Translate this page into:
Interferon-γ-inducible protein-10 in chronic hepatitis C: Correlations with insulin resistance, histological features & sustained virological response
Reprint requests: Dr. Mircea Grigorescu, Regional Institute of Gastroenterology & Hepatology “Prof. Dr. Octavian Fodor”, 19-21 Croitorilor Street, Cluj-Napoca 400 162, Romania e-mail: mgrigorescu@umfcluj.ro
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
One of the multiple factors contributing to virological response in chronic hepatitis C (CHC) is interferon-gamma-inducible protein-10 (IP-10). Its level reflects the status of interferon-stimulated genes, which in turn is associated with virological response to antiviral therapy. The aim of this study was to evaluate the role of serum IP-10 levels on sustained virological response (SVR) and the association of this parameter with insulin resistance (IR) and liver histology.
Methods:
Two hundred and three consecutive biopsy proven CHC patients were included in the study. Serum levels of IP-10 were determined using ELISA method. IR was evaluated by homeostasis model assessment-IR (HOMA-IR). Histological features were assessed invasively by liver biopsy and noninvasively using FibroTest, ActiTest and SteatoTest. Predictive factors for SVR and their interrelations were assessed.
Results:
A cut-off value for IP-10 of 392 pg/ml was obtained to discriminate between responders and non-responders. SVR was obtained in 107 patients (52.70%). Area under the receiver operating characteristic curve for SVR was 0.875 with a sensitivity of 91.6 per cent, specificity 74.7 per cent, positive predictive value 80.3 per cent and negative predictive value 88.7 per cent. Higher values of IP-10 were associated with increasing stages of fibrosis (P<0.01) and higher grades of inflammation (P=0.02, P=0.07) assessed morphologically and noninvasively through FibroTest and ActiTest. Significant steatosis and IR were also associated with increased levels of IP-10 (P=0.01 and P=0.02). In multivariate analysis, IP-10 levels and fibrosis stages were independently associated with SVR.
Interpretation & conclusions:
Our findings showed that the assessment of serum IP-10 level could be a predictive factor for SVR and it was associated with fibrosis, necroinflammatory activity, significant steatosis and IR in patients with chronic HCV infection.
Keywords
Fibrosis
hepatitis C
insulin resistance
IFN-γ-inducible protein-10
steatosis
sustained virological response
Hepatitis C virus (HCV) infection leads to increased morbidity and mortality. It was a difficult to treat infection till the discovery of new direct antivirals which increased the rate of response to 80-90 per cent1. Classical standard of care with pegylated interferon-alpha and ribavirin yielded a sustained virological response (SVR) in 42-52 per cent of patients chronically infected with HCV of genotype 123. It has been shown that factors that are independently associated with a favourable outcome after accomplishing the antiviral treatment are virus and host dependent. The infection with genotype 1, high viral load, increased body mass index (BMI), advanced age, presence of severe fibrosis and viral kinetics (lack of rapid virologic response) are unfavourable for achieving a SVR4567.
Studies have indicated another independent predictor of therapeutic outcome, namely interferon-gamma (IFN-γ)-inducible protein-10 (IP-10), a chemotactic CXC chemokine of 10 kDa, with 77 amino acids. Unlike other CXC chemokines, IP-10 lacks chemotactic activity for neutrophils rather it appears to target T lymphocytes, natural killer (NK) cells and monocytes8910 through its receptor, CXCR311. IP-10 can be produced by a variety of cells including hepatocytes12. In HCV infection, IP-10 mRNA expression in the liver has been reported to be associated with the presence of lobular necroinflammatory activity in liver biopsy samples12. It has been suggested that IP-10 may be involved in the immune response to chronic hepatitis13. Baseline levels of IP-10 before antiviral treatment were reported to be higher in patients who did not achieve an SVR after the end of treatment1314, especially in HCV genotype 1 infection. Though the association of IP-10 with SVR has already been studied, the correlation with liver histology is partially controversial, particularly when it comes to the degree of steatosis14. Moreover, the IP-10 association with insulin resistance (IR) in chronic hepatitis C (CHC) has not been thoroughly studied, nor the association with metabolic syndrome (MS).
