Translate this page into:
Inhibition of hepatitis B virus gene expression & replication by crude destruxins from Metarhizium anisopliae var. dcjhyium
Reprint requests: Dr Changjin Dong, College of Life Science, Hubei Normal University, Huangshi 435002, Hubei, PR China e-mail: dongchangjin2005@163.com; or
Dr Ying Zhu, State Key Laboratory of Virology, College of Life Science, Wuhan University, Wuhan 430072, Hubei, PR China e-mail: yingzhu@whu.edu.cn
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Destruxin A, destruxin B and destruxin E isolated from entomopathogenic fungus Metarhizium anisopliae showed a strong suppressive effect on the replication of hepatitis B virus (HBV) in human hepatoma cells. In this study, the anti-HBV effects of the crude destruxins extracted from M. anisopliae var. dcjhyium were detected both in vitro and in vivo.
Methods:
HepG2.2.15 cells were cultured to observe the inhibitory effects of the crude destruxins on the gene expression and replication of HBV by radioimmunoassay detection and real-time quantitative PCR. In vivo, duck HBV (DHBV)-infected ducks were treated with the crude destruxins at 2.0, 4.0, 6.0 μg/kg once a day for 15 days, DHBV DNA was examined by real-time quantitative PCR.
Results:
The crude destruxins suppressed the replication of HBV-DNA and the production of HBsAg and HBeAg with IC50 of about 1.2 and 1.4 μg/ml. Transcript of viral mRNA was significantly suppressed by the crude destruxins in HepG2.2.15 cells. In vivo, the duck serum DHBV-DNA levels were markedly reduced in the group of the crude destruxins.
Interpretation & conclusions:
The crude destruxins inhibited the gene expression and replication of HBV both in vitro and in vivo, and their anti-HBV effect was stronger than that with destruxin B. Our results indicate that the crude destruxins from M.anisopliae var. dcjhyium may be potential antivirus agents. Further studies need to be done to confirm these findings.
Keywords
Anti-hepatitis B virus
cude destruxins
DHBV
HBV
M. anisopliae var. dcjhyium
Chronic hepatitis B virus (HBV) infection is a major cause of liver disease affecting millions of people worldwide12. Chronic infection is associated with a high risk of developing primary liver carcinoma and liver cirrhosis in human345. Although immunization against HBV has been used to prevent chronic infection, effective drugs to eradicate HBV are still not available and need to be developed.
New antiviral compounds suitable for monotherapy or combination therapy are highly desired. In vitro and in vivo liposome-encapsulated matrine, Ganoderma lucidum, wogonin can evidently inhibit the replication of hepatitis B virus and TAT-HBV targeted ribonuclease showed a significant anti-HBV activity678. Destruxins are cyclic hexadepsipeptides first isolated from the culture filtrate of insect-pathogenic fungus Metarhizium anisopliae910. Over 35 different structurally related destruxins have been isolated, and 15 of these were isolated from M. anisopliae11. The most abounding components are destruxin A, destruxin B and destruxin E, several destruxins showed a strong suppressive effect on the replication of hepatitis B virus in human hepatoma cells1213. Destruxin B could be a specific inhibitor of vacuolar-type H+ translocating ATPase and showed a strong suppressive effect on hapatitis B virus surface antigen gene expression in human hepatoma cells1415.
M. anisopliae var. dcjhyium, a Chinese strain of M. anisopliae was isolated from subterranean termite, Odontoternes formosanus16. As compared with other virulent M. anisopliae isolates, this new variety was highly virulent causing 100 per cent mortality when the termites were treated with 3×108 conidia/ml M. anisopliae var. dcjhyium for 3 days17. The new variety can produce high yields of crude destruxins from liquid cultures of M. anisopliae var. dcjhyium. In the shaker-flask cultivation of M. anisopliae var. dcjhyium on SDAY (dextrose 10 g, peptone 2.5 g, yeast extract 5 g/l), the production of crude destruxins was 2.30 g/l (unpublished data), but the production of crude destruxins by the other M. anisopliae isolates were only 30-118 mg/l1819. This study was aimed to assess the anti-HBV activity of crude destruxins extracted from M. anisopliae var. dcjhyium in vitro in human HBV-transfected cell line HepG2.2.15 and in vivo in duck hepatitis B virus (DHBV)-infected ducks.
Material & Methods
This study was conducted in Hubei Normal University and State Key Laboratory of Virology, Wuhan University, China.
