Translate this page into:
Incidence & outcomes of clinically significant bleeding events in critically ill COVID-19 patients receiving Therapeutic dose AntiCoagulanTs: A retrospective cohort study (INTerACT study)
For correspondence: Dr Venkata Ganesh, Department of Anaesthesia & Intensive Care, Postgraduate Institute of Medical Education & Research, Sector 12, Chandigarh 160 012, India e-mail: bjfiero@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The high mortality associated with the thrombotic events in hospitalized COVID-19 patients resulted in the usage of anticoagulants in varying doses. Whether high-dose anticoagulants have led to better outcomes or higher incidence of clinically significant bleeding events is debatable. Thus, this study was conducted to find the incidence of clinically significant bleeding events in moderate-to-severe COVID-19 ARDS (acute respiratory distress syndrome) patients on therapeutic anticoagulation and their outcomes.
Methods:
In this retrospective, single-centre study of 155 critically ill COVID-19 patients, the incidence of clinically significant bleeding was observed. Multivariate regression models were used to evaluate the association between anticoagulant regimen, coagulation and inflammatory markers with the incidence of bleeding and thrombotic events.
Results:
The incidence of clinically relevant non-major bleeding was 33.54 per cent (26.17-41.46%) and major bleeding was 9.03 per cent (5.02-14.69%). The anticoagulation intensity at baseline had a high odds of major bleeding when enoxaparin and dual antiplatelet therapy were used together [adjusted odds ratio OR of 434.09 (3.81-49502.95), P<0.05]. At admission, bleeders had a poorer PaO2/FiO2 ratio with more patients on invasive ventilation. At the time of bleeding, the bleeders had a higher D-dimer, ferritin, C-reactive protein and procalcitonin compared to non-bleeders. The subhazard ratio for death in bleeders was 3.35 (95% confidence interval, 1.97-5.65; P<0.001).
Interpretation & conclusions:
The incidence of bleeding in critically ill COVID-19 patients on therapeutic anticoagulation may increase with the severity of the disease as well as with concurrent use of dual antiplatelets. Major bleeding may also contribute to higher mortality.
Keywords
Anticoagulants
COVID-19
critical illness
haemorrhage – thrombosis
The COVID-19 disease caused by the novel coronavirus is a state of hyperinflammation and hypercoaguability1. The autopsy studies of COVID-19 patients suggested that pulmonary vascular thrombosis is one of the pathological hallmarks of the disease2. A high incidence of thrombotic events at other vascular beds was observed in patients with moderate to severe COVID-193. Hence, pre-emptive anticoagulation in hospitalized patients with COVID-19 is considered standard of care. However, the dosing strategy, as well as duration of anticoagulation, are a matter of debate and various trials recommend different doses of anticoagulation45.
Randomized trials have also demonstrated an increased bleeding incidence among hospitalized patients with COVID-19 with a recent meta-analysis reporting an incidence of 7.8 per cent56. The high incidence of bleeding may be attributed to the use of therapeutic anticoagulation or coagulopathy due to severe disease per se. The coagulation abnormalities manifest in form of prolongation of prothrombin time (PT) and activated partial thromboplastin time (aPTT), elevated D-dimers and fibrinogen. Thrombocytopenia has also been reported7. Despite the increasing awareness on bleeding risk in COVID-19, definitive clinical predictors of abnormal bleeding are not well established.
The present study was undertaken to retrospectively investigate the incidence of bleeding and thrombotic complications in patients with moderate-to-severe COVID-19 ARDS (acute respiratory distress syndrome) disease on therapeutic low-molecular-weight or unfractionated heparin. The incidence of major bleeding and clinically relevant non-major bleeding (CRNMB), risk predictors for bleeding and correlation with outcomes in terms of mortality and discharge were also studied using standard definitions89.
Material & Methods
Patients selection and dosing of therapeutic anticoagulation: After taking approval from the Institute Ethics Committee (Reference no NK/7438/Study/223), the data were retrieved from the hospital information system of admitted patients to intensive care unit (ICU) between March 1 and June 5, 2021 in the Postgraduate Institute of Medical Education & Research, Chandigarh, a dedicated COVID tertiary care centre in northern India. A total of 167 patients met the inclusion criteria. They were more than 18 yr of age with moderate-to-severe COVID-19 ARDS [P/F (PaO2/FiO2) ratio <200] confirmed by reverse transcription-polymerase chain reaction (RT-PCR), admitted to ICU and treated with therapeutic anticoagulation [low-molecular-weight heparin (LMWH) at curative dose (100 IU/kg/12 h SC based on actual weight, without exceeding 10,000 IU/12 h or unfractionated heparin (UFH) 500 IU/kg/24 h if creatinine clearance <30 ml/min)]. Twelve patients were excluded due to incomplete data. The patients who were already on prophylactic anticoagulant dosing (LMWH <100 IU/kg/24 h SC or UFH <500 IU/kg/24 h) at admission, who had any bleeding disorder or were bleeding at the time of admission were excluded (Fig. 1). The follow up was till discharge or death.

