Translate this page into:
Inhibitors of Gq signalling down-regulate β-catenin expression & function in human colon cancer cells
For correspondence: Dr S. Mahmoud A. Najafi, Department of Cell & Molecular Biology, School of Biology, College of Sciences, University of Tehran, P.O. Box 14155-6455, Tehran, Iran e-mail: smarabnajafi@ut.ac.ir
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
β-catenin signalling plays a key role in maintaining normal cellular physiology, and therefore, its deregulation can lead to many human diseases including cancers. Previously, we have shown that the activation of Gq signalling positively regulates β-catenin by inhibiting glycogen synthase kinase-3 beta and increasing the stability of β-catenin protein, however, these results were mainly based on overexpression experiments in either Xenopus oocytes or HEK293T cells. The present study was undertaken to evaluate the modulation of Gq signalling in human colon cancer cells.
Methods:
Gq signalling in SW480 and HT-29 colon cancer cells was specifically blocked to investigate the interaction between β-catenin and the Gq signalling pathways. GP antagonist-2A (a commercially available peptide) and a minigene expression construct encoding a peptide corresponding to the C-terminal 11 amino acids of Gαq were used to block Gq signalling. β-catenin expression and function were examined by western blotting, immunofluorescence microscopy, and quantitative real-time PCR experiments.
Results:
Transfection of cells with either of the blockers significantly decreased both β-catenin protein levels and β-catenin-mediated transcriptional activities. In addition, the migration of SW480 cells was reduced in the presence of the Gq blockers.
Interpretation & conclusions:
The results of this study further support the positive role of Gq signalling in regulating β-catenin expression and function and may provide a new means of preventing β-catenin-mediated carcinogenesis by blocking heterotrimeric G proteins.
Keywords
β-catenin
colon cancer
G proteins
Gq
signal transduction
Colon cancer is among the most common human malignancies and is often fatal1. Many colon cancer patients carry genetic mutations in the APC (Adenomatous polyposis coli) gene, leading to complete or partial loss of the APC protein function2. It is believed that these APC mutations lead to up-regulation of the Wnt/β-catenin pathway and therefore cause cellular and nuclear accumulation of β-catenin3. β-catenin is the product of the proto-oncogene, CTNNB1 (catenin beta 1), with at least two important biological functions4. On the one hand, β-catenin is a component of the E-cadherin-mediated cell adherens junctions in the epithelial cells, and on the other hand, as a signalling molecule, it interacts with the Tcf/Lef family members of transcription factors and regulates transcription of several important cellular genes. Therefore, there are at least two cellular pools of β-catenin protein, membrane and cytoplasmic4. The cytoplasmic β-catenin has been considered to have a more important role in human carcinogenesis because it appears to be a ready source of this protein for nuclear translocation and transcriptional activities56. However, it has been shown that dissociation of β-catenin from the cell membrane can also be used for nuclear translocation of this protein7.
Given the involvement of Wnt/β-catenin signalling in the initiation and progression of colorectal cancer and some other human malignancies, further investigations of the regulation of this signalling pathway may help us find new and safer approaches for the prevention and/or treatment of these human cancers. For years, various groups including ours have been studying the interaction between heterotrimeric G proteins and Wnt signalling891011. The original idea behind these investigations was based on the topological similarities between G protein coupled receptors (GPCRs) and frizzled receptors (both families of receptors have seven hydrophobic transmembrane domains)12. We have previously shown, using Xenopus oocytes and mammalian HEK-293 cells, that the Gq class of G proteins positively regulates β-catenin signalling by reducing glycogen synthase kinase-3 beta (GSK-3β) activity and increasing intracellular protein levels of β-catenin1011. In the present study, HT-29 and SW480 colon cancer lines were used to further investigate the interaction between the Gq and the β-catenin-mediated signalling pathways, with the help of two specific inhibitors of Gq signalling, a commercially available peptide, GP antagonist-2A13, and a minigene plasmid construct encoding a short peptide corresponding to the carboxy-terminal eleven amino acids of Gαq111415. These colon cancer cell lines carry genetic mutations in the APC gene and therefore have higher cellular protein levels of β-catenin16 than normal colon cells. This feature makes these cell lines ideal cell systems to study the regulators of the Wnt/β-catenin pathway.
Material & Methods
Cell culture and transfection: SW480 and HT-29 cell lines were procured from Iranian Biological Resource Center (Tehran, Iran). The cells were grown in RPMI 1640 (Gibco, Thermo Fisher Scientific, USA) supplemented with 10 per cent foetal bovine serum, streptomycin (100 µg/ml) and penicillin (100 U/ml). Cells were grown under five per cent CO2 at 37°C. For cell transfection, 1×105 SW480 or HT-29 cells were seeded in each well of a 12 well plate and grown to about 70 per cent confluency. The medium was then replaced by serum-free medium, and one hour later, the cells were transfected with the indicated amounts of the Gαq minigene plasmid or GP antagonist-2A (Calbiochem, Germany). Lipofectamine 2000 (Invitrogen, MA, USA) was used for cell transfection based on a method described by the supplier.
Indirect immunofluorescence staining: SW480 and HT-29 cells were grown in 12 well plates and transfected as described above. The cells were washed in phosphate-buffered saline (PBS) and fixed in chilled methanol (−20°C) for 15 min. The cells were washed again and permeabilized in 0.2 per cent Triton X-100 in PBS for three minutes. The cells were washed after fixation (with PBS) and then incubated in three per cent BSA (w/v in PBS) for 30 min at RT to block non-specific binding. The primary antibody was diluted (1:500) in blocking buffer, and the cells were incubated in this solution for one hour at RT. The cells were again washed three times (10 min each) in PBS and then incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (diluted 1:250 in blocking buffer) for one hour at RT. The cells were finally washed three times and visualized with a fluorescence microscope.
Real-time PCR experiments: Total RNA was isolated by using RNX-Plus Solution (Sinaclon, Tehran, Iran) and a method described by the supplier. Further, complementary DNA (cDNA) synthesis was carried out using HyperScript™ RT Master Mix (GeneAll, Seoul, South Korea) according to the manufacturer’s instructions.
