Translate this page into:
Inhibitory effect of polyunsaturated fatty acids on apoptosis induced by Streptococcus pneumoniae in alveolar macrophages
Reprint requests: Prof. Sanjay Chhibber, Department of Microbiology, Panjab University, Chandigarh 160 014, India e-mail: sanjaychhibber8@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Apoptosis is considered as a major defense mechanism of the body. Multiple pathogens induce macrophage apoptosis as a mode of immune evasion. In earlier studies, n-3 polyunsaturated fatty acids (PUFA) have been reported to be protective against neuronal apoptosis and neuronal degeneration, seen after spinal cord injury. In this study, we tried to evaluate the role of n-3 polyunsaturated fatty acids on the process of macrophage phagocytic activity and apoptosis in mice.
Methods:
Mice were divided into three groups (n=60); Group I was fed on sea cod oil; Group II on flaxseed oil supplementation for 9 wk along with standard laboratory chow diet. Group III was fed on standard diet and served as control. After supplementation, phagocytic and apoptotic (morphological staining: acridine orange plus ethidium bromide; H-33342 plus propidium iodide staining and DNA ladder formation) activities of mouse alveolar macrophages were assessed.
Results:
Alveolar macrophages (obtained from sea cod oil and flaxseed oil fed group mice) showed significant increase in bacterial uptake as well as intracellular killing (P< 0.05) of Streptococcus pneumoniae. Significant decrease (P<0.05) in apoptotic cells was observed among alveolar macrophages from sea cod and flaxseed oil fed mice whereas maximum apoptosis was observed in control alveolar macrophages on interaction with bacteria in vitro which was confirmed by DNA laddering.
Interpretation & conclusions:
These findings suggest that dietary supplementation with n-3 polyunsaturated fatty acids to mice led to enhanced phagocytic capability of their alveolar macrophages as well as provided protection against apoptosis upon challenge with S. pneumoniae.
Keywords
Apoptosis
flaxseed oil
phagocytosis
pneumonia
polyunsaturated fatty acids
sea cod oil
Streptococcus pneumoniae
Pneumococcus usually colonizes the nasopharynx of humans asymptomatically, although sometimes it moves to the lungs, brain, and blood. This can lead to diseases associated with high morbidity and mortality such as pneumonia, septicemia, and meningitis12. Pneumococci are capable of inducing apoptosis in respiratory tree epithelium, endothelium, neuronal cells and alveolar macrophages (AM)13. AM are the first line defenders in the lungs and play an essential role against infections because of their capability to phagocytose and kill the invading microorganisms. To induce and potentiate inflammatory immune processes, AM release the required cellular mediators, such as tumour necrosis factor-alpha (TNF-α) and eicosanoids4. The critical role of AM in lung defense against infection has been reported on the basis of high frequency of pneumonia in severely neutropenic patients and in cases of lung macrophage dysfunction4.
Several compounds in or derived from the diet modulate apoptosis in cell cultures in vitro. These observations have important implications concerning the mechanism whereby dietary components affect health. Proapoptotic compounds could protect against cancer by enhancing elimination of initiated, precancerous cells and antiapoptotic compounds could promote tumour formation by inhibiting apoptosis in genetically damaged cells5. Oxidative stress activates apoptosis, and antioxidants protect against apoptosis in vitro; thus, a central role of dietary antioxidants may be to protect against apoptosis5. However, little data are available to directly link diet with altered apoptosis as an underlying determinant of disease. Omega-3 polyunsaturated fatty acids are important nutritional elements for humans, and these have the potential to inhibit excessive inflammatory responses; hence are widely recommended against atherosclerosis, coronary heart diseases, arrhythmias and in allergic conditions like asthma67. In an attempt to understand the role of dietary n-3 polyunsaturated fatty acids (PUFA) on phagocytosis and apoptosis, we studied interaction of alveolar macrophages obtained from the mouse fed on n-3 PUFA with Streptococcus pneumoniae, in vitro.
Material & Methods
Bacterial strain: A standard strain of S. pneumoniae D39 serotype 2 (provided by Dr Dong Kwon Rhee, College of Pharmacy, Sungkyunkwan University, South Korea) was used in this study. Organism was maintained on blood agar plates. The strain was found to be virulent in mice as confirmed by intra-peritoneal inoculation8. Experimental pneumonia was developed in mice by intra- tracheal administration as described by Zeng et al9.