Extracts of crude destruxins from the culture solution of M. anisopliae var. dcjhyium: M. anisopliae var. dcjhyium was supplied by State Key Laboraroty of Virology, College of Life Science, Wuhan University, China. Conidia were grown in a liquid cultural medium (dextrose 10 g, peptone 2.5 g, yeast extract 5 g/l; 100-ml flasks) on a shaker at ambient temperature (26°C) for 14 days. The culture medium was filtered through four layers of Miracloth and Whatman filter paper no.2. For destruxins extraction, the culture filtrates were mixed with acetonitrile (1:1, v/v) and NaCl (5%, w/v) and shaken vigorously for 20 min. The pH value of the filtrates was adjusted to 7.0. After an hour, acetonitrile was separated from broth and completely evaporated using a rotary evaporator, leaving a crude destruxin2021.
Cell culture and treatment: Cell line HepG2.2.15 was obtained from State Key Laboratory of Virology in Wuhan University, China. The human HBV-transfected cell line HepG2.2.15 was maintained in Dulbecco’ s modified Eagle medium (DMEM) supplemented with 10 per cent foetal calf serum (FCS), 100 unit/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine (all from Sino-American Biotech, China). For bioassay, crude destruxins or destruxin B was first dissolved in ethanol (vehicle), filtered through a 0.25 μm fluoropore filter (Millipore, USA), and added to cell cultures15. Cells were treated with crude destruxins, or destruxin B (Sigma, USA) at various concentration for a specified period in serum-free DMEM medium supplemented with 100 unit/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine in 24-well plate at a density of 2×104 per well. The culture medium was replaced with a fresh one on day 2, with or without (negative control conditions) different concentrations of crude destruxins or destruxin B during the 8 days experiment.
Detection of HBsAg and HBeAg: HepG2.2.15 cells were seeded in 24-well plates at a density of 2×104 per well for measurement of hepatitis B surface (HBsAg) and e antigen (HBeAg), and HBV-DNA. After incubation with various concentrations of crude destruxins, or destruxin B for 2 or 8 days, the culture medium was collected and cell debris removed. HBsAg and HBeAg in culture supernatants of HepG2.2.15 cells were measured by radioimmunoassay (RIA) kits (Sino-American Biotech, China).
Determination of survival rates of cells: HepG2.2.15 cells were plated into 24-well plates at a density of 2×104 per well. After 24 h, the culture medium was replaced by the culture medium with drugs (crude destruxins or destruxin B) at the final concentration of 1.0, 2.0, 3.0, 4.0 μg/ml, or CK1(blank control without ethanol or drugs), CK2 (vehicle control with ethanol). After 48 h, the morphology of cells was observed through inverted microscopy and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sino-American Biotech, China) was applied in each well at the final concentration of 5 mg/ml. After culturing for another 4 h, 150 μl DMSO was added into all wells and the light absorbance at A490 was detected8.
Measurement of HBV-DNA by fluoresent quantitative PCR: The intracellular HBV-DNA from HepG2.2.15 cells and the HBV DNA in cell culture supernatants were extracted22 using DNA Extraction Kit (Sino-American Biotech, China), and real-time quantitative PCR78 was performed in Lightcycler (Bio-Rad, USA) using HBV Fluoresent Quantitative PCR Detection Kit (Sino-American Biotech, China).
RNA isolation and Northern blotting analysis: Total cellular RNA was isolated by centrifugation through cesium chloride23. The RNA (20 μg) was denatured in 6.5 per cent formaldehyde and fractionated by electrophoresis in 1 per cent agarose gel. The RNA was transferred to a nitrocellulose filter by capillary blotting and immobilized by heating at 80°C for 2 h1524. The membrane filter was prehybridized for 6 h at 42°C in a solution containing 35 per cent formamide, 5x Denhardt's reagent (1x: 0.02% Ficoll, 0.2% BSA, 0.02% polyvinylpyrrolidone), 5x SSPE (1x: 0.15 M NaCl, 0.01 M NaH2PO4, 1 mM EDTA, 1% SDS, and 500 μg/ml denatured salmon sperm DNA, pH 7.4). Transcripts were detected by hybridization with 32P-labelled HBV DNA probes. Denatured 32P-labelled probes (106 cpm/μg) were added directly to the prehybridization buffer, and hybridization was carried out at 42°C for 36 h. The membrane filter was washed twice in 0.2x SSC(1x: 0.15 M sodium chloride, 0.05 M sodium citrate, pH 7.0), 0.2 per cent SDS at 42°C for 15 min, and once in 0.1x SSC (0.1% SDS at 65% for 15 min. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA level was used as an internal control to normalize for the amount RNA loaded1524. Autoradiography was performed at -70°C with an intensifying screen for 12 h on X-ray film.