- Flow diagram showing the data collection and patients selection process.
Outcomes
Bleeding events: The primary outcome was the incidence of bleeding. The bleeding incidence was defined as major bleeding events and CRNMB events according to the International Society on Thrombosis and Haemostasis (ISTH) criteria89. Fatal bleeding, symptomatic bleeding in critical area or organ (intracranial, gastrointestinal, intra-abdominal and haemoptysis) or any bleeding causing a fall in haemoglobin level of 2 g/dl or more or leading to transfusion of two or more units of red cells were considered as major bleeding events. Any other minor bleeding event which required the attention of a healthcare professional was included in CRNMB.
Thrombotic events: Myocardial infarction was diagnosed utilizing clinical criteria plus biomarker elevation and electrocardiographic changes. The diagnosis of pulmonary thromboembolism was presumed based on echocardiographic findings with clinical correlation. Cerebrovascular accident was confirmed using computed tomography.
Data collection: The data were collected from the online hospital information system. Data collected included demographics, comorbidities, duration of hospital stay, status at discharge, need for intubation and mechanical ventilation, oxygen devices used at varying time points, blood investigations such as haemoglobin, D-dimer, PT, aPTT, INR, fibrinogen, platelet count, C-reactive protein (CRP), ferritin, procalcitonin and P/F ratio among others at different time points during the hospital stay. The incidence of bleeding was observed from the day of admission to the day of discharge.
Statistical analysis: We collected data for 155 patients. Statistical analysis was performed using Stata 15 (StataCorp. LLC. Stata Statistical Software: Release 15. College Station, TX, USA). Imputation methods were not used. The patients were divided into two groups, bleeders and non-bleeders. Bleeders were the patients who had any form of bleeding (both major bleed and CRNMB) during their hospital stay. The demographic characteristics, laboratory parameters were compared between the two groups using standard descriptive statistics (Table I). Univariate comparisons were made using the Chi-square test/Fisher’s exact test and Mann–Whitney U test for inter-group comparison as most continuous variables were skewed, as per Q-Q plots. Categorical and count data were presented as numbers and percentages. Continuous variables have been represented as median with interquartile range.
| Parameters | All patients (n=155) | Non-bleeders (n=103) | Bleeders (n=52) | P |
|---|---|---|---|---|
| Age (yr), median (IQR) | 50 (42-60) | 52 (42-61) | 48 (41.5-59) | 0.545 |
| BMI (kg/m2), median (IQR) | 25.72 (23.94-28.73) | 25.72 (23.94-28.41) | 25.79 (24.05-28.87) | 0.950 |
| Gender (n) (male/female) | 89/66 | 56/47 | 33/19 | 0.280 |
| DM, n (%) | 20 (12.90) | 14 (13.5) | 6 (11.54) | 0.719 |
| HTN, n (%) | 27 (17.42) | 16 (15.43) | 11 (21.15) | 0.384 |
| CKD, n (%) | 8 (5.16) | 4 (3.88) | 4 (7.69) | 0.312 |
| CLD, n (%) | 2 (1.20) | 0 (0.00) | 2 (3.85) | 0.046* |
| Hospital stay (days), median (IQR) | 8 (6-13) | 8 (6-11) | 14 (6-18) | <0.001* |
| Mortality, n (%) | 59 (38.06) | 24 (23.30) | 35 (67.31) | <0.001* |
| Intubated on admission, n (%) | 15 (9.68) | 9 (8.74) | 6 (11.54) | 0.578 |
| Mechanical ventilation, n (%) | 111 (71.61) | 67 (65.6) | 44 (84.62) | 0.014* |
| SOFA | 6 (4-8) | 5 (3-7) | 7 (4-8) | 0.005* |