Real-time PCR (qPCR) analysis was performed to measure the mRNA levels of the β-catenin/Tcf-target genes. A real-time PCR Master Mix was used for these experiments (RealQ Plus 2x Master Mix Green without ROX™, Ampliqon, Odense M, Denmark). Briefly, 1 µl of template cDNA, 5 µl of the master mix and 0.5 µl of each forward and reverse primer were mixed in a total volume of 10 µl. The amplification protocol involved denaturation at 95°C for five minutes, annealing at 60°C for 30 sec and extension at 72°C for 30 sec. A Mic (magnetic induction cycler) real time-PCR machine was used for these experiments. Relative expression of each gene was normalized to ribosomal protein lateral stalk subunit P0 (RPLP0) expression. Expression levels were evaluated according to the ∆∆CT method17, and all reactions were performed in duplicate, with non-template controls based on the instructions provided by the RealQ Plus 2x Master Mix manufacturer (Odense M, Denmark).
For measuring mRNA levels of the luciferase reporter gene, SW480 or HT-29 cells grown in 12-well plates were transfected with 500 ng/well of the TOPFlash plasmid18 with or without 500 ng/well of the Gαq minigene plasmid or GP antagonist-2A (50 µM). Luciferase gene transcription was measured by real-time PCR as described above. The sequence of the primers used for real-time PCR experiments are listed in Supplementary Table.
| Genes | Forward | Reverse |
|---|---|---|
| RPLP0 | 5'-'TGGTCATCCAGCAGGTGTTCGA- 3' | 5'-ACAGACACTGGCAACATTGCGG-3' |
| C-MYC | 5'-GGCTCCTGGCAAAAGGTCA- 3' | 5'-AGTTGTGCTGATGTGTGGAGA-3' |
| CCND1 | 5'-GAGGGTTGTGCTACAGATGA- 3' | 5'-CGCCTCCTTTGTGTTAATGC- 3' |
| FGF20 | 5'-GGACCACAGCCTCTTCGGTA- 3' | 5' -TGTCCACACCTCTAATACTGACCAG-3' |
| LUC | 5'-CTCATAGAACTGCCTGCGTG- 3' | 5'-GGCGAAGAAGGAGAATAGGG- 3' |
RPLP0, ribosomal protein lateral stalk subunit P0; LUC, luciferase
Cell fractionation and western blotting: Cytoplasmic proteins were isolated from HT-29 and SW480 cells by lysing the cells from each well of a six-well plate into 100 µl of an ice cold buffer (50 mM Tris-Cl pH=7.8, 100 mM NaCl, 2 mM EDTA and 0.1 v/v of 1X protease inhibitor cocktail (Sigma-Aldrich, USA)) by passing the cells through a 27-gauge needle 25 times. The cell suspension was centrifuged at a maximum speed in a cold microfuge for 15 min and the supernatant was saved as cytoplasmic protein.
The pellet containing membrane proteins and cellular debris was dissolved in 120 µl of a lysis buffer containing 60 µl of buffer A (50 mM Tris-HCl pH=7.8, 200 mM NaCl, 2 mM EDTA, 0.05% SDS and 0.1 v/v of 1X protease inhibitor cocktail) and 60 µl buffer B (100 µM Tris-HCl, 200 mM beta mercaptoethanol, 4% SDS, 20% glycerol and 0.1 v/v of 1X protease inhibitor cocktail). The mixture was heated for four minutes at 95°C, vortexed well and heated again for three minutes at 95°C. The suspension was then centrifuged at 14,000 rpm for 30 min at RT. The supernatant containing the membrane proteins was collected and transferred to a new tube and stored at −70°C until use.
Protein concentration was measured as described previously11, and 20 µg of proteins was separated by SDS-PAGE (8%). After electrical transfer of proteins to a nitrocellulose membrane, the gel was stained for 12 h with Coomassie blue to verify the efficiency of transfer. The membrane was washed for 15 min in TBS (25 mM Tris-HCl pH=7.5, 137 mM NaCl, 2.7 mM KCl) and incubated in five per cent fat-free milk (in TBS) for one hour at RT to block non-specific binding. The blot was incubated with a polyclonal β-catenin antibody at a 1:1000 dilution for one hour at RT. The blot was washed four times with TBS (1, 5, 10 and 15 min, respectively) and incubated for one hour with HRP-labelled secondary antibody (goat anti-rabbit IgG, 1:1000 diluted in blocking buffer) at RT. The membrane was washed as above and the antigen–antibody complex was visualized with an ECL (enhanced chemiluminescence) detection kit (Pierce, USA)1119.
Intracellular calcium analysis by confocal microscopy and spectrofluorometry: Intracellular calcium concentration was measured using Fura-2/AM. SW480 and HT-29 cells were seeded onto the centre of glass coverslips and grown to reach about 70 per cent confluency. The cells were then transfected with the Gαq minigene plasmid or GP antagonist-2A. After transfection, the coverslips were loaded with 5 µM Fura-2/AM in Ringer solution for 15-30 min at 37°C, after which the cells were incubated in fresh Ringer solution for 30 min at 37°C in 5 per cent CO2. The cells were then visualized under a confocal laser scanning microscope (Leica TCS SPE, Wetzlar, Germany). For spectrofluorometry analysis, manufacturer instructions were followed for Fura-2/AM (Abcam, Cambridge, USA). The transfected cells were trypsinized and briefly centrifuged. The cell pellets were washed with Ringer solution and re-suspended in the same buffer containing 5 μM Fura-2/AM for 15 to 30 min at 37°C. The Fura-2/Ringer solution was removed and the cells were incubated in fresh Ringer solution for 30 min at 37°C in 5 per cent CO2. The cell suspensions were placed into the cuvettes of a spectrofluorometer (Carry100Bio, Varrian, Australia) present at excitation wavelengths of 340 and 380 nm and emission wavelength of 510 nm.
Wound healing experiments to measure cell migration: SW480 cells (1.0×105 cells) were seeded in each well of a 12-well plate and grown to 100 per cent confluency. The cells were starved overnight in a medium containing 0.5 per cent FBS before being transfected with the Gαq minigene plasmid or GP antagonist-2A. Five-hour post-transfection a scratch was made with a sterile pipette tip. The cells were cultured for an additional 24 and 48 h and the wound images were recorded using a phase-contrast microscope (OPTICAL, Germany). The horizontal migration abilities of the cells were quantified by measuring the wound widths using the Image J analysis software as described previously20.