Measurement of DHBV DNA in duck serum by real-time quantitative PCR: Three day old ducklings (with no restrictions of sex; from Guangzhou Yangsheng Duck Farm, China) were randomly chosen. They were abdominally inoculated with 0.1 ml DHBV-DNA positive viral serum (from Guangzhou University of Chinese Medicine, China). The infected positive ducklings were selected by means of PCR amplification. The DHBV-positive ducks were randomly divided into 3 groups with 5 ducks in each group. Crude destruxins and destruxin B was given to DHBV-infected ducks by gastric perfusion. Three groups were observed: group of crude destruxins (2.0, 4.0, 6.0 μg/kg), group of destruxin B (4.0 μg/kg) and group of blank model. The drug was given once daily for 15 days continuously, and normal saline was used as control. After the treatment, the ducks were maintained for additional 10 days. The duck serum HBV DNA was extracted with DNA Extraction Kit (Sino-American Biotech, China) and used for real-time quantitative PCR. The sense and antisense primers used were 5′-TCGGATTACTGCTAAGCT-3′and 5′-CCCGTTGTCCGTCAGATACAG-3’7. The final real-time quantitative PCR78 reaction consisted of 10 mM Tris-HCl, 2 mM MgCl2, 0.3 μM dNTP, 600 nM primer R, 600nM primer L, and 2.0 U Taq DNA polymerase (all the PCR reagents obtained from Sina-American Biotech, China). After denaturation at 95°C for 3 min, amplifications with 42 cycles of denaturation (94°C for 2 min), annealing (60°C for 30s) and polymerization (72°C for 2 min) were done. HBV DNA was quantified using a standard curve.
The study protocol was approved by the Hubei Animal Ethical Committee.
Statistical analysis: The results were expressed as mean ± SD (n=3). The data obtained were processed by Origin software or SPSS software (IBM, USA). T test was used to analyze the statistical difference between the treatment and the control groups.
Results
Inhibitory effects of crude destruxins on HBsAg and HBeAg in HepG2.2.15 cells: M. anisopliae var. dcjhyium was grown in a liquid cultural medium at ambient temperature (26°C) for 14 days. Crude destruxins extracted from the culture solution looked like white diamond crystals which could be dissolved in ethanol. The results showed that crude destruxins suppressed HBsAg and HBeAg production with IC50 of about 1.2 and 1.4 μg/ml (Figs 1, 2). The inhibitory effects on the generation of HBsAg and HBeAg increased with concentration of crude destruxins. The production of HBsAg and HBeAg was suppressed with 2.0 μg/ml crude destruxins (Figs 3, 4).
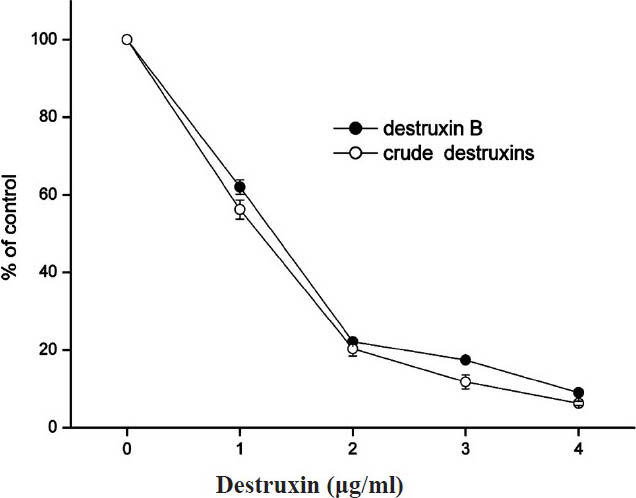
- Inhibitory effect of different concentrations of crude destruxins on the production of HBsAg in HepG2.2.15 cells after 48 h of treatment. HepG2.2.15 cells were cultured and treated with various concentrations (0.0, 1.0, 2.0, 3.0, 4.0 μg/ml) of crude destruxins or destruxin B in serum-free DMEM medium for 48 h. The amount of HBsAg in culture medium was then determined by radioimmunoassay. Control cells produced 41.4 ng of HBsAg/ml/48 h. Data are expressed as mean ± SD (n=3).