| Number of days of mechanical ventilation, median (IQR) | 3 (0-8) | 2 (0-4) | 8.5 (3-13.5) | <0.001* |
| Anticoagulant use at baseline, n (%) | ||||
| Enoxaparin 100 IU/kg bd or heparin tds | 140 (90.32) | 96 (93.20) | 44 (84.62) | 0.200 |
| Enoxaparin 100 IU/kg bd and aspirin 75 mg od | 12 (7.74) | 6 (5.83) | 6 (11.54) | |
| Enoxaparin 100 IU/kg bd and DAPT | 3 (1.94) | 1 (0.97) | 2 (3.85) |
*Refers to significant difference. Comparisons were made using the Mann–Whitney U test and Chi-square test/Fisher’s exact test as appropriate. DAPT, dual anti platelet therapy; IQR, interquartile range; BMI, body mass index; DM, diabetes mellitus; HTN, hypertension; CKD, chronic kidney disease; CLD, chronic liver disease; SOFA, sequential organ failure assessment
Analysis of coagulation and inflammatory parameters and predictors of bleeding events and death: Univariate and multivariate regression models were used to evaluate whether the anticoagulant regimen, coagulation, biochemical parameters and inflammatory markers obtained at the time of admission and at the time of bleeding had any influence on the outcome as well as prediction of the incidence of overall bleeding. In addition, the influence of these markers and the incidence of bleeding on mortality and discharge rates were also assessed using competing risk regression and relevant sub-hazard ratios were presented. Each parameter was assessed in a univariable model and a multivariable model controlled for age, body mass index, the anticoagulant intensity (i.e. number of anticoagulants used, as detailed in Table I), the status of being intubated at baseline, P/F ratio at baseline and comorbidities. These parameters were selected based on domain knowledge that influence severity of disease as well as from variables that are significantly different (P<0.05) between groups at baseline. The goodness-of-fit and robustness of estimates were assessed using the Hosmer–Lemeshow and Omnibus tests. The laboratory parameters were not adjusted for each other. The thresholds used to categorize the markers were based on clinical relevance, the distribution of data and the standard reference ranges in the laboratory. Further, a generalized linear model (GLM) was selected, with five predictors (11 reduced to 5 after recursive feature elimination), as the best performing model from K-fold cross-validation repeated 10 times on 80 per cent of the dataset as a training set. The model performance was then evaluated on the remaining 20 per cent of the dataset as the test dataset.
Results
There was no difference in demographic characteristics in patients with or without bleeding (Table I). The study included a total of 1577 patient-days (225 patient-weeks) analyzed including 866 patient-days (124 patient-weeks) in the non-bleeder group and 711 patient-days (102 patient-weeks) in the bleeder group.
Bleeding events and event rates: The overall frequency of patients with any form of bleeding was 33.54 per cent [95% confidence interval (CI), 26.17-41.56%, 132 bleeding events in 52 of 155 patients] or an event rate of 8.37 (95% CI, 7.03-9.89) per 100 patient-days. This included CRNMB in 33.5 per cent (95% CI, 26.17-41.46%, 109 CRNMB events in 52 of 155 patients) or an event rate of 6.91 (95% CI, 5.70-8.30) per 100 patient-days. The frequency of patients with major bleeding was 9.03 per cent (95% CI, 5.0-14.69%, 23 major bleeding events in 14 of 155 patients) or an event rate of 1.46 (95% CI, 0.94-2.15) per 100 patient-days. Major bleeding events are summarized in Table II.