Data analysis: Statistical analyses were carried out using one-way analysis of variance using SPSS 25 software (IBM Corp., Chicago, IL, USA). Post hoc power analysis was done to determine the observed power for each variable. Differences at P <0.05 were considered statistically significant. The statistical data are presented in Tables I and II.
| Cell lines | Subcellular fractionation | Mean inhibitora | Mean control | ANOVA | Observed powerc | ||
|---|---|---|---|---|---|---|---|
| Mq | GP | f | Pb | ||||
| β-catenin expression (A) | |||||||
| SW-480 | Cytoplasm | 0.32±0.02 | 0.27±0.02 | 0.60±0.04 | 98.18 | 0.000** | 0.999 |
| Membrane | 0.43±0.02 | 0.44±0.04 | 0.41±0.05 | 0.328 | 0.723 | 0.165 | |
| HT-29 | Cytoplasm | 0.86±0.02 | 0.83±0.04 | 0.87±0.06 | 0.738 | 0.217 | 0.121 |
| Membrane | 0.59±0.06 | 0.65±0.05 | 1.19±0.06 | 93.467 | 0.000** | 1.000 | |
| Ca2+concentration (B) | |||||||
| SW-480 | 1.35±0.05 | 1.55±0.05 | 1.84±0.06 | 63.52 | 0.000** | 1.000 | |
| HT-29 | 1.45±0.05 | 1.25±0.05 | 1.80±0.09 | 50.34 | 0.000** | 1.000 | |
P **<0.01. aAll values are mean±SD; bResulted from one-way ANOVA; cObserved power for the current study. Mq, Gαq minigene; GP, G-protein antagonist-2a; SD, standard deviation; ANOVA, analysis of variance
| Cell line/genes | Mean inhibitorsa | ANOVA | Observed powerc | ||
|---|---|---|---|---|---|
| Mq | GP | f | Pb | ||
| SW-480 | |||||
| Luc | 0.101±0.05 | 0.356±0.22 | 112.47 | 0.000** | 1.000 |
| C-MYC | 1.845±0.37 | 0.456±0.04 | 96.70 | 0.001** | 1.000 |
| CCND1 | 0.476±0.26 | 0.950±0.15 | 32.16 | 0.020* | 0.999 |
| FGF20 | 0.301±0.29 | 0.101±0.09 | 100.84 | 0.001** | 1.000 |
| HT-29 | |||||
| Luc | 0.322±0.09 | 0.740±0.10 | 159.93 | 0.000** | 1.000 |
| C-MYC | 0.580±0.15 | 0.542±0.15 | 22.24 | 0.011* | 1.000 |
| CCND1 | 0.576±0.26 | 2.130±0.69 | 31.43 | 0.020* | 0.996 |
| FGF20 | 0.556±0.05 | 0.295±0.24 | 54.06 | 0.003** | 0.939 |
P * <0.05, ** <0.01. aAll values are mean±SD; bResulted from one-way ANOVA; cObserved power for the current study
Results
Gq signalling is active in colon cancer cells: To examine the activity of Gq signalling in SW480 and HT-29 cells, the cellular expression of three known GPCRs (the protease-activated receptor 1 (PAR1), PAR2 and m3-muscaranic acetylcholine receptor) was measured. These receptors are all Gq-coupled and therefore can activate Gq signalling212223. The results of reverse-transcriptase PCR experiments showed that both cell lines express all three receptors and Gαq itself (Fig. 1A). In addition, it has been shown that some other Gq-coupled receptors including lysophosphatidic acid receptor 2 and metabotropic glutamate receptors can be expressed by colon cancer cells or tissues2425. Release of calcium from the endoplasmic reticulum is one of the main results of Gq signalling1126. We found that pre-treatment of HT-29 or SW480 cells with Fura-2 (a fluorescent dye which binds free intracellular calcium) followed by treatment with carbachol (a muscarinic acetylcholine receptor agonist) led to release of intracellular calcium (Fig. 1B). Taken together, these results show that Gq signalling is an active pathway in colon cancer cells.
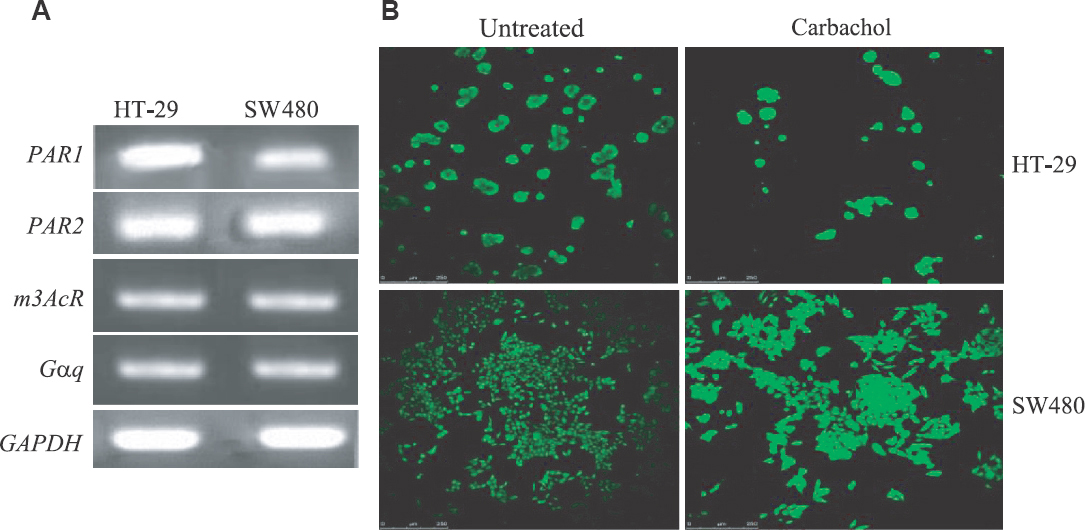