The aim of the present study was therefore, to investigate plasma IP-10 levels in patients with chronic HCV infection before antiviral treatment in relation with SVR and to report a cut-off value predictive for SVR in the population. Another objective was to evaluate the association of IP-10 with liver histology and also to check for some possible associations with IR and MS.
Material & Methods
This study prospectively included 203 consecutive patients who were previously diagnosed with CHC and underwent liver biopsy at 3rd Medical Clinic, Regional Institute of Gastroenterology and Hepatology, Cluj-Napoca, Romania, between January 2011 and August 2014. Aiming a power of the study of 80 per cent and a significance level of 0.05, the minimum sample size was estimated to be 199 patients.
CHC infection was defined as the presence of anti-HCV for at least six months plus the positivity for HCV-RNA. Patients with other aetiologies of chronic liver disease, such as hepatitis B, autoimmune liver disease, Wilson's disease, haemochromatosis, α1-antitripsin deficiency, HIV infection and those with a history of hepatotoxic or steatosis-inducing drug use or alcohol consumption (more than 20 g/day for women and 30 g/day for men) were excluded. All patients were naive for antiviral treatment. The study was approved by the local Ethical Committee of the Regional Institute of Gastroenterology and Hepatology ‘Prof. Dr. Octavian Fodor’ in Cluj-Napoca, and written informed consents were obtained from all patients.
Anthropometric data: To assess the metabolic features, height, weight and waist circumference (WC) were determined, and based on height and weight, BMI was calculated (kg/m2). Evaluation of the MS was performed according to the Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention 200915. It implies the presence of any three of the following five features: elevated WC ≥94 cm for men and ≥80 cm for women and elevated triglyceride (TG) levels ≥150 mg/dl; reduced high-density lipoprotein (HDL)-cholesterol <40 mg/dl in males and <50 mg/dl in females; raised blood pressure: systolic ≥130 or diastolic ≥85 mmHg; raised fasting plasma glucose ≥100 mg/dl or previously diagnosed type 2 diabetes.
Laboratory investigations: All patients underwent haematological, biochemical and virological examinations on the day of their participation. To exclude biliary obstruction and the presence of liver focal lesions, abdominal ultrasonography was performed for all patients on inclusion into the study. A blood sample (3 ml) was obtained after eight hours of overnight fasting for routine investigations [aspartate transaminase (AST), alanine transaminase (ALT), platelet count, gamma-glutamyl transpeptidase (GGT), bilirubin, urea, prothrombin time, cholesterol, fasting plasma glucose, HDL-cholesterol, and TGs]. All assessments were made on an automatic analyzer (Konelab 30 I - Thermo Electron Corp, Finland). Fasting insulin and C-peptide levels were measured by ELISA (Mercodia AB, Sweden). The degree of IR was calculated according to the homeostasis model assessment for IR (HOMA-IR) by the formula: fasting insulin level (mUI/l)×fasting glucose level (mg/dl)/40516. A HOMA-IR index value of more than 2.7 was considered as the criterion of IR and that higher than 4.0 as a pre-diabetic state17.
Serum HCV-RNA was measured by PCR (Cobas Amplicor HCV 2.0 version, Roche, Germany). Serum samples from each patient were stored at -70°C for further biochemical analysis. All biochemical tests and their scores were assessed without knowledge of the liver biopsy results. The serum biochemical markers: α2-macroglobulin, haptoglobin and apolipoprotein A1 were assessed by nephelometry. The following blood tests were also evaluated: FibroTest18, ActiTest19 and SteatoTest20 (BioPreditive, France).
Interferon-gamma-inducible protein-10 (IP-10) quantification: Quantification of human IP-10 was performed using Quantikine (R&D Systems, UK), a solid-phase ELISA, on plasma samples obtained during the week before the start of therapy. All samples were stored at -70°C until assayed.
Antiviral treatment: Patients included in the study received standard antiviral treatment (standard of care, SOC): Peg-interferon (Peg-IFN) alpha 2a or 2b and ribavirin, 48 wk. SVR was defined as serum HCV-RNA undetectable 24 wk after the end of treatment. Patients were classified as having a relapse if the serum viral load was undetectable at the end of treatment but detectable 24 wk after the completion of treatment. Patients were classified as non-responders (NR) if HCV-RNA was detectable in serum at the end of treatment and 24 wk after the completion of treatment and response relapsers (RR) if the viral load was undetectable at the end of treatment, but positive at 24 wk of follow up. Non-responders (NR) and RR were included in the same group.