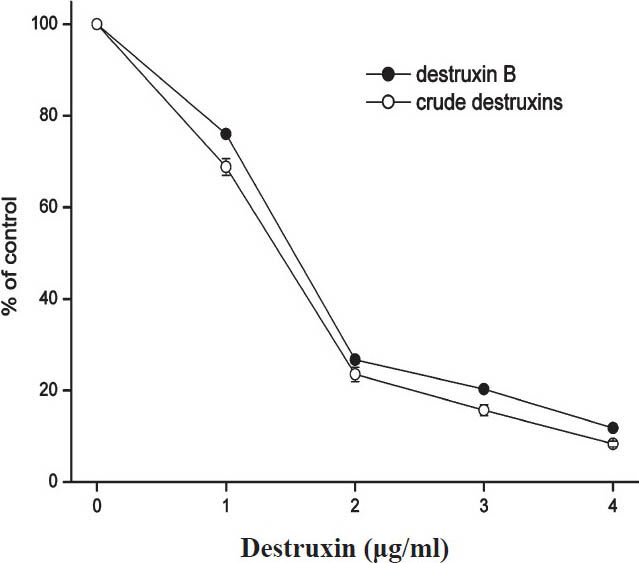
- Inhibitory effect of different concentrations of crude destruxins on the production of HBeAg in HepG2.2.15 cells after 48 h of treatment. HepG2.2.15 cells were cultured and treated with various concentrations (0.0, 1.0, 2.0, 3.0, 4.0 μg/ml) of crude destruxins or destruxin B in serum-free DMEM medium for 48 h. The amount of HBeAg in culture medium was then determined by radioimmunoassay. Control cells produced 13.5 NCU of HBeAg/ml/48 h. Data are expressed as mean ± SD (n=3). NCU, National clinical unit (China).
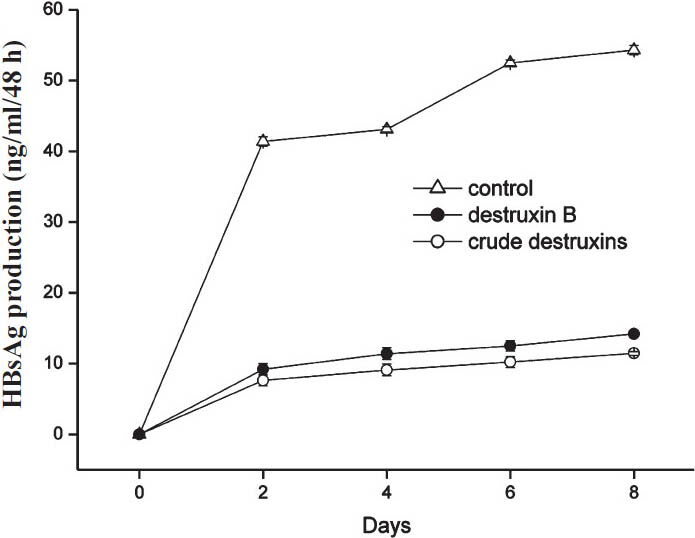
- Inhibitory effect of 2.0 μg/ml of crude destruxins on the production of HBsAg in HepG2.2.15 cells after a different time of treatment. HepG2.2.15 cells were treated without or with 2.0 μg/ml of crude destruxins or 2.0 μg/ml of destruxin B and incubated for 2, 4, 6, 8 days. The medium with drug was changed every two days and the amount of HBsAg in culture medium was determined by radioimmunoassay. Date are expressed as mean ± SD (n=3).