Test animals: Both male and female BALB/c mice, 6-8 wk old, healthy weighing 25 ± 5 g were procured from Central Animal House, Panjab University, Chandigarh, India. Animals in groups of eight were randomly housed in propylene cages and had free access to an antibiotic free diet (Hindustan Lever Limited, Mumbai) and water ad libitum. The experiments were performed in the Department of Microbiology, Panjab University, Chandigarh, after taking the permission from the Institutional Animal Ethics committee.
Feeding with n-3 polyunsaturated fatty acid (n-3 PUFA) to mice: Mice (n=60) were divided into three groups and consisted of 20 mice each. Group I was fed on standard laboratory chow diet by daily supplementation of 0.5 ml sea cod oil (Seven seas R Seacod TM, Universal Medicare, India) providing 900 mg per human body weight per day of n-3 PUFA. Group II was fed with daily supplementation of 0.5 ml flaxseed oil (Flax Oil, Nature's Bounty, USA) providing 2000 mg per human body weight per day of n-3 PUFA administered orally with a feeding catheter (Romsons Ltd., India) for 9 wk along with standard laboratory chow diet. Control mice (Group III) were fed on standard diet along with daily oral intake of 0.5 ml of normal saline.
Phagocytosis: Uptake and intracellular killing of S. pneumoniae D39 type 2 by mouse alveolar macrophages was studied as described earlier10.
Relative uptake value was expressed as percentage of viable bacteria taken up by the macrophages at respective sampling time interval. For intracellular killing, bacterial suspension (108 cfu/ml) was mixed with normal mouse serum and kept for 30 min at room temperature. Macrophages (106 cells/ml) were added to above bacterial suspension, incubated and centrifuged. The cells were lysed by the addition of normal saline containing 0.5 per cent sodium deoxycholate at time intervals of 1, 2 and 3 h. The colony forming units (cfu) were counted after overnight incubation at 37 °C.
Apoptosis studies: Alveolar macrophages were collected from the lungs of different groups of mice supplemented with dietary n-3 PUFA following the method of Morissette et al11. Briefly, Broncheoalveolar lavage (BAL) was performed using 1.5 ml of sterile phosphate buffer saline (PBS) containing 0.1 per cent (w/v) EDTA. The lavage cells were centrifuged, washed and suspended in RPMI-1640. The cell density of alveolar macrophages was adjusted after checking the viability with 0.2 per cent (w/v) trypan blue staining. A ratio of prokaryotic to eukaryotic cell 100:1 was achieved1213 by incubating macrophages (106 cells/ml) with 108 cfu/ml of bacteria for 3 h in humidified atmosphere containing 5 per cent CO2 at 37°C.
Acridine orange plus ethidium bromide staining: For morphological evaluation of alveolar macrophage apoptosis, staining of cells with acridine orange/ethidium bromide (Sigma, USA) was performed by the method of Singhal et al14. Briefly, at the end of the scheduled incubation, 2 μl of a combined dye of 100 μg/ml acridine orange and 100 μg/ml ethidium bromide were added to 20 μl of the cell suspension (106 cells/ml), and 5 μl of the stained cell suspension was rapidly transferred to a glass slide for immediate analysis using an ultraviolet fluorescence microscope (Olympus BH-2, Japan). Staining with acridine orange plus ethidium bromide combined with fluorescent microscopy was used to distinguish early apoptotic cells from necrotic cells. Ten random fields were counted and percentage of apoptotic cells was recorded by the observer unaware of experimental condition.
H-33342 plus propidium iodide staining: H-33342 stains the nuclei of live cells and identifies apoptotic cells by increased fluorescence, whereas propidium iodide costain the dead cells. Double staining by these two agents provides the percentage of live, apoptotic and necrosed cells under control and experimental conditions. For this the method of Kapasi et al15 was followed. Briefly, at the end of the scheduled incubation periods, aliquots of methanol containing H-33342 (final concentration, 1 μg/ml) were added and incubated for 10 min at 37°C. Subsequently, cells (without a wash) were placed on ice, and propidium iodide (final concentration, 1 μg/ml) was added to the cell suspension. Cells were incubated with the dyes for 10 min on ice, protected from light, and then examined under ultraviolet light. Percentage of apoptotic cells was recorded in ten random fields by the observer unaware of experimental conditions.