| Serial number | Age/gender | Outcome | Platelets on the day of bleeding (×109/l) | Anticoagulation on day of major bleed | Type/site of major bleed | Comments |
|---|---|---|---|---|---|---|
| 1 | 45/male | Death | 254.5 | MT | ET bleed | Required ET tube change once |
| 2 | 38/male | Death | 91 | MT | ET and upper GI bleed | Had overt DIC at the time of bleeding, needed triple vasopressor |
| 3 | 38/male | Death | 144 | MT | Lower GI bleed | |
| 4* | 51/male | Alive | 191 | UFH bd | Lower GI and Haemoptysis | Recurrent, controlled with transfusions, ** sepsis |
| 5*,# | 69/male | Death | 175 | UFH bd | Haematuria | Recurrent, septic shock |
| 6 | 35/male | Death | 101 | Enoxaparin bd | Catheter site and ET bleed | **Septic shock on 2 vasopressors |
| 7ᴪ | 34/male | Death | 351.5 | Enoxaparin bd | Lower GI bleed | |
| 8*,¥ | 68/male | Death | 66.5 | Enoxaparin bd | Epistaxis and ET bleed | Recurrent |
| 9*,# | 80/female | Death | 36 | MT | Lower GI bleed | Had overt DIC at the time of bleeding |
| 10 | 29/female | Death | 176.5 | Enoxaparin bd | Upper GI bleed and epistaxis | CT angiography revealed diffuse upper GI bleed, was in severe** sepsis with procalcitonin over 30 ng/ml |
| 11* | 60/male | Death | 345.5 | Enoxaparin bd | Upper GI and ET bleed | |
| 12*,¥ | 52/male | Death | 87 | MT | Haematuria and ET bleed | Recurrent |
| 13ᴪ,€ | 45/female | Death | 169.5 | MT | ET bleed and haematuria | Was in ** sepsis with procalcitonin of 3.1 at the time of bleeding |
| 14 | 65/female | Death | 123.5 | MT | Lower GI bleed | **Septic shock on 2 vasopressors |
**The diagnosis of secondary sepsis was made with raised total leucocyte counts, cultures and procalcitonin; ¥CKD; €CLD; #DM; *HTN; ᴪObesity. DM, diabetes mellitus; HTN, hypertension; CKD, chronic kidney disease; CLD, chronic liver disease; MT, mechanical thromboprophylaxis; UFH, unfractionated heparin; GI, gastrointestinal; ET, endotracheal; bd, twice daily; od, once daily; tds, thrice daily
Disseminated intravascular coagulation and thrombocytopenia: Three patients had overt disseminated intravascular coagulation (DIC) as per the International Society in Thrombosis and Haemostasis (ISTH) scientific subcommittee scoring system for DIC10. Two of them had major bleeding. One patient who had a score of six did not have any form of bleeding. However, at admission, he was on two vasopressors and died within 24 h of ICU admission. Despite an overall frequency of bleeding of 33.54 per cent, none of the patients had a fibrinogen <1 g/l at any point during the hospital stay. Thrombocytopenia with platelets <100×109 and <50×109 was present in 8.39 (13/155) and 2.58 (4/155) per cent of all patients, respectively, at admission. Among those who had any form of bleeding platelets were <100×109 and <50×109 in 9.62 (5/52) and 1.92 (1/52) per cent of patients, respectively, at the time of bleeding.
Coagulation and inflammatory parameter analysis: Table III lists the various coagulation and inflammatory markers at the time of admission and at the time of the bleeding event. At baseline, all laboratory parameters were similar between those who had no bleeding and those who had at least one bleeding event during their hospital stay. The bleeders, however, had a worse P/F ratio at baseline [median difference −26, (95% CI, −54.14-2.14), P=0.003]. This has been used as an adjustment variable in multivariate analysis. At the time of bleeding, however, the bleeders had a significant higher D-dimer [median difference 669, (95% CI, 261.28-1076.72)], CRP [median difference 42.4, (95% CI, 24.14-60.66)], ferritin [median difference 349, (95% CI, 81.2-616.8)] and procalcitonin [median difference 0.33, (95% CI, 0.01-0.65)]. There was no difference in platelets, PT, aPTT or fibrinogen at the time of bleeding between non-bleeders and bleeders. The spearman rank correlation coefficients between inflammatory markers are represented in Fig. 2.