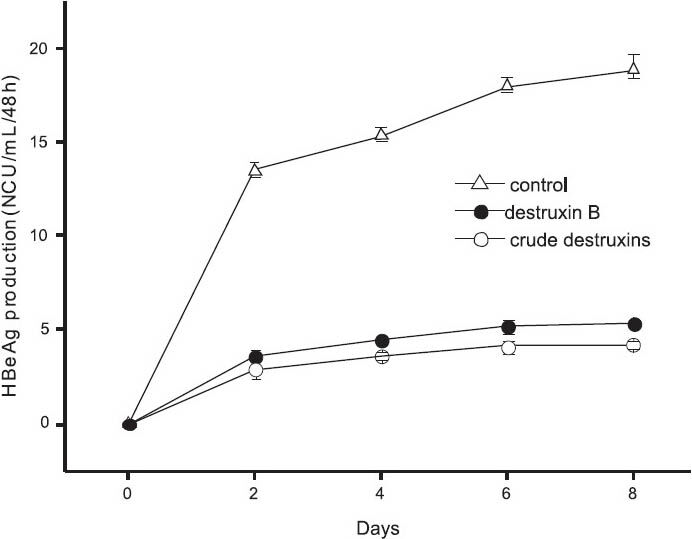
- Inhibitory effect of 2.0 μg/ml of crude destruxins on the production of HBeAg in HepG2.2.15 cells after a different time of treatment. HepG2.2.15 cells were treated without or with 2.0 μg/ml of crude destruxins or 2.0 μg/ml of destruxin B and incubated for 2, 4, 6, 8 days. The medium with drug was changed every two days and the amount of HBeAg in culture medium was determined by radioimmunoassay. Date are expressed as mean ± SD (n=3). NCU, National clinical unit (China).
Inhibitory effect of crude destruxins on HBV DNA replication in HepG2.2.15 cells: Consistent with the inhibitory effect on HBsAg and HBeAg secretion, crude destruxins treatment led to a significant (P<0.05) reduction in the intracellular HBV-DNA from HepG2.2.15 cells and the extracellular HBV-DNA in cell culture supernatants compared with the no drug control (Figs 5, 6). With the increasing concentrations, the inhibitory effects of crude destruxins on the replication of HBV-DNA were more obvious. When HepG2.2.15 cells were treated with 4.0 μg/ml of crude destruxins or destruxin B, the mean differences between crude destruxins and destruxin B treatment was significant (P <0.05).
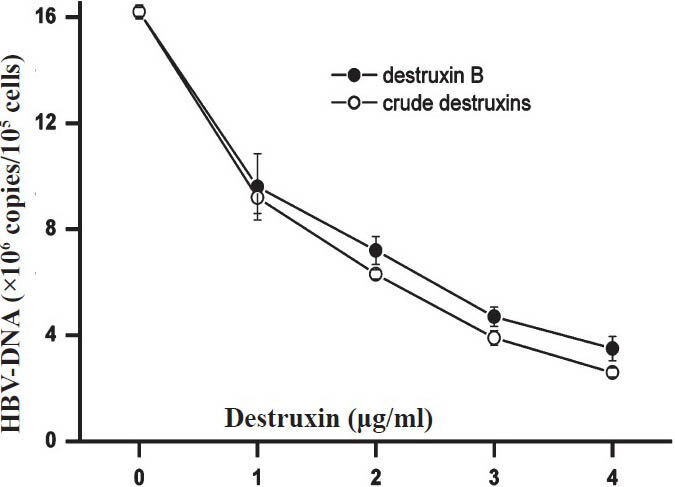
- Inhibitory effect of different concentrations of crude destruxins on the replication of intracellular HBV-DNA from HepG2.2.15 cells after 48 h of treatment. HepG2.2.15 cells were cultured in the presence of crude destruxins or destruxin B at various concentrations (0.0, 1.0, 2.0, 3.0, 4.0 μg/ml) for 48 h and then the intracellular HBV DNA from HepG2.2.15 cells were extracted and quantified by real-time quantitative PCR. Data are expressed as mean ± SD (n=3).

- Inhibitory effect of different concentrations of crude destruxins on the replication of HBV-DNA in cell culture supernatants from HepG2.2.15 cells after 48 h of treatment. HepG2.2.15 cells were cultured in the presence of crude destruxins or destruxin B at various concentrations (0.0, 1.0, 2.0, 3.0, 4.0 μg/ml) for 48 h and then HBV DNA in cell culture supernatants from HepG2.2.15 cells were extracted and quantified by real-time quantitative PCR. Data are expressed as mean ± SD (n=3).
HepG2.2.15 cells were treated with or without 2.0μg/ml of crude destruxins or destruxin B as described in Fig. 1. After 2, 4, 6 and 8 days of incubation, the HBV-DNA from HepG2.2.15 cells was extracted from culture supernatants and amplified by real-time quantitative PCR. The results showed that crude destruxins significantly suppressed the replication of HBV-DNA with the different time of incubation (Fig. 7).