Assessment of apoptosis by DNA laddering: DNA isolation and gel electrophoresis are simple techniques to confirm the occurrence of apoptosis1617, as these provide morphologic evidence of DNA fragmentation. To confirm the occurrence of macrophage apoptosis when incubated with S. pneumoniae, cells were lysed in DNA lysis buffer [10mM Tris-HCl (pH-8,) 0.1M EDTA, 0.5% SDS and 20 μg/ml RNase]. DNA was extracted and run on a 1.6 per cent (w/v) agarose gel and electrophoresed.
Statistical analysis: Data were analyzed for significant differences by two way ANOVA. Individual group comparisons were made by two tailed Student's t test. Each experiment was done in triplicates.
Results & Discussion
Alveolar macrophages are critical to early defence in the lung and their ability to phagocytose bacteria represents an integral part of the host immune defense against S. pneumoniae18. Following phagocytosis, macrophages recruit activated inflammatory cells to the site of infection, as well as are involved in the processing and presentation of bacterial antigens, thereby linking innate and adaptive immunity19. In the present study, phagocytosis of S. pneumoniae with mouse alveolar macrophages obtained from animals supplemented with n-3 PUFA for 9 wk was studied. When compared with control, macrophages showed significant increase in uptake as well as intracellular killing (P<0.05) of S. pneumoniae (Fig. 1). Macrophages from sea cod oil fed group showed 30 per cent increase in phagocytic uptake which was slightly higher than the flaxseed oil group (27%). Similarly, increase in intracellular killing of S. pneumoniae by alveolar macrophages from sea cod oil as well as flaxseed oil fed groups was 36 and 30 per cent, respectively. Previous studies have observed that macrophages enriched with saturated fatty acids such as myristate or palmitate showed decrease of 28 and 21 per cent, respectively in their ability to phagocytose unopsonized zymosan particles. Those enriched with polyunsaturated fatty acids showed 25-55 per cent enhancement of phagocytic capacity20. In another study, short-term enteral feeding with an eicosapentaenoic acid-enriched or eicosapentaenoic with gamma-linolenic acid-enriched diet rapidly modulated the fatty acid composition of alveolar macrophage phospholipids, promoted a shift toward formation of less inflammatory eicosanoids by stimulated macrophages, but did not impair alveolar macrophage bactericidal function relative to responses observed after feeding a linoleic acid diet21.

- Uptake (%, solid line) and intracellular killing (%, dotted line) of macrophages obtained from control (square) unsupplemented mice and flaxseed oil (triangle), sea cod oil (circle) supplemented mice infected with S. pneumoniae.
Tissue macrophages, including AM of the lung, exhibit low levels of constitutive apoptosis22. Therefore, induction of apoptosis is a major mechanism by which macrophage function is regulated during infection. Morphologically, apoptosis is characterized by intact plasma membranes and cellular organelles, nuclear condensation, DNA fragmentation and formation of apoptotic bodies22. DNA fragmentation can be detected by TUNEL labeling of breaks at 3-OH ends in DNA strands. However, this technique lacks specificity for apoptosis since DNA strand breaks have also been seen in necrotic cell death. Ethidium bromide (EB) intercalates and stains DNA, providing a fluorescent red-orange stain. Although it will not stain healthy cells, it is used to identify cells that are in the final stages of apoptosis - such cells have much more permeable membranes. The stain may also be used in conjunction with acridine orange (AO) in viable cell counting. This EB/AO combined stain causes live cells to fluoresce green whilst apoptotic cells retain the distinctive red-orange fluorescence14. Hoechst 33342 (H-33342) binds preferentially to DNA rich in thymidine and adenine base pairs and is widely used as a marker for DNA condensation that accompanies apoptotic cell death in various cell types. The difference in H-33342 fluorescence emission between normal and apoptotic cells is attributed to a more rapid membrane uptake of H-33342 by cells undergoing apoptosis. This stain is used in conjunction with propidium iodide15. The potential of S. pneumoniae to induce apoptosis in alveolar macrophages of n-3 PUFA supplemented mice was evaluated. In order to quantify the apoptotic population, the nuclear morphology of cells was observed following acridine orange plus ethidium bromide staining and H-33342 plus propidium iodide staining. The macrophages in the control samples were moderate green (Fig. 2.1 A). The apoptotic cells were greenish yellow in colour whereas the necrotic cells were orange-red (Fig. 2.1 B). With H-33342 plus propidium iodide staining viable cells showed moderate blue fluorescence while apoptotic cells showed nuclei with bright pink (Fig. 2.1 D, E). Significant decrease (P<0.05) in apoptotic cells was observed among alveolar macrophages from sea cod and flaxseed oil fed mice (Fig. 2.1 C & F) whereas maximum apoptosis was observed in control alveolar macrophages (Fig. 2.2) on interaction with bacteria in vitro which was confirmed by DNA laddering (Fig. 2.3).