| Parameters | All patients (155) | Non-bleeders (n=103) | Bleeders (n=52) | P |
|---|---|---|---|---|
| Parameters at admission, median (IQR) | ||||
| Haemoglobin (g/dl) | 11.5 (10.3-13.1) | 11.7 (10.3-13.1) | 11.3 (10.4-12.9) | 0.570 |
| D dimer (ng/ml) | 949.69 (606-2625) | 897 (606-2625) | 1021.71 (614.91-3264.19) | 0.273 |
| PT (sec) | 15 (14.1-15.9) | 15.1 (14.2-16) | 14.7 (13.7-15.6) | 0.114 |
| aPTT (sec) | 29.2 (26.6-31.5) | 29.1 (26.5-31.5) | 29.55 (27.4-31.7) | 0.465 |
| INR | 1.12 (1.05-1.2) | 1.14 (1.06-1.21) | 1.1 (1.04-1.18) | 0.155 |
| Fibrinogen (g/l) | 5.5 (4.7-6.4) | 5.53 (4.7-6.4) | 5.4 (4.8-6.5) | 0.696 |
| Platelet count (×109/l) | 212.5 (162.5-280) | 199 (156-266) | 238 (184-292) | 0.056 |
| CRP (mg/l) | 83 (45-129) | 84 (41.76-137) | 80.5 (53.32-112.85) | 0.782 |
| Ferritin (µg/ml) | 787 (424-1193) | 787 (409-1168) | 815 (486-1469) | 0.288 |
| Procalcitonin (ng/ml) | 0.23 (0.1-0.68) | 0.26 (0.1-0.72) | 0.19 (0.09-0.591) | 0.417 |
| P/F ratio | 120 (91-176) | 128.17 (100-187) | 103 (64-126) | 0.003* |
| Number on invasive ventilation, n (%) | 15 (9.68) | 9 (8.74) | 6 (11.54) | 0.557 |
| Parameters on the day of bleeding, median (IQR)‡ | ||||
| Haemoglobin (g/dl) | 10.83 (9.8-12.1) | 10.6 (9.8-11.7) | 11.3 (10.2-12.6) | 0.075 |
| D dimer (ng/ml) | 790.25 (401.78-1692.5) | 629.5 (326-1231) | 1305.5 (613.27-2284) | 0.002* |
| PT (sec) | 14.7 (14-15.68) | 14.65 (13.85-15.6) | 14.8 (14-15.8) | 0.332 |
| aPTT (sec) | 29.55 (27.6-32.6) | 29.8 (27.5-32.5) | 29.2 (27.6-33.6) | 0.765 |
| INR | 1.13 (1.05-1.23) | 1.13 (1.04-1.2) | 1.13 (1.06-1.25) | 0.388 |
| Fibrinogen (g/l) | 5.6 (4.9-6.38) | 5.61 (5-6.4) | 5.47 (4.8-6.38) | 0.709 |
| Platelet count (×109/l) | 242 (165-320) | 256 (178-312) | 191 (110-333) | 0.214 |
| CRP (mg/l) | 22.5 (6.08-71.31) | 12 (3.27-40.75) | 53.68 (21.37-105.30) | 0.001* |
| Ferritin (µg/ml) | 620.5 (282.5-988.5) | 507 (254-855.4) | 856 (442.5-1297.5) | 0.008* |
| Procalcitonin (ng/ml) | 0.15 (0.05-0.75) | 0.07 (0.04-0.24) | 0.4 (0.15-5.39) | <0.001* |
| P/F ratio | 141.5 (68-288.35) | 128 (67-306) | 178.63 (82.2-266) | 0.607 |
| Number on invasive ventilation, n (%) | 66 (42.58) | 25 (24.27) | 41 (78.85) | <0.001* |
‡For non-bleeders, this corresponded to the last day of hospital stay when the parameter was measured; *was considered significant. Comparisons were made using the Mann–Whitney U-test and Chi-square test/Fisher’s exact test as appropriate. IQR, interquartile range; PT, prothrombin time; aPTT, activated partial thromboplastin time; INR, international normalized ratio; CRP, c-reactive protein; P/F, PaO2/FiO2 ratio
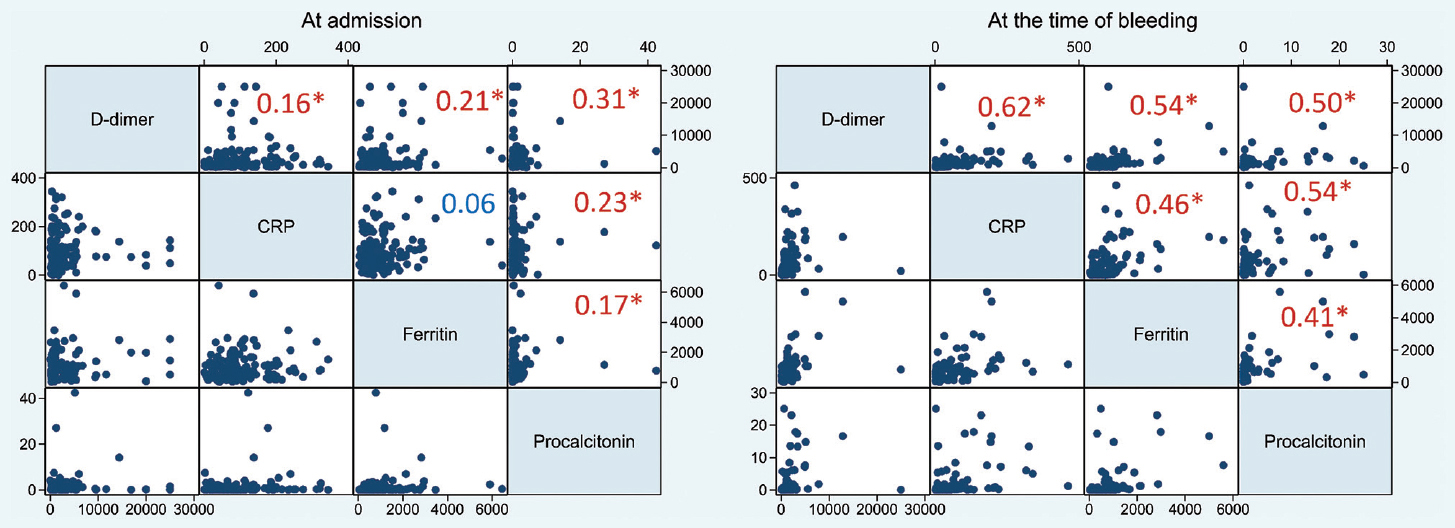
- Graph-matrix of inflammatory markers at the time of admission (left) and at the time of bleeding event (right). The large numbers inside the graph-matrix represent corresponding correlation coefficients (Spearman). Those marked with* have a P<0.05. D-dimer in ng/ml, CRP in mg/l, Ferritin µg/ml, Procalcitonin ng/ml. CRP, c-reactive protein.