- Gq signalling is active in HT-29 and SW480 cells. (A) A reverse transcriptase PCR (RT-PCR) showing the expression of different genes in HT-29 and SW480 colon cancer cells. (B) HT-29 and SW480 cells visualized by a confocal microscope. PAR, protease activated receptor; m3AcR, m3-muscarinic receptor; Gαq, Gαq minigene; GAPDH, glyceraldehyde 3-phosphate dehydrogenase
Blockade of Gq signalling decreases β-catenin protein levels in colon cancer cells: Two specific blockers of Gq signalling were used in this study, a commercially available peptide (GP antagonist-2A), and a minigene expression plasmid encoding a peptide corresponding to the C-terminal 11 amino acids of Gαq (Gαq minigene). The suggested mechanism for the inhibitory function of both peptides relies on their ability to block the interaction between the Gq-coupled receptor and Gαq protein131415. The specificity of both peptides has been verified to some extent by experiments performed in different laboratories including ours1011131415. To further verify the function of these two Gq inhibitors, their effects on calcium release from intracellular stores were examined. Gαq is a potent activator of the beta isoforms of phospholipase C (PLCβ)1126. The active form of PLCβ converts phosphatidylinositol 4,5-bisphosphate (PIP2) into two second messengers, inositol 1,4,5-trisphosphate (IP3) and diacyl glycerol (DAG), and the interaction of IP3 with its receptors on endoplasmic reticulum then leads to the release of calcium into the cytosol. HT-29 and SW480 cells were transfected with either GP antagonist-2A or the Gαq minigene plasmid, and calcium release was measured by treatment of cells with Fura-2. As shown in Fig. 2 and Table I, both inhibitors could significantly decrease the release of calcium from intracellular stores, indicating that the inhibitors are capable of blocking Gq signalling. The lack of complete blockade of calcium release by the inhibitors could, however, be due to the activity of other cellular phospholipases, which are not under the regulation of Gαq.
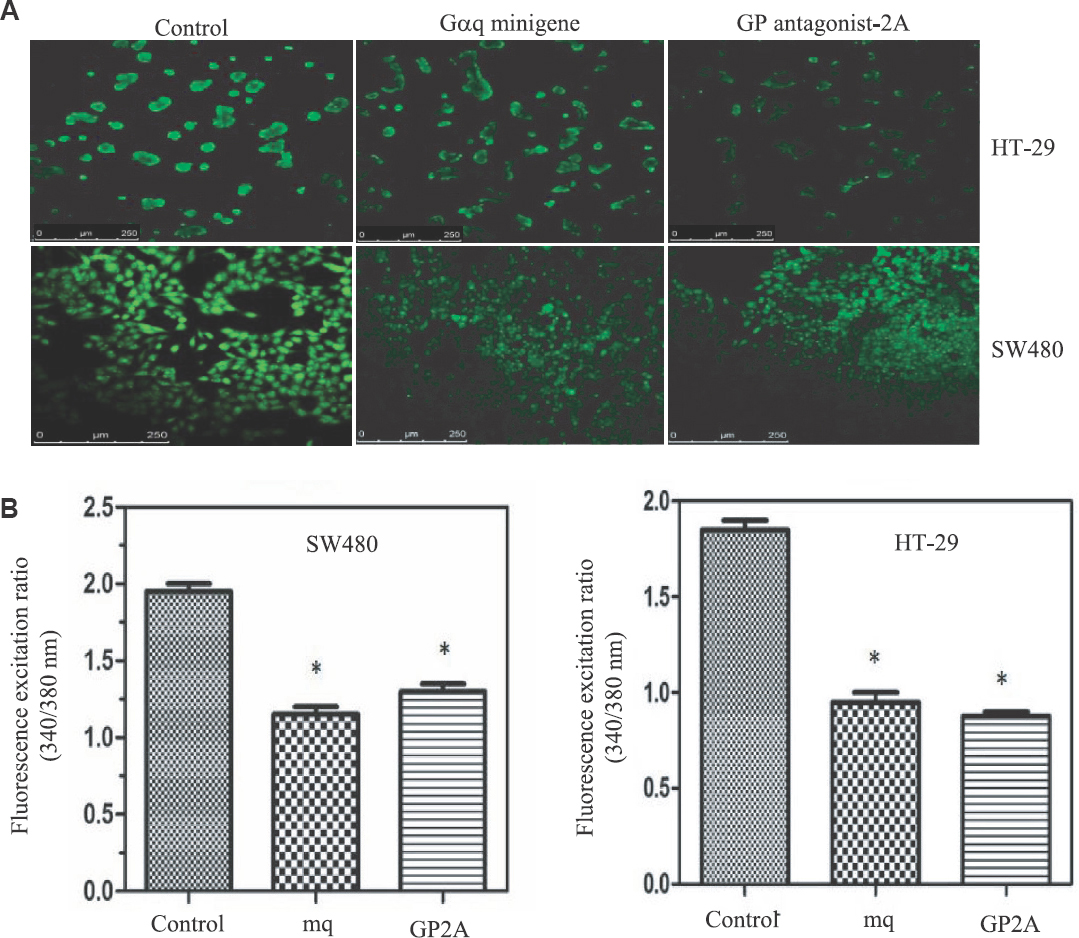
- (A) Reduction of intracellular calcium by inhibitors of Gq signalling. Empty vector-transfected cells were used as control. (B) Fluorescence was quantified using spectrofluorometry analysis. Values plotted are an average of three independent experiments. (P *<0.05 vs. control). mq, Gαq minigene; GP2A, GP antagonist-2A.
To examine the role of Gq signalling blockade on β-catenin protein expression, cells (SW480 and HT-29) were transfected with the Gαq minigene plasmid or GP-antagonist 2A and then visualized β-catenin protein by immunofluorescence microscopy (Fig. 3). Compared to cells transfected with empty vector, cells transfected with GP-antagonist 2A or the Gαq minigene appeared to have lower amounts of cellular β-catenin (Fig. 3). HT-29 cells have an epithelial phenotype and grow like grape clusters in vitro. In these cells, β-catenin clearly could be seen at the cell membrane, although intracellular β-catenin could also be weakly detected (Fig. 3). Interestingly, in HT-29 cells, the decrease of β-catenin protein by the Gq blockers was mainly observed at the cell membrane, and this decrease was not associated with a reduction in the size of HT-29 clusters or the number of cells in each cluster, suggesting that the blockade of Gq signalling did not affect HT-29 cell-cell junctions or the epithelioid phenotype of these cells.