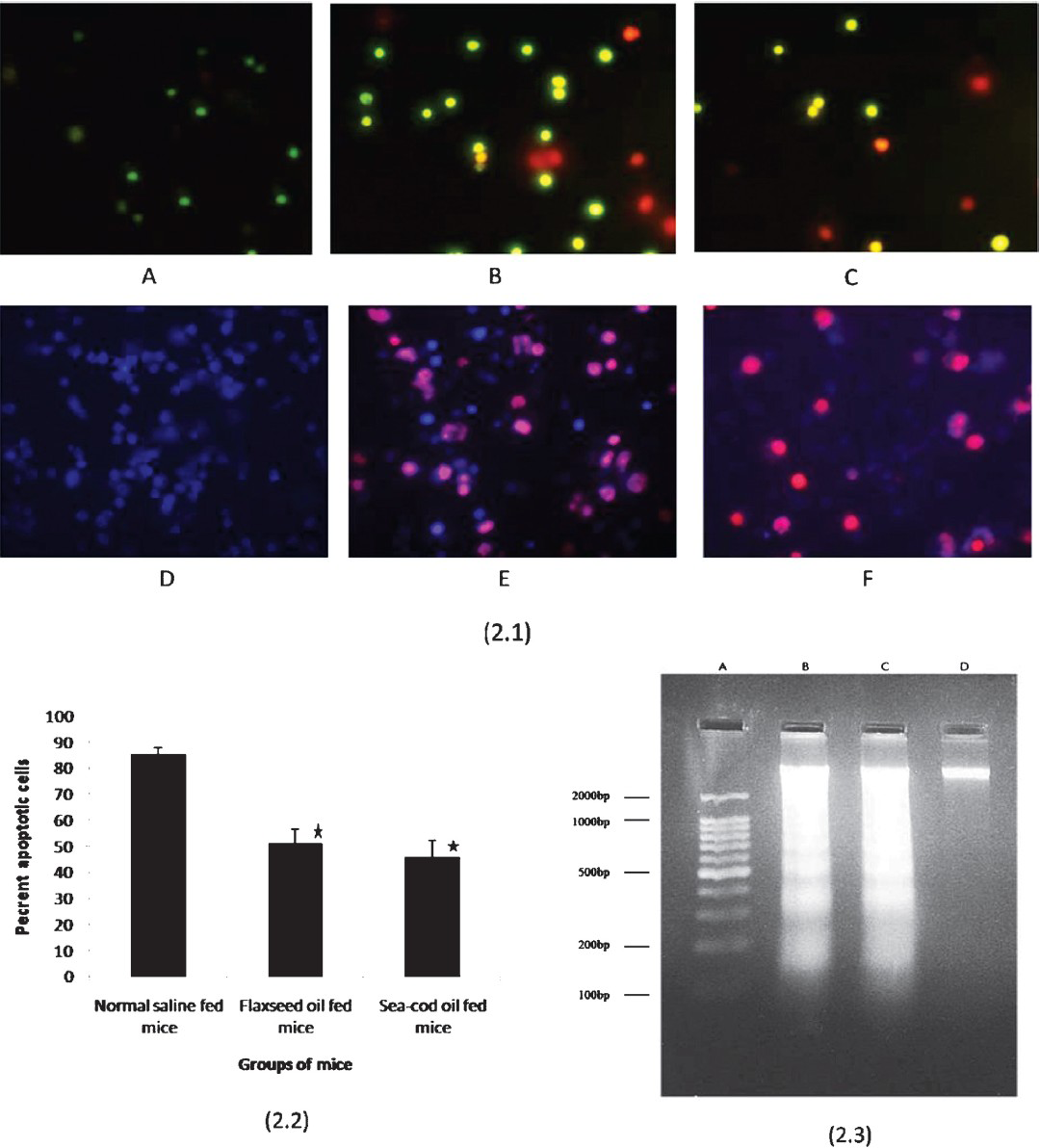
- Representative photographs showing presence of apoptotic cells (2.1) and per cent of apoptotic cells (2.2) among alveolar macrophage obtained from normal as well as n-3 PUFA fed mice on interaction with S. pneumoniae in vitro. Macrophages were stained with acridine orange plus ethidium bromide and Hoechst-33342 plus propidium iodide. (2.1 A, D: macrophages from normal mice (negative control); 2.1 B, E: macrophages from normal mice after interaction with S. pneumoniae (positive control); 2.1 C, F: macrophages from n-3 PUFA supplemented mice after interaction with S. pneumoniae). Live cells stain green or blue; necrotic cells stains orange-red or red; apoptotic cells stain greenish yellow or pink depending on the stain used. DNA ladder formation (2.3) in alveolar macrophages obtained from normal as well as n-3 PUFA fed mice upon exposure to whole cells of S. pneumoniae (Lane A: 100bp DNA ladder marker; Lane B: DNA of alveolar macrophages extracted from flaxseed oil supplemented mice; Lane C: DNA of alveolar macrophages extracted from sea cod oil supplemented mice showing characteristic ladder formation; Lane D: DNA of alveolar macrophages extracted from normal mice (negative control).
The present study demonstrated that dietary supplementation with n-3 PUFA not only enhanced phagocytic capability of alveolar macrophages but also decreased alveolar macrophage apoptosis by S. pneumoniae D39 type 2. In earlier studies, it has been reported that regulation of the inflammatory infiltrate is critical for the successful outcome of pneumonia and alveolar macrophage apoptosis is a feature of pneumococcal infection and this aids disease resolution223. Previous studies have also found that the degree of apoptosis in the lungs correlates with the severity of injury223. Thus, modulation of macrophage life span can be an important mechanism for the regulation