Predictors of all bleeding events: In our dataset, none of the inflammatory or coagulation markers was significantly predictive of bleeding events. The markers were adjusted for anticoagulation intensity at baseline, but not for each other. Table IV lists the relevant adjusted and unadjusted odds ratios (OR).
| Parameter | Initial value | Number of cases | Number of non-cases | Any bleeding event | Major bleed | ||
|---|---|---|---|---|---|---|---|
| Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI)‡ | ||||
| Number of anticoagulants | Enoxaparin 100 IU/kg bd or UFH tds | 44 | 96 | 1 | 1 | 1 | 1 |
| Enoxaparin 100 IU/kg bd and aspirin 75 mg od | 6 | 6 | 2.18 (0.66-7.14) | 1.11 (0.26-4.78) | 2.6 (0.49-13.52) | 0.28 (0.01-5.88) | |
| Enoxaparin 100 IU/kg bd and DAPT | 2 | 1 | 4.36 (0.39-49.41) | 5.56 (0.38-81.1) | 26 (2.16-312)* | 434.09 (3.81-49502.95)* | |
| D dimer (ng/ml) | ≤300 | 2 | 9 | 1 | 1 | 1 | 1 |
| 300-2500 | 36 | 68 | 2.38 (0.49-11.62) | 1.67 (0.28-9.92) | 1.06 (0.12-9.19) | 0.54 (0.02-16.34) | |
| >2500 | 14 | 26 | 2.42 (0.46-12.8) | 1.38 (0.21-8.96) | 0.81 (0.08-8.67) | 0.17 (0.004-6.34) | |
| PT (sec) | ≤16 | 44 | 80 | 1 | 1 | 1 | 1 |
| >16 | 8 | 23 | 0.63 (0.26-1.53) | 0.57 (0.20-1.61) | 1.1 (0.28-4.22) | 4.17 (0.63-27.33) | |
| aPTT (sec) | ≤40 | 49 | 103 | Omitted due to collinearity and perfect prediction of outcome (by aPTT ≤40) | 1 | 1 | |
| >40 | 3 | 0 | 5.34 (0.45-63) | 8.03 (0.41-156.77) | |||
| INR | ≤1.2 | 44 | 75 | 1 | 1 | 1 | 1 |
| >1.2 | 8 | 28 | 0.49 (0.2-1.16) | 0.46 (0.16-1.34) | 0.52 (0.11-2.46) | 1.36 (0.17-10.76) | |
| Fibrinogen (g/l) | <4.5 | 6 | 17 | 0.66 (0.24-1.78) | 0.81 (0.26-2.56) | 0.41 (0.05-3.34) | 0.17 (0.01-3.94) |
| ≥4.5 | 46 | 86 | 1 | 1 | 1 | 1 | |
| Platelet count (×109/l) | <75 | 4 | 5 | 1.63 (0.42-6.36) | 1.66 (0.34-8.02) | 1.27 (0.14-11.03) | 7.11 (0.52-96.52) |
| ≥75 | 48 | 98 | 1 | 1 | 1 | 1 | |
| CRP (mg/l) | ≤50 | 13 | 30 | 1 | 1 | 1 | 1 |
| >50 | 39 | 73 | 1.23 (0.58-2.93) | 0.82 (0.34-1.92) | 0.96 (0.28-3.22) | 0.32 (0.05-1.64) | |
| Ferritin (µg/ml) | ≤500 | 15 | 31 | 1 | 1 | 1 | 1 |
| >500 | 37 | 72 | 1.06 (0.51-2.21) | 0.76 (0.33-1.75) | 2.72 (0.58-12.68) | 1.24 (0.21-7.28) | |
| Procalcitonin (ng/ml) | ≤0.5 | 38 | 70 | 1 | 1 | 1 | 1 |
| >0.5 | 14 | 33 | 0.78 (0.37-1.63) | 0.67 (0.28-1.59) | 1.82 (0.59-5.6) | 1.9 (0.44-8.35) | |
‡The adjusted binary logistic regression models were a significant improvement over the null model; P*<0.05. Variables used in the adjusted models include age, BMI, comorbidities, P/F ratio on day 1, status of being intubated on day 1. The markers were not adjusted for each other but were adjusted for the number of anticoagulants at baseline and the above-mentioned variables. BMI, body mass index; PT, prothrombin time; aPTT, activated partial thromboplastin time; INR, international normalized ratio; CRP, c-reactive protein; DAPT, dual anti platelet therapy; UFH, unfractionated heparin; CI, confidence interval