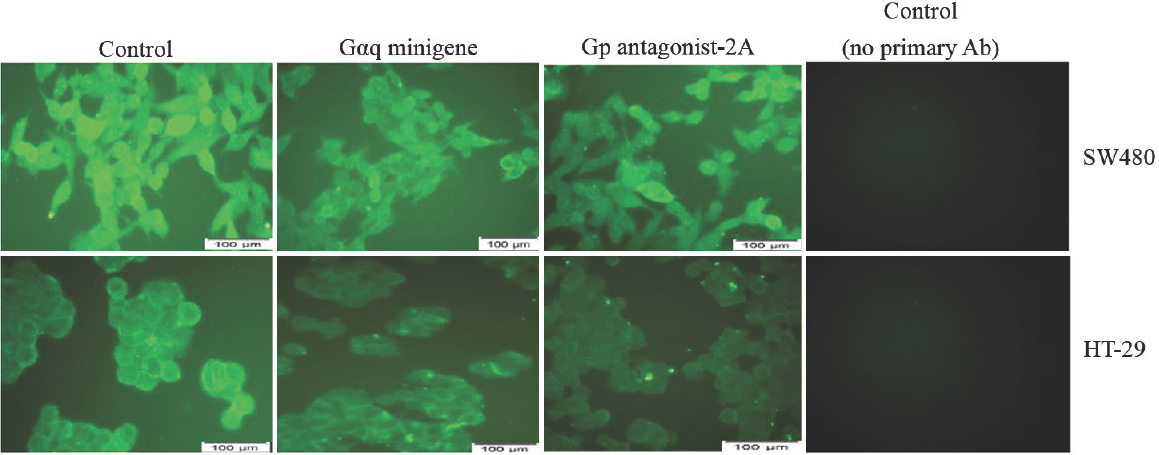
- Inhibitors of Gq signalling decrease cellular β-catenin protein levels.
SW480 cells have a fibroblastic phenotype and look like isolated cells. In these cells, β-catenin staining was ubiquitous, suggesting that the protein is present throughout the cell (Fig. 3). Expression of the Gq inhibitors in SW480 cells led to an overall decrease in β-catenin protein levels. In addition, the inhibitors of Gq promoted the formation of small populations of epithelioid cells having β-catenin at the cell membrane (Fig. 3). Thus results were confirmed by western blotting. As shown in Fig. 4, compared to the cells transfected with empty vector, the membrane levels of β-catenin in HT-29 cells decreased by approximately 50 per cent in the cells transfected with GP-antagonist 2A or Gαq minigene plasmid. The cytoplasmic β-catenin levels in HT-29 cells appeared to be similar in the presence and absence of the Gq inhibitors (Fig. 4). In SW480 cells, the cytoplasmic β-catenin levels were decreased by 50 and 40 per cent in the presence of GP-antagonist 2A and Gαq minigene, respectively. β-catenin was easily detected in the membrane extracts of SW480 cells, and the levels of this protein did not change significantly in the presence of the Gq inhibitors (Fig. 4 and Table I).
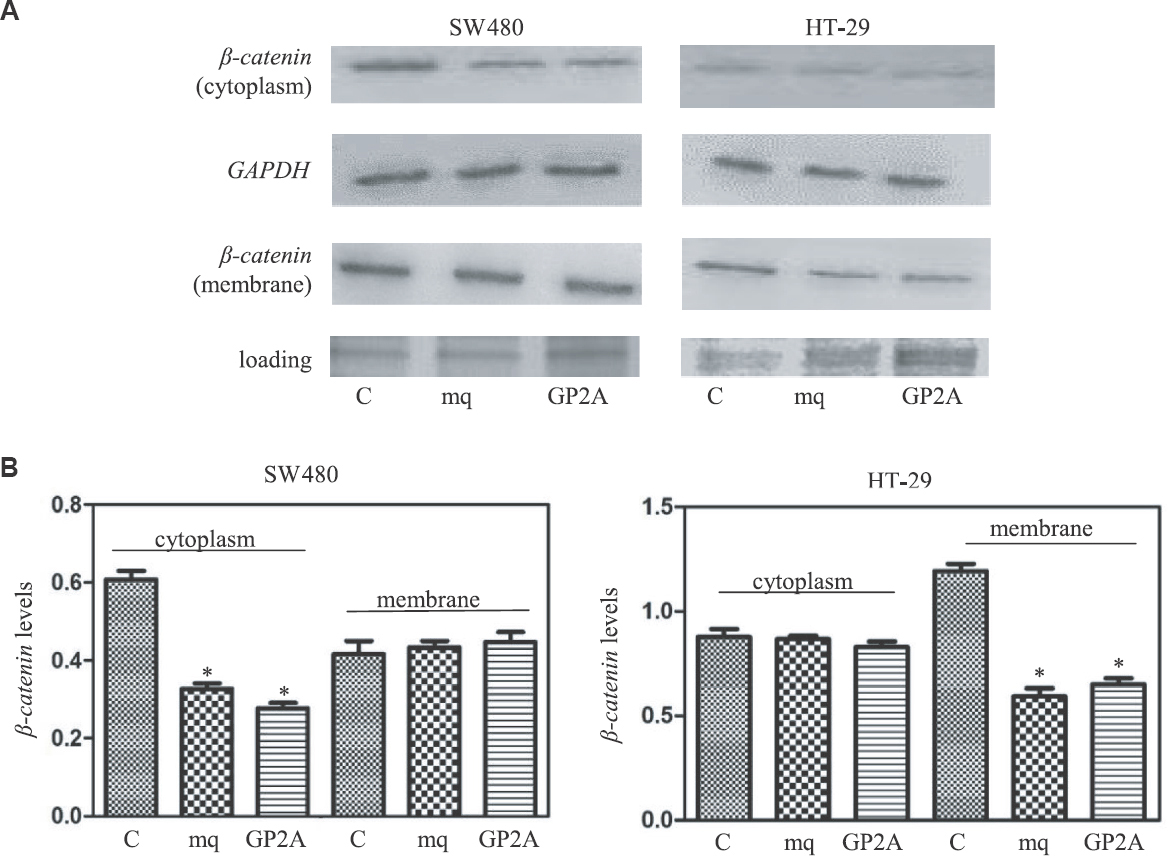
- (A) Western blotting experiments to measure cellular β-catenin protein levels in the presence of inhibitors of Gq signalling. (B) Values plotted represent average of three quantitative western blot experiments (P *<0.05 vs. control).