of macrophage function. The incorporation of n-3 PUFA in immune cell membranes may influence the membrane fluidity, structure and function of several membrane receptors, transporters, enzymes and ionic channels2124. These alterations can in turn indirectly modulate macrophage function as has been reported in earlier studies related to central nervous system associated problems. Wu et al25 have suggested that dietary supplementation with n-3 PUFA may be helpful in spinal cord injury by inhibiting neuronal apoptosis and thus has a potential means to delay the onset of the disease and/or the rate of progression. PUFA can also block apoptosis2627 and several studies have documented their neuroprotective effects in vitro and in vivo1928. In neurodegenerative diseases of retina, docosahexaenoic acid (DHA), the major retinal polyunsaturated fatty acid, prevents photoreceptor apoptosis during early development in vitro, and upon oxidative stress2930.
In conclusion, the present observations are important as AM are the first line of defence in respiratory tract infections. These observations, therefore, form the basis for future experimentation to study the molecular basis of altered macrophage function on exposure to n-3 PUFA.
Acknowledgement
Authors acknowledge the ICMR (Indian Council of Medical Research), New Delhi, India for financial support and thank Dr Dong Kwon Rhee, Prof. of Microbiology from College of Pharmacy, Sungkyunkwan University, South Korea, for providing the bacterial strain.
References
- Pneumonia research to reduce childhood mortality in the developing world. J Clin Invest. 2008;118:1291-300.
- [Google Scholar]
- The role of pneumolysin in mediating lung damage in a lethal pneumococcal pneumonia murine model. Respir Res. 2007;8:3.
- [Google Scholar]
- Bacterial programmed cell death of cerebral endothelial cells involves dual death pathways. J Clin Invest. 2005;115:1607-15.