The anticoagulation intensity at baseline had a very high odds of major bleeding when enoxaparin and dual antiplatelet therapy were used together [adjusted OR of 434.09 (95% CI, 3.81-49502.95), P<0.05]. As the number of days on assisted ventilation increased, patients were at an odds of 1.23 (95% CI, 1.14-1.33, P<0.001) of developing any bleeding event. The adjusted OR came to 1.19 (95% CI, 1.09-1.29, P<0.001). This could be interpreted as the odds of bleeding increased with the increasing severity of critical illness.
K-fold cross-validation was done with 15 folds, repeated 10 times on the training dataset (80% of the 155 samples) with recursive feature elimination on 10 admission predictors [platelet count, SOFA scores, P/F ratio, Charlson Comorbidity Index (CCI), D-dimer, procalcitonin, age, gender, CRP and anticoagulation intensity (categorized as heparins + DAPT and only heparins). These predictors were chosen based on domain knowledge and after assessing other parameters for colinearity. Variables which had high internal correlation variables (oxygen device at admission, PT, INR and APTT) were excluded from the assessment. The models assessed with these 10 variables included a generalized linear model, random forest, elastic net and simulated annealing. Of these, the generalized linear model (binary logistic regression, GLM) gave the best accuracy of 67.2 per cent. The top five predictors after recursive feature elimination were platelet count, SOFA score, P/F ratio, CCI and D-dimer at admission. A reduced GLM using these five predictors was then applied to the training dataset (125 samples) with 15 fold cross-validation which yielded an accuracy of 70.9 per cent. The predicted probabilities from this model were assessed on the remaining test dataset which gave a prediction accuracy of 70 per cent (95% CI 50.6-85.3%). This model was then applied to the full dataset of 155 samples and yielded a prediction accuracy for bleeding as 70.97 per cent (95% CI 63.14-77.97%), positive predictive value of 62.96 per cent and a negative predictive value of 72.66 per cent. Thus, a lower platelet count, lower P/F ratio, higher SOFA score and CCI at admission can predict a high chance of having a bleeding event. D-dimer had the least contribution among the five variables in the predictive model.
Trend analysis of inflammatory markers: Data of serum levels of ferritin, procalcitonin, D-dimer and CRP were collected on the day of admission to ICU, days three, seven, 14 and the last day of ICU stay. A post-hoc generalized estimating equations model with time, group (non-bleeder/bleeder) and group time interaction as covariates were applied to each marker with gamma distribution, identity link function and an exchangeable correlation matrix for repeated measures. The within-group repeated measures were Bonferroni adjusted. Ferritin did not show any significant change over time (P=0.127) or between groups (P=0.148). Ferritin continued to remain high among bleeders even on the last day of ICU stay with a difference from baseline of −169.09 (95% CI, −454.57-776.47, P=0.25). There was no significant difference in procalcitonin at any time point from baseline in either group.