To examine whether the blockers of Gq signalling had any effect on β-catenin-mediated gene transcription, the mRNA levels of the three cellular β-catenin/TCF-target genes CCND1, c-MYC and FGF-20 were measured. As shown in Fig. 5, although it appeared that there was an overall decrease in transcription of the target genes in the presence of the inhibitors of Gq signalling, there were also a few contradictory results. In HT-29 cells, the expression of CCND1 was increased by GP-antagonist 2A but decreased by the Gαq minigene. In SW480 cells, the Gαq minigene enhanced c-MYC expression, but GP-antagonist 2A decreased it (Fig. 5).
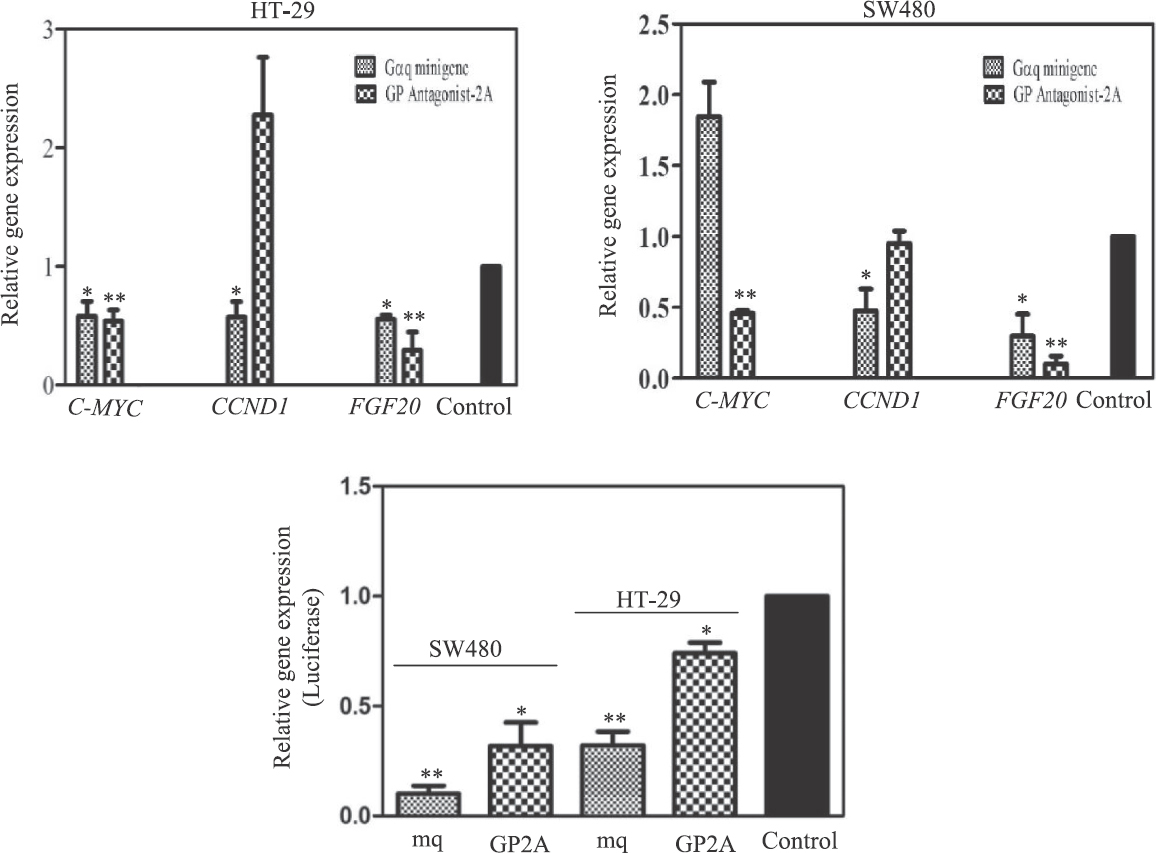
- Expression of β-catenin-target genes in the presence of Gq inhibitors by real time PCR. The cells transfected with empty vector were used as control. The charts represent the average of 3 or 4 independent experiments (P *<0.05; **<0.01 vs. control).
The three target genes mentioned above are among those genes which are under tight regulation in animal cells; several signalling pathways (including the Wnt/β-catenin signalling) may take part in the regulation of transcription of these genes. It is also possible that different cells such as SW-480 and HT-29 do not use exactly similar mechanisms for regulating the expression of these β-catenin-target genes. Therefore, we took advantage of the TOPflash plasmid, in which the reporter luciferase gene is placed downstream of some β-catenin/TCF recognition elements18. Expression of the luciferase gene was decreased in the presence of Gq inhibitors in both SW480 and HT-29 cells (Fig. 5 and Table II) but could not be detected in the cells transfected with the FOPflash plasmid18 which carries mutations in the β-catenin/TCF binding elements (data not shown).
The blockers of Gq signalling decrease SW480 cell migration: In context to the inhibition of this signalling pathway and how it affects biological activities of colon cancer cells such as growth, proliferation, differentiation and migration, whether inhibition of Gq signalling had any effect on the cell migration of SW480 cells was examined. As shown in Fig. 6, compared to cells transfected with an empty vector (100%), the wound closure values (after 48 h) were decreased significantly for the SW480 cell monolayers transfected with Gαq minigene plasmid (30%) or GP-antagonist 2A (26%).