- [Google Scholar]
- Apoptosis contributes to the decrement in numbers of alveolar macrophages from rats with polymicrobial sepsis. J Microbiol Immunol Infect. 2002;35:71-7.
- [Google Scholar]
- Enrichment of RAW264.7 macrophages with essential 18-carbon fatty acids affects both respiratory burst and production of immune modulating cytokines. J Nutr Biochem. 2010;21:556-60.
- [Google Scholar]
- Importance of n-3 fatty acids in health and disease. Am J Clin Nutr. 2000;71(Suppl 1):171S-5S.
- [Google Scholar]
- Effect of n-3 polyunsaturated fatty acids in asthma after low-dose allergen challenge. Int Arch Allergy Immunol. 2009;148:321-9.
- [Google Scholar]
- Contribution of a response regulator to the virulence of Streptococcus pneumoniae is strain dependent. Infect Immun. 2003;71:4405-13.
- [Google Scholar]
- Interferon- inducible protein 10, but not monokine induced by gamma interferon, promotes protective type 1 immunity in murine Klebsiella pneumoniae pneumonia. Infect Immun. 2005;73:8226-36.
- [Google Scholar]
- Protective efficacy of Emblica officinalis against Klebsiella pneumoniae induced pneumonia in mice. Indian J Med Res. 2008;128:188-93.
- [Google Scholar]
- Lung phagocyte bactericidal function in strains of mice resistant and susceptible to Pseudomonas aeruginosa. Infect Immun. 1996;64:4984-92.
- [Google Scholar]
- Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect Immun. 1993;61:4064-71.
- [Google Scholar]
- Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833-8.
- [Google Scholar]
- Ethanol-induced macrophage apoptosis: the role of TGF-β. J Immunol. 1999;162:3031-6.
- [Google Scholar]
- HIV-1 gp 120 induced tubular epithelial cell apoptosis is mediated through p38-MAPK phosphorylation. Mol Med. 2002;8:676-85.
- [Google Scholar]
- HIV-1 gp 160 envelope protein modulates proliferation and apoptosis in mesangial cells. Nephron. 1997;76:284-95.
- [Google Scholar]
- Phagocytosis and killing of common bacterial pathogens of the lung by human alveolar macrophages. J Infect Dis. 1985;152:4-13.
- [Google Scholar]
- Macrophages and polymorphonuclear neutrophils in lung defense and injury state of the art. Am Rev Respir Dis. 1990;141:471-501.
- [Google Scholar]
- n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(Suppl 6):1505S-19S.
- [Google Scholar]
- Effect of short- term enteral feeding with eicosapentaenoic and gamma-linolenic acids on alveolar macrophage eicosanoid synthesis and bactericidal function in rats. Crit Care Med. 1999;27:1908-15.
- [Google Scholar]
- Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-κB, Bad, or caspase activation. J Exp Med. 2001;194:113-26.
- [Google Scholar]
- Differential patterns of apoptosis in resolving and nonresolving bacterial pneumonia. Am J Resp Crit Care Med. 2000;161:2043-50.
- [Google Scholar]
- Neuroprotective effect of docosahexaenoic acid on glutamate-induced cytotoxicity in rat hippocampal cultures. Neuroreport. 2003;14:2457-61.
- [Google Scholar]
- Inhibitory effect of polyunsaturated fatty acids on apoptosis induced by etoposide, okadaic acid and AraC in Neuro2a cells. Acta Med Okayama. 2007;61:147-52.
- [Google Scholar]
- Inhibition of neuronal apoptosis by polyunsaturated fatty acids. J Mol Neurosci. 2001;16:223-7. discussion 279-84
- [Google Scholar]
- Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J Neurosci. 2006;26:4672-80.
- [Google Scholar]
- Docosahexaenoic acid prevents apoptosis of retina photoreceptors by activating the ERK/MAPK pathway. J Neurochem. 2006;98:1507-20.
- [Google Scholar]
- Protective effect of docosahexaenoic acid on oxidative stress-induced apoptosis of retina photoreceptors. Invest Ophthalmol Vis Sci. 2003;44:2252-9.
- [Google Scholar]