Pathological study: Liver biopsy obtained under ultrasonographic guidance and stained with haematoxylin–eosin and Masson's trichrome were blindly assessed according to the METAVIR scoring system21. According to the METAVIR system, fibrosis was staged on a scale from F0 to F4, as follows: F0: no fibrosis; F1: portal fibrosis, without septa; F2: few septa; F3: many septa without cirrhosis and F4: cirrhosis. The patients were divided depending on the presence or absence of advanced fibrosis in two groups: F0-F2 stages were considered as non-severe fibrosis and F3-F4 as severe fibrosis. Liver biopsies were obtained from all patients within 12 months before inclusion in the study. Only biopsies with a length exceeding 1.2 cm and containing more than 6 portal tracts were considered to be eligible for the study.
Statistical analysis: Comparison between groups was performed using Student's t test for continuous variables with normal distribution and Chi-square test for categorical variables. The continuous variables with non-normal distribution were expressed as median and 25th-75th percentiles and the differences were analyzed with Mann–Whitney test. For the comparison of more than two values of different variables depending on the stage of fibrosis, ANOVA was used. Performance of IP-10, significant fibrosis and HOMA-IR in predicting the rate of SVR was determined as the area under the receiver operating characteristic curve (AUROC). The cut-off values were chosen so that the sum of sensitivity and specificity was maximum. The variables that were found to be significantly associated in univariate analysis with SVR were included in a multivariate analysis, using logistic regression. For the statistical analysis the MedCalc® 13.3.9.0. software and SPSS software version 15.0 (SPSS Inc. Chicago, IL, USA) were used.
Results
A total of 203 patients with CHC were included in the study (mean age of 53.5±9.9 yr, of which 38.42% were male). The baseline features of the study group are presented in Table I. The mean BMI was 27.12±8.77 kg/m2, 62.06 per cent of the enrolled patients were overweight. The assessment of liver function showed a slight increase in the level of ALT, AST and GGT, with normal level of platelets and cholesterol. The metabolic features were not significantly modified, with a normal level of fasting glucose and a median (IQR) level of HOMA-IR of 2.20 (1.66-3.28). The liver morphology revealed a predominance of non-severe fibrosis (F02 - 68.96%), in contrast with an increased occurrence of significant inflammation and steatosis (A01-33.00%, respectively, S01-43.34%). After accomplishing a 48 wk treatment with Peg-IFN and ribavirin, the SVR rate was 53.69 per cent. The median value of IP-10 in CHC study group was 309.00 (IQR 195.70-460.00).
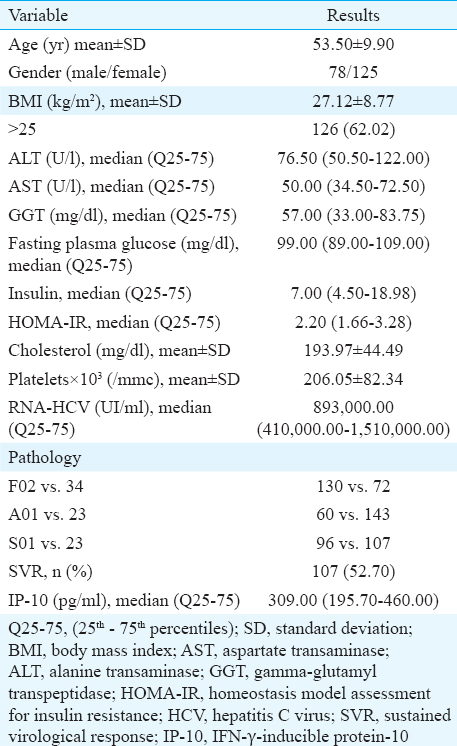
To assess the association of IP-10 with the liver morphology, the characteristics evaluated directly through liver biopsy and also the morphology indirectly and non-invasively assessed by FibroTest, ActiTest and SteatoTest, were used. Before testing the association of IP-10 with morphology, the association between non-invasive and invasive tests to reveal the liver modifications in CHC was assessed. A significant association was observed between fibrosis and steatosis (P<0.001) and also for necroinflammatory activity (P=0.006). When testing the association of IP-10 with morphology, a significant association was found between IP-10 and each stage of fibrosis or each grade of necroinflammatory activity evaluated through both invasive and non-invasive methods (for the association with fibrosis P<0.001, for the association with inflammation on biopsy sample, P=0.02, respectively, with ActiTest P=0.007 for the association with activity, Table II). No significant association was found when testing the relation of IP-10 with each grade of steatosis, but there was a significant association between IP-10 and steatosis (P=0.01) (Table II).
