inoculated into the skin. Contaminated tattoo ink, a surgical procedure where tap water or other contaminated solutions inadvertently enter the surgical field, or injections where injectable materials are contaminated18. Therefore, strict sterilization procedures like the introduction of gas plasma in surgical OT for non-autoclavable instruments, proper mechanical scrubbing of instruments to remove biofilms, avoidance of tap water, and overall stringent infection control measures in OT can prevent the risk of iatrogenic surgical site infections (SSI) by rapid grower mycobacteria. In our study, all isolates of RGM were sensitive to amikacin, tobramycin, doxycycline, and 80 per cent were sensitive to minocycline. These antibiotics were, therefore, considered for the treatment of M. abscessus infection. Clarithromycin is an important agent for the treatment of pulmonary and cutaneous infections caused by M. abscessus, M. chelonae, and M. fortuitum3. Several studies demonstrated that Clarithromycin was found to be active against nearly all RGM isolates19-21. But our study showed resistance in 83.33 per cent of cases. In our hospital, we used to prescribe a combined drug regimen of clarithromycin and ciprofloxacin for RGM infections before antibiotic sensitivity tests as empiric treatment. However, from this investigation, it may be concluded that empirical overuse of clarithromycin could be the cause of resistance of RGM infections22,23 to this antibiotic. In the present study, RGM appeared to be resistant organisms against meropenem, imipenem, and cephalosporin groups. Based on the antibiotic susceptibility test, we have formulated an antibiotic policy for RGM infections in our institute that is combination therapy with injection amikacin with oral moxifloxacin or combination therapy with oral moxifloxacin and doxycycline and local instillation of injection amikacin24.
Overall, rapid-grower mycobacteria is an emerging hospital-acquired infection, often resistant to existing empiric therapy. Clinicians should be aware of NTM when diagnosing surgical site infections. It is mandatory to do an antibiotic sensitivity test before initiation of treatment, and strict infection control measures must be followed to prevent iatrogenic NTM infection. Our study warrants the need for patient management and the development of prevention strategies against RGM. It may provide useful information on antibiotics that are effective against RGM and may help to identify suitable therapy for patients infected with such organisms.
Acknowledgment
Authors acknowledge the department of General Surgery, Institution of Postgraduate Medical Education and Research, Kolkata, the Infection Control Committee for active cooperation, and the Saha Institute of Nuclear Physics, Kolkata, for the atomic force microscopy facility.
Financial support &sponsorship
This work was supported by the NTEP, Govt. of India (RNTCP/35/2017/890/322045).
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript, and no images were manipulated using AI.
References
- Port site infection in laparoscopic surgery: A review of its management. World J Clin Cases. 2015;3:864-71.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Analysis of laparoscopic port site complications: A descriptive study. J Minim Access Surg. 2013;9:59-64.
- [CrossRef] [PubMed] [Google Scholar]
- Post surgical non-tuberculous mycobacterium: A case series. Cureus. 2022;14:e24701.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- An official ATS/IDSA statement: a diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment strategy for skin and soft tissue infections caused by nontuberculous mycobacteria following various procedures. Arch Aesthetic Plast Surg. 2021;27:3-11.
- [CrossRef] [Google Scholar]
- Laboratory aspects of clinically significant rapidly growing mycobacteria. Indian J Med Microbiol. 2011;29:343-52.
- [CrossRef] [PubMed] [Google Scholar]
- M24. Susceptibility testing of mycobacteria, Nocardia spp., and other aerobic actinomycetes. (3rd ed). Available from: https://clsi.org/standards/products/microbiology/documents/m24/, accessed on January 18, 2024.
- An overview of pulmonary infections due to rapidly growing mycobacteria in South Asia and impressions from a subtropical region. Int J Mycobacteriol. 2020;9:62-70.
- [CrossRef] [PubMed] [Google Scholar]
- Atypical mycobacterial infection, a diagnostic and therapeutic challenge-Case report and review of literature. J NTR Univ Health Sci. 2019;8:138-40.
- [CrossRef] [Google Scholar]
- Prevalence of non-tuberculous mycobacterial infection in surgical site infections and their antibiotic susceptibility profile. Med J Armed Forces India. 2021;77:343.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Epidemic of post-surgical infections caused by Mycobacterium massiliense. J Clin Microbiol. 2009;47:2149-55.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Large-scale outbreak of infection with Mycobacterium chelonae subsp. abscessus after penicillin injection. J Clin Microbiol. 2002;40:2626-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Hospital outbreak of post caesarean wound infection with atypical mycobacteria. J Evol Med Dent Sci. 2016;5:3416-9.
- [CrossRef] [Google Scholar]
- Surgical site infections by atypical mycobacteria: prevalence and species characterization using MALDI-TOF and molecular LCD chip array. Infection. 2022;50:1557-63.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evaluation of an immunochromatographic test for discrimination between Mycobacterium tuberculosis complex and non tuberculous mycobacteria in clinical isolates from extra-pulmonary tuberculosis. Indian J Med Res. 2012;135:901-6.
- [PubMed] [PubMed Central] [Google Scholar]
- Chronic urinary tract infection by biofilm-producing Mycobacterium abscessus following a posttraumatic laparotomy wound infection. Ann Med Sci Res. 2022;1:150.
- [CrossRef] [Google Scholar]
- Disinfection and sterilization in health care facilities: what clinicians need to know. J Clin Infect Dis. 2004;39:702-9.
- [CrossRef] [Google Scholar]
- Laboratory diagnosis and antimicrobial susceptibility testing of nontuberculous mycobacteria. In: Griffith DE, ed. Nontuberculous Mycobacterial Disease. Respiratory Medicine. Switzerland: Humana Press, Cham; 2018. p. :15-59.
- [Google Scholar]
- Antibiotic susceptibility pattern of rapidly growing mycobacteria. J Postgrad Med. 2010;56:76-8.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical site infections due to rapidly growing mycobacteria in Puducherry. India J ClinDiagn Res. 2015;9:5-8.
- [CrossRef] [Google Scholar]
- Antimicrobial susceptibility testing, drug resistance mechanisms, and therapy of infections with nontuberculous mycobacteria. Clin Microbiol Rev. 2012;25:545-82.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evaluation of antimicrobial susceptibilities of rapidly growing mycobacteria. Mikrobiyol Bul. 2023;57:220-37.
- [CrossRef] [PubMed] [Google Scholar]
- Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am J Respir Crit Care Med. 2018;198:1559-69.
- [CrossRef] [PubMed] [Google Scholar]