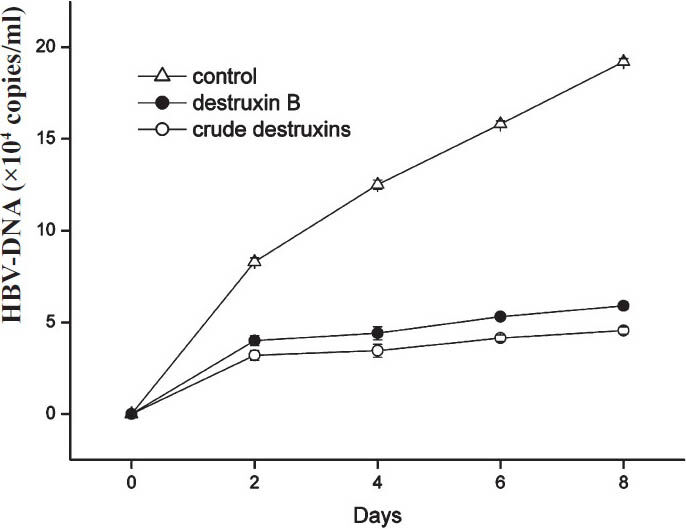
- Inhibitory effect of 2.0 μg/ml of crude destruxins on HBV DNA replication in HepG2.2.15 cells after a different time of treatment. HepG2.2.15 cells were treated without or with 2.0 μg/ml of crude destruxins or 2.0 μg/ml of destruxin B and incubated for 2, 4, 6, 8 days. The medium with drug was changed every two days and the amount of HBV DNA in culture medium was determined by real-time quantitative PCR. Data are expressed as mean ± SD (n=3).
Transcript of total viral mRNA in crude destruxins treated HepG2.2.15 cells: The total HBV mRNA which included 3.5kb for precore mRNA and pgRNA, 2.4 kb for preS mRNA and 2.1 kb for HBV S mRNA was detected in the HepG2.2.15 cells. The crude destruxins significant decreased 3.5 kb, 2.4/2.1 kb HBV-DNA transcripts in a dose-dependent manner. With increasing concentrations (1.0, 2.0, 3.0, 4.0 μg/ml) of crude destruxins treatment, the dramatic decrease of total virus mRNA in the HepG2.2.15 cells suggested that the suppression of HBV gene expression in HepG2.2.15 cells was mainly at the mRNA level (Fig. 8).
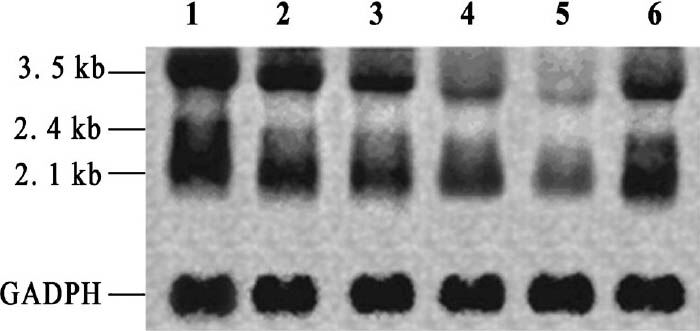
- Effect of crude destruxins on total HBV mRNA level in HepG2.2.15 cells. HepG2.2.15 cells were plated into 24-well plates at a density of 2×104 per well and treated with drugs (crude destruxins or destruxin B) or without (negative control conditions) in serum free DMEM medium for 48 h. Total HBV mRNA was extracted and analyzed by Northern hybridization. In Lane 1-CK (without drugs), 2, 3, 4, 5 - crude destruxins (1.0, 2.0,3.0, 4.0 μg/ml), 6 - destruxin B (2.0 μg/ml). The crude destruxins decreased 3.5 kb, 2.4/2.1 kb HBV transcripts in a dose-dependent manner. The constitutively expressed glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA level was used as an internal control.