One of the objectives of our study was to analyze the relation between IP-10 and metabolic features. The presence of IR, defined as HOMA-IR ≥2.7 was found to be associated with higher values of IP-10 (P=0.02). Another important observation was the association of IP-10 with severe steatosis (P=0.01). No significant association was found between IP-10 and the presence of MS, overweight or obesity.
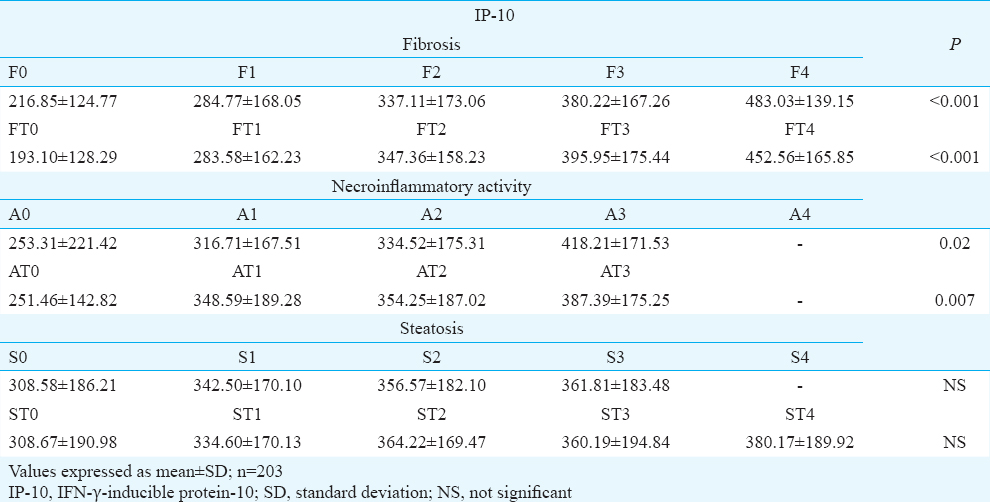
When assessing the factors that influence the presence of SVR, a significant association was found with IP-10. Of the histological features, significant fibrosis was seen to be associated with SVR, whereas of metabolic features, IR was significantly (P= 0.02) associated with virological response. The high level of cholesterol favoured the response to treatment (Table III). In multivariate analysis, only IP-10 and severe fibrosis were independently associated with SVR (P<0.001 and P=0.008, respectively) (Table IV).
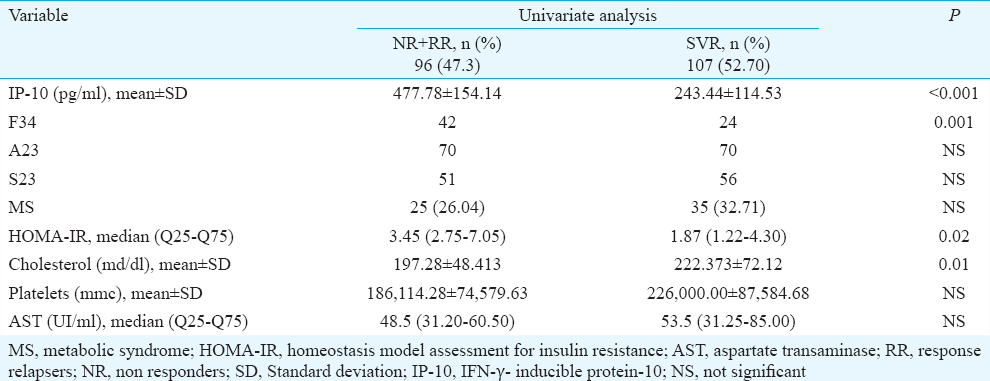
To assess the diagnostic accuracy of the factors influencing the virological response, we determined the AUROC, sensitivity and specificity of the factors associated with univariate analysis with SVR. IP-10 had the highest AUROC (0.875) with a sensitivity of 91.6 per cent, specificity of 74.7 per cent, positive predictive value=80.3 per cent and negative predictive value=88.7 per cent. The cut-off value of IP-10 for discriminating between responders and non- responders was 392 ng/ml. The next in line to predict SVR was severe fibrosis and HOMA-IR with the same diagnostic accuracy (AUROC=0.64) (Table V).