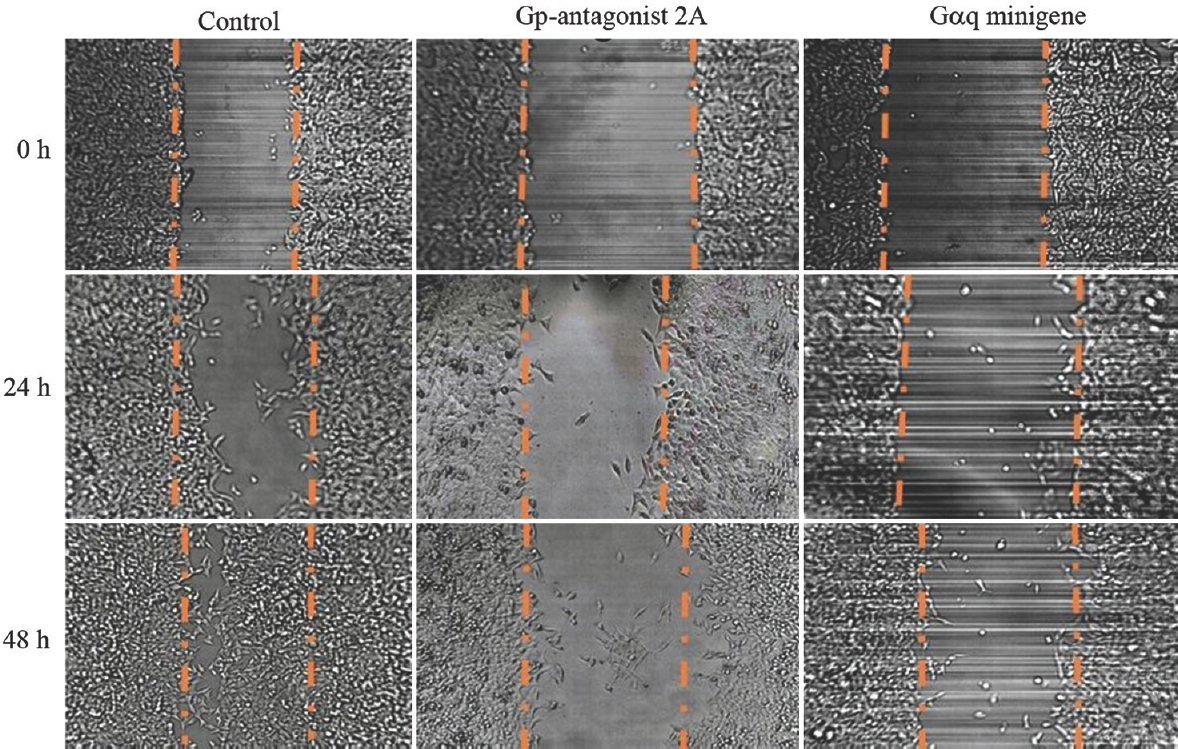
- Inhibitors of Gq signalling decrease migration of SW480 cells. The figure represents the results of wound-healing experiments.
Discussion
The present study investigated the interaction between the Gq class of heterotrimeric G proteins and the β-catenin signalling pathways in HT-29 and SW480 colon cancer cells. Two specific Gq blocking strategies were employed. Gp Antagonist-2A which specifically inhibits Gq pathway13 and introduction of a minigene expression plasmid into the cells, which was originally described by Gilchrist et al14. The minigene encodes a short peptide (11 amino acids) corresponding to the extreme C-terminal portion of Gαq, the region that is required for interaction of the Gαq subunit of heterotrimeric G proteins with the corresponding receptors. The synthesized peptide appears to compete with the Gα protein for interaction with the receptor1415. In the present study, the two Gq inhibitors produced generally consistent results, and the minor differences could be related to the difference in their original nature. When introduced into the cells, GP-antagonist 2A was available to function, but the availability of the other inhibitor was dependent on the expression of the Gαq minigene.
Transfection of HT-29 or SW480 cells with the Gq blockers significantly reduced β-catenin cellular protein levels and β-catenin transcriptional activities (Figs 3-5). These results are consistent with our previous work showing that the expression of Gαq in HEK-293T cells increases both cellular β-catenin protein levels and β-catenin transcriptional activities1011.
In addition to the decrease in protein levels of β-catenin in the presence of the Gq blockers, immunofluorescence microscopy and western blotting experiments yielded two results which need to be further investigated. Despite a decrease in membrane protein levels of β-catenin in HT-29 cells transfected with the Gq blockers, change was not observed in the epithelial phenotype of these cells (Fig. 3). It is possible that HT-29 cells have extra β-catenin at the cell membrane to make these cells ready for cell proliferation when needed. Moreover, SW480 cells achieved a more distinct epithelial phenotype in the presence of the Gq inhibitors.
These two results further support an oncogenic role for Gq signalling in cancer cells. These observations also raise a possibility that the activation of Gq signalling may promote a phenomenon similar to epithelial–mesenchymal transition in colon cancer cells supported by our previous observation that treatment of HT-29 cells with thrombin (a PAR1 agonist) or carbachol (a muscarinic acetylcholine receptor agonist) leads to dissociation of HT-29 cells and a significant increase in intracellular β-catenin protein levels (Jasemi and Najafi, unpublished results).
The results of the gene expression experiments clearly showed that the decrease in β-catenin protein levels in the presence of the Gq blockers accompanied a decrease in transcription of the β-catenin-target genes, indicating a functional link between Gq signalling and β-catenin activities. For this part of the study, we used three cellular β-catenin-target genes (CCND1, c-MYC and FGF-20) and a known reporter luciferase gene18.
The results of functional studies of transcription factors detection (like β-catenin) using reporter gene experiments always need to be interpreted cautiously. In these experiments, investigators normally use a segment of a responsive element (alone or in multiple copies) in an isolated state and cloned in a plasmid. These might not represent the real-responsive elements in the cellular genome which probably have other cis-regulatory sequences together with distinct chromatin structures plus multiple other regulators. Despite these differences, the results from qRT-PCR experiments measuring transcription of the reporter luciferase and the three β-catenin-responsive cellular genes were consistent (Fig. 5).