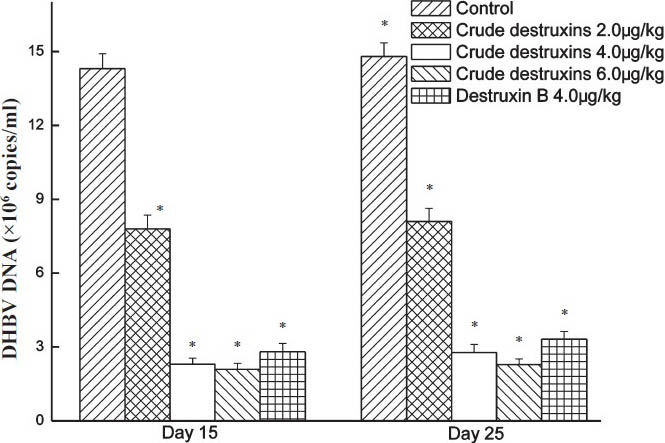
In vivo inhibitory effect of crude destruxins on DHBV DNA replication in ducks: DHBV-infected ducks were treated with crude destruxins (2.0, 4.0, 6.0 μg/kg) or destruxin B (4.0 μg/kg) and the duck serum DHBV-DNA levels were measured by real-time quantitative PCR. Compared to the controls, the duck serum DHBV-DNA levels markedly reduced in the groups of crude destruxins and destruxin B after treating for 15 days (Fig. 9) after withdrawal of the drug for 10 days, the levels of DHBV-DNA remained at the same level. To the suppression on the replication of DHBV-DNA was more with crude destruxins than with destruxin B in vivo.
-
In vivo inhibitory effect of crude destruxins on the replication of duck serum DHBV-DNA. Ducks were treated with crude destruxins (2.0, 4.0, 6.0 μg/kg) or destruxin B (4.0 μg/kg) once a day for 15 days. After the treatment, ducks were maintained for additional 10 days. Serum DHBV-DNA levels were quantified by real-time quantitative PCR. Data are expressed as mean ± SD (n=5). *P<0.05 compared to control.
Survival rates of cells by MTT assay: Crude destruxins showed no cytotoxic effect on the viability of HepG2.2.15 cells. After 48 h of culture, the morphology of cells was observed under inverted microscopy. There was no difference among the drug groups (crude destruxins or destruxin B) at the final concentration of 1.0, 2.0, 3.0, 4.0 μg/ml, or CK1 (blank control without ethanol nor drugs), CK2 (vehicle control with ethanol). MTT assay showed no significant difference among the three groups, crude destruxins had no effect on the division and the viability of HepG2.2.15 cells (data not shown).
Discussion
Hepatitis B virus causes a spectrum of liver diseases including acute hepatitis, chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma25. In the recent studies, thiazolylbenzimidazole derivatives, hepatocyte nuclear factor 4-alpha specific short hairpin RNA, toll-like receptor signaling and cellular inhibitor of apoptosis protein 2 (cIAP2) inhibited hepatitis B virus replication and gene expression26272829. Destruxins are cyclic hexadepsipeptides first isolated from the culture filtrate of insect-pathogenic fungus M. anisopliae9. Destruxins A, B, and E have been reported to markedly reduce HBsAg production in human hepatoma cells131530, and the inhibitory effect of destruxin B on HBsAg production was more obvious.
HepG2.2.15 cells and DHBV-infected ducks are two main models used for evaluating the anti-HBV effect of drugs8, and earlier studies have shown that destruxin B has a good suppressive effects on hepatitis B virus surface antigen gene expression in human hepatoma cells1315. In this study, the crude destruxins extracted from M. anisopliae var. dcjhyium strongly inhibited the production of HBsAg and HBeAg in HepG2.2.15 cells. The suppressive activity of the crude destruxins on the production of HBsAg and HBeAg in HepG2.2.15 cells was highly specific and was not due to the general cytotoxic effect of the drug. MTT assay showed that with the increase in the crude destruxins concentrations, the proliferation rate of HepG2.2.15 cells was not changed. Our observations strongly suggest that the crude destruxins directly suppress the replication and expression of HBV-DNA in HepG2.2.15 cells. In DHBV-infected ducks, the crude destruxins extracted from M. anisopliae var. dcjhyium was effective in suppressing DHBV replication by gastric perfusion. Compared with blank control group, the duck plasma DHBV-DNA levels markedly decreased in ducks treated with 2.0, 4.0, 6.0 μg/kg concentration of the crude destruxins for 15 days, and after withdrawal of the drug for 10 days.
In conclusion, the present study showed anti-HBV effect of the crude destruxins extracted from M. anisopliae var. dcjhyium both in vitro and in vivo. The crude destruxins were more effective than destruxin B, the reason may be that the crude destruxins contain many complex components (such as destruxins A, B and E, etc.) and their synergistic effect shows a strong suppressive effect on the replication of hepatitis B virus and viral gene expression. The crude destruxins extracted from M. anisopliae var. dcjhyium may have potential antivirus activity, however, further studies are required to purify and evaluate the mechanism of action.