Discussion
It has been reported that pre-treatment IP-10 is a predictor factor of SVR following interferon and ribavirin therapy in HCV-infected patients14. This study confirmed the predictive value of pre-treatment IP-10 levels in patients chronically infected with HCV, and the relation of IP-10 with other baseline characteristics reported to be associated with SVR. A significant association was found between the high levels of IP-10 and increasing stages of fibrosis, increasing grades of necroinflammatory activity and with significant steatosis.
The association of IP-10 with SVR has already been confirmed in some earlier studies1422. Our study revealed an AUROC of 0.875 for predicting SVR for a cut-off of 392 pg/ml. Other studies used two cut-offs to increase the predictive value of IP-10 (<150 pg/ml and >600 pg/ml23. Diago et al14 reported a pre-treatment IP-10 concentrations <594.1 pg/ml as being predictive for SVR.
In our study, besides the level of IP-10, other factors found to be associated with SVR were severe fibrosis, HOMA-IR and cholesterol. In multivariate analysis, only IP-10 and severe fibrosis were independently associated with SVR. The results were concordant with data reported by other authors1423.
A significant association of higher values of IP-10 with increasing grades of inflammation and stages of fibrosis was observed and this was also seen using non-invasive methods for assessing necroinflammatory activity and fibrosis. These results were similar with other studies2224.
The association between IP-10 levels and necroinflammatory activity and fibrosis stage suggests that IP-10 plays a role in the natural pathogenesis of HCV-induced liver damage13. It has been demonstrated that HCV-infected hepatocytes produce IP-10. Thus, it is possible that after IP-10 production by infected hepatocytes, a large number of inflammatory cells are attracted to the hepatocyte. If the infection becomes chronic, the inflammatory response will result in increasing fibrotic changes25. Moreover, an association has been demonstrated between the expression of IP-10 mRNA in the liver and lobular necroinflammatory activity and fibrosis1226.
A significant association has been reported between high levels of IP-10 at baseline and steatosis in HCV genotypes (other than 3)22. The association of IP-10 with overweight patients has also been reported, but the underlying mechanism remains unclear at present and warrants further investigation22. It was shown that visceral adiposity was associated with higher IP-10 and hepatocyte growth factor (HGF), irrespective of BMI. In contrast, subcutaneous adiposity was not associated with IP-10 and HGF. Furthermore, the presence of MS was associated with IP-10 and HGF27.
In our study, a significant association was observed between IP-10 and significant steatosis, but not between IP-10 and BMI or MS. An important observation was that of significant association with IR. Tisato et al28 showed an association of IP-10 with MS in overweight patients, compared to overweight group without MS.
The link between MS, overweight, steatosis and hepatitis C is IR. Patients chronically infected with HCV are at an increased risk of IR and frank type 2 diabetes29. We found a significant association of IP-10 with IR as has been shown by Berenguer et al30 in their study on HCV/HIV coinfection and the variables associated with IP-10. Beside the relationship with HOMA-IR, HCV genotype, HIV viral load and FIB-4 (fibrosis-4 as an expression of fibrosis) were independently associated with IP-1030.
The limitation of the study was that the follow up of IP-10 trend during and after treatment was not done as it would have been useful for the complete understanding of the pathogenic mechanism and eventually for the need of following this marker as a long term prognosis factor.