Taken together, the results presented in this paper, together with our previous results and those from other laboratories, provide evidence that the Gq class of G proteins positively regulate cellular β-catenin protein levels as well as transcriptional activities1011272829. We have already provided a mechanism by which Gαq activates β-catenin and that is through inhibition of GSK-3β kinase activity1011. Gαq signalling activates the beta isoform of PLCβ, an enzyme which converts PIP2 to IP3 and DAG which are two important cellular second messengers101126. IP3 interacts with its receptors on endoplasmic reticulum to facilitate calcium release into the cytosol. Both calcium and DAG can activate protein kinase C (PKC)2629. PKC is among the protein kinases that can phosphorylate and inactivate GSK-3β3031. Inhibition of GSK-3β by Gαq signalling has also been related to the Gαq-mediated phosphatidylinositol signalling and generation of inositol pentakisphosphate (IP5)29. It was then suggested that transient accumulation of IP5 inhibits GSK-3β and therefore leads to accumulation of β-catenin and Tcf/Lef-dependent transcription29. β-catenin is known as a potent proto-oncoprotein whose up-regulation has been reported in a wide range of human cancers including colon cancer. Currently, several pre-clinical studies are being carried out using compounds which target β-catenin protein stability or β-catenin function32.
Interestingly, in addition to Gq signalling, the interaction of some other classes of G proteins with β-catenin expression and function has also been reported9. The involvement of G proteins in β-catenin regulation opens up a new direction toward targeting β-catenin in human cancers. Many colon cancer cells appear to have higher levels of expression of some GPCRs which are thought to preferentially couple to Gq22232425. This feature is consistent with our results which show that HT-29 and SW480 colon cancer cells express several known Gq-coupled receptors. We also demonstrate that treatment of HT-29 and SW480 cells with the cholinergic agonist carbachol leads to release of calcium from intracellular stores (Figs 1 and 2). These observations suggest that activation of Gq signalling is required for formation and progression of colon cancer cells. Despite a broad diversity of signalling pathways mediated by different GPCRs and G proteins, the role of G proteins in cancer biology has not been investigated much.
In summary, the present study demonstrates that Gq signalling positively regulates β-catenin expression and function in HT-29 and SW480 colon cancer cells. These results also support the idea that heterotrimeric G proteins and their receptors are among potential targets for cancer therapeutics.
Financial support & sponsorship: This work was supported in part by a grant from Iran National Science Foundation (93004354) to SMAN and also by the University of Tehran Research department, Tehran, Iran.
Conflicts of Interest: None.
References
- Genetics, diagnosis and management of colorectal cancer (review) Oncol Rep. 2015;34:1087-96.
- [Google Scholar]
- APC, signal transduction and genetic instability in colorectal cancer. Nat Rev Cancer. 2001;1:55-67.
- [Google Scholar]
- Biology of the adenomatous polyposis coli tumor suppressor. J Clin Oncol. 2000;18:1967-79.
- [Google Scholar]
- The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell. 2001;105:391-402.
- [Google Scholar]
- Cadherin-bound β-catenin feeds into the Wnt pathway upon adherens junctions dissociation: Evidence for an intersection between β-catenin pools. PLoS One. 2009;4:e4580.
- [Google Scholar]
- Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111-22.
- [Google Scholar]
- Activation of β-catenin signaling pathways by classical G-protein-coupled receptors: Mechanisms and consequences in cycling and non-cycling cells. Cell Cycle. 2006;5:2295-300.
- [Google Scholar]
- Activators of G proteins inhibit GSK-3βand stabilize β-catenin in Xenopus oocytes. Biochem Biophys Res Commun. 2009;382:365-9.
- [Google Scholar]
- Regulation of GSK-3βand β-catenin by Gαq in HEK293T cells. Biochem Biophys Res Commun. 2010;395:577-82.
- [Google Scholar]
- G ptotein antagonists: A novel hydrophobic peptide compete with receptor for G protein binding. J Biol Chem. 1992;267:16237-43.
- [Google Scholar]
- A dominant-negative strategy for studying roles of G proteins in vivo. J Biol Chem. 1999;274:6610-6.
- [Google Scholar]
- Gαminigenes expressing C-terminal peptides serve as specific inhibitors of thrombin-mediated endothelial activation. J Biol Chem. 2001;276:25672-9.
- [Google Scholar]
- Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2013;2:e71.
- [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;24:402-8.
- [Google Scholar]
- Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275:1787-90.
- [Google Scholar]
- Crude subcellular fractionation of cultured mammalian cell lines. BMC Res Notes. 2009;10:243.
- [Google Scholar]
- Anticancer properties of teucrium persicum in PC-3 prostate cancer cells. Asian Pac J Cancer Prev. 2014;15:785-91.
- [Google Scholar]
- Muscarinic receptor subtypes: Physiology and clinical implications. N Engl J Med. 1989;321:1022-9.
- [Google Scholar]
- Initiation of human colon cancer cell proliferation by trypsin acting at protease-activated receptor-2. Br J Cancer. 2001;85:772-9.
- [Google Scholar]
- Aberrant expression and activation of the thrombin receptor protease-activated receptor-1 induces cell proliferation and motility in human colon cancer cells. Am J Pathol. 2003;162:1503-13.
- [Google Scholar]
- Lysophosphatidic acid (LPA) receptors: Signaling properties and disease relevance. Prostaglandins Other Lipid Mediat. 2010;91:130-8.
- [Google Scholar]
- G protein signaling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science. 2001;292:1718-22.
- [Google Scholar]
- Rapid, Wnt-induced changes in GSK3βassociations that regulate β-catenin stabilization are mediated by Gαproteins. Curr Biol. 2005;15:1989-97.
- [Google Scholar]
- Inositol pentakisphosphate mediates Wnt/β-catenin signaling. J Biol Chem. 2007;282:26490-502.
- [Google Scholar]
- Differential regulation of glycogen synthase kinase 3-beta by protein kinase C isotypes. J Biol Chem. 2007;267:16878-82.
- [Google Scholar]
- Protein kinase C inhibits amyloid beta peptide neurotoxicity by acting on members of the Wnt pathway. FASEB J. 2002;16:1982-4.
- [Google Scholar]
- Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985-99.
- [Google Scholar]