Acknowledgment
This study was funded by Open Research Fund Program of the State Key Laboratory of Virology of China (NO.2010001), Major Project of Hubei Provincial Department of Education, China (NO.2008Z001) and Production-Study-Research Key Project of Hubei Province, China (NO.C2010058).
References
- Expression of nuclear factor-kappa B in hepatocellular carcinoma and its relation with the X protein of hepatitis B virus. World J Gastroenterol. 2001;7:340-4.
- [Google Scholar]
- Anti-hepatitis B activities of ganoderic acid from Ganoderma lucidum. Biotechnol Lett. 2006;28:837-41.
- [Google Scholar]
- Anti-hepatitis B virus activity of wogonin in vitro and in vivo. Antiviral Res. 2007;74:16-24.
- [Google Scholar]
- Anti-HBV effect of TAT-HBV targeted ribonuclease. World J Gastroenterol. 2003;9:1525-8.
- [Google Scholar]
- Toxic substances to insects, produced by Aspergillus ochraceus and Oospora destructor. Agric Biol Chem. 1961;25:261-7.
- [Google Scholar]
- New destruxins from the entomopathogenic fungus Metarhizium anisopliae. J Nat Prod. 1993;56:643-7.
- [Google Scholar]
- Insecticidal and cytotoxic effects of natural and hemisynthetic destruxins. Comp Biochem Physiol. 1994;108C:195-203.
- [Google Scholar]
- The novel desmethyldestruxin B2, from Metarhizium anisopliae, that suppresses HBV surface antigen production in human hepatoma cells. J Nat Prod. 1995;58:527-31.
- [Google Scholar]
- Destruxin B, a specific and readily reversible inhibitor of vacular-type H+ translocation ATPase. Biochem Biophys Res Commum. 1994;205:1555-63.
- [Google Scholar]
- Suppressive effects of destruxin B on hepatitis B virus surface antigen gene expression in human hepatoma cells. Antiviral Res. 1997;34:137-44.
- [Google Scholar]
- Characterization of a newly discovered China variety of M. anisopliae (M. anisopliae var. dcjhyium) for virulence to termites, isoenzyme, and phylogenic analysis. Microbiol Res. 2007;162:53-61.
- [Google Scholar]
- Pathogenicity of a new China variety of M. anisopliae (M. anisopliae var. dcjhyium) to subterranean termite Odontotermes formesanus. Microbiol Res. 2009;164:27-35.
- [Google Scholar]
- Toxicity testing of destruxins and crude extracts from the insect-pathogenic fungus M. anisopliae. FEMS Microbiol Lett. 2005;251:23-8.
- [Google Scholar]
- A quick method for M. anisopliae isolation from cultural soils. Am J Agri Biol Sci. 2009;4:152-5.
- [Google Scholar]
- A sensitive bioassay for destruxins, cyclodepsipeptide from the culture filtrates of the entomopathogenic fungus M. anisopliae Sorok. An Soc Entomol Bras. 1998;27:229-35.
- [Google Scholar]
- Fluorometric quantification of DNA in cells and tissue. Anal Biochem. 1983;131:538-47.
- [Google Scholar]
- Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974;13:2633-7.
- [Google Scholar]
- Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Nati Acad Sci USA. 1980;77:5201-4.
- [Google Scholar]
- Management of chronic hepatitis B patients: efficacy & limitation of nucleos(t)ide analogues. Indian J Med Res. 2011;133:11-3.
- [Google Scholar]
- Synthesis and anti-hepatitis B virus activity of novel class of thiazolylbenzimidazole derivatives. Arch Pharm Chem Life Sci. 2011;2:78-83.
- [Google Scholar]
- Inhibition of hepatitis B virus replication by cIAP2 involves accelerating the ubiquitin-proteasome-mediated destruction of polymerase. J Virol. 2011;85:11457-67.
- [Google Scholar]
- Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79:7269-72.
- [Google Scholar]
- Inhibition of hepatitis B virus replication by hepatocyte nuclear factor 4-alpha specific short hairpin RNA. Liver International. 2012;32:742-51.
- [Google Scholar]
- Study of structure-activity correlation in destruxins, a class of cyclodepsipeptides possessing suppressive effect on the generation of HBV surface antigen in human hepatoma cells. Biochem Biophys Res Commun. 1996;229:65-72.
- [Google Scholar]