In conclusion, the serum IP-10 concentration was associated with the severity of fibrosis and necroinflammation in patients with chronic HCV infection. Steatosis and IR were also significantly associated with IP-10 but not with MS. Pretreatment plasma IP-10 levels may be a reliable marker in predicting the outcome of antiviral treatment in patients infected with HCV, low baseline IP-10 levels being associated with SVR. The prognostic value of IP-10, in conjunction with other prognostic factors, should be used to define the group of patients difficult to treat, needing a longer treatment or new antivirals added or replacing the classical standard of care.
Acknowledgment
This study was conducted under the frame of European Social Fund, Human Resources Development Operational Programme 2007-2013, project no. POSDRU/159/1.5/S/138776.
Conflicts of Interest: None.
References
- Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-87.
- [Google Scholar]
- Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-82.
- [Google Scholar]
- Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: A randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-55.
- [Google Scholar]
- Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358:958-65.
- [Google Scholar]
- Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343:1666-72.
- [Google Scholar]
- Predicting the therapeutic response in patients with chronic hepatitis C: The role of viral kinetic studies. J Antimicrob Chemother. 2004;53:15-8.
- [Google Scholar]
- Early viral kinetics and treatment outcome in combination of high-dose interferon induction vs. pegylated interferon plus ribavirin for naive patients infected with hepatitis C virus of genotype 1b and high viral load. J Med Virol. 2005;75:27-34.
- [Google Scholar]
- The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): A novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 1997;8:207-19.
- [Google Scholar]
- Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155:3877-88.
- [Google Scholar]
- Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809-14.
- [Google Scholar]
- Binding and functional properties of recombinant and endogenous CXCR3 chemokine receptors. J Biol Chem. 1998;273:18288-91.
- [Google Scholar]
- Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol. 2003;74:360-9.
- [Google Scholar]
- Plasma chemokine levels correlate with the outcome of antiviral therapy in patients with hepatitis C. Blood. 2005;106:1175-82.
- [Google Scholar]
- Association of pretreatment serum interferon gamma inducible protein 10 levels with sustained virological response to peginterferon plus ribavirin therapy in genotype 1 infected patients with chronic hepatitis C. Gut. 2006;55:374-9.
- [Google Scholar]
- Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-5.
- [Google Scholar]
- Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-9.
- [Google Scholar]
- Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: A prospective study. Lancet. 2001;357:1069-75.
- [Google Scholar]
- Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol. 2004;3:8.
- [Google Scholar]
- The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol. 2005;4:10.
- [Google Scholar]
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20(1 Pt 1):15-20.
- [Google Scholar]
- Interferon (IFN)-gamma-inducible protein-10: Association with histological results, viral kinetics, and outcome during treatment with pegylated IFN-alpha 2a and ribavirin for chronic hepatitis C virus infection. J Infect Dis. 2006;194:895-903.
- [Google Scholar]
- IP-10 predicts viral response and therapeutic outcome in difficult-to-treat patients with HCV genotype 1 infection. Hepatology. 2006;44:1617-25.
- [Google Scholar]
- Serum IP-10 levels correlate with the severity of liver histopathology in patients infected with genotype-1 HCV. Gut Liver. 2011;5:506-12.
- [Google Scholar]
- Progression of fibrosis in untreated patients with hepatitis C virus infection. Liver. 2002;22:136-44.
- [Google Scholar]
- The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection. J Viral Hepat. 2007;14:675-87.
- [Google Scholar]
- Hepatocyte growth factor and interferon-γ inducible protein-10 are related to visceral adiposity. Eur J Clin Invest. 2013;43:369-78.
- [Google Scholar]
- Patients affected by metabolic syndrome show decreased levels of circulating platelet derived growth factor (PDGF)-BB. Clin Nutr. 2013;32:259-64.
- [Google Scholar]
- Metabolic syndrome, insulin resistance and adiponectin level in patients with chronic hepatitis C. J Gastrointestin Liver Dis. 2008;17:147-54.
- [Google Scholar]
- High plasma CXCL10 levels are associated with HCV-genotype 1, and higher insulin resistance, fibrosis, and HIV viral load in HIV/HCV coinfected patients. Cytokine. 2012;57:25-9.
- [Google Scholar]