D-dimer did not show any significant change over time (P=0.903) or between groups (P=0.958). There was a significant decrease in D-dimer on day 14 compared to baseline among the non-bleeders (−1139.98, 95% CI −2207.9-−72.08, P=0.02). CRP showed a significant reduction from baseline among the non-bleeders by day seven (−55.10, 95% CI −76.30-−33.89, P<0.001) and this decreasing trend continued till the last day of hospital stay. Among the bleeders, CRP continued to remain high (last day CRP – admission CRP of −8.48, 95% CI −39.53-22.69, P=0.59). This suggested that a persistent heightened inflammatory state could have contributed to bleeding events.
Mortality: The overall mortality was 38.06 per cent (67.3% in bleeders and 23.3% in non-bleeders). Those with major bleeding had a mortality of 92.86 per cent and those with exclusive CRNMB had a mortality of 57.89 per cent. This is summarized in Figure 3A.
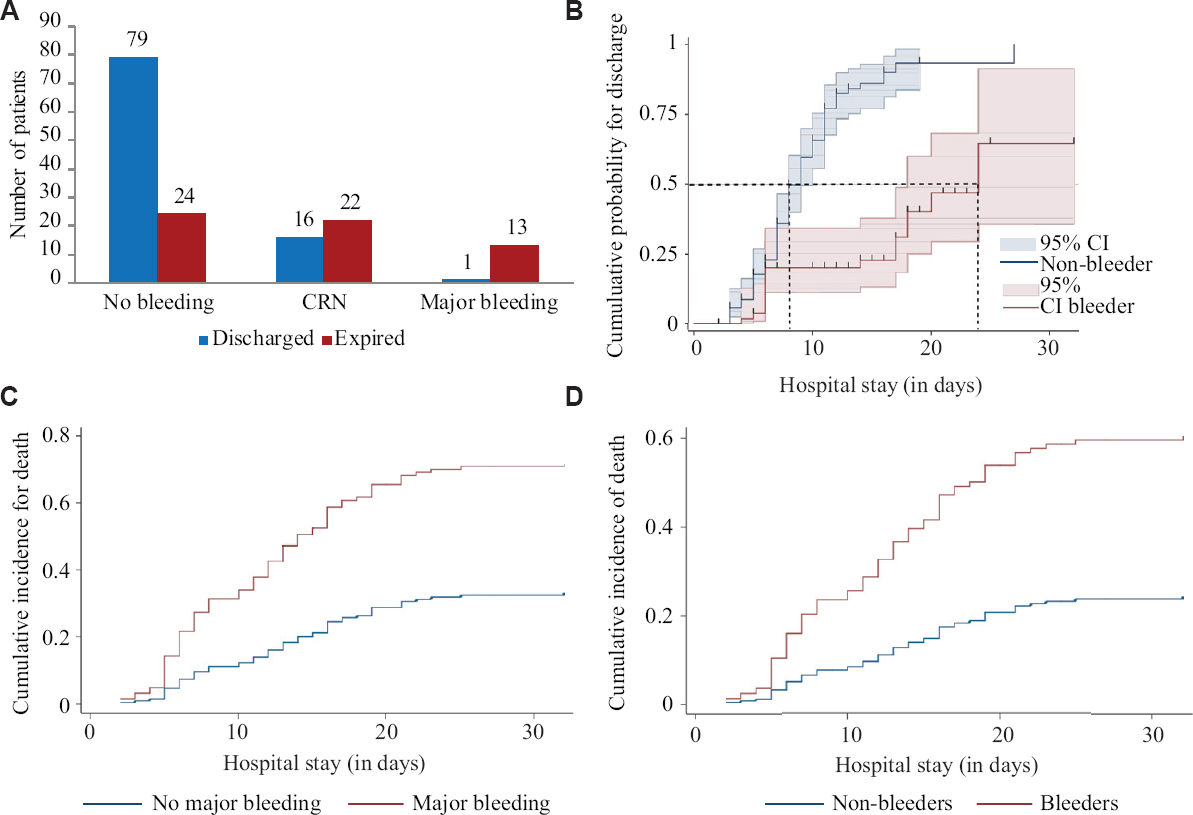
- (A) Bar chart showing outcome (discharge vs. expired) classified by bleeding status; (B) 1 minus Kaplan–Meier estimates showing the cumulative probability of discharge among bleeders versus non-bleeders; (C) Competing-risk regression curves for death (the competing event being discharge) in major bleeders and the rest of the patients; (D) Competing-risk regression curves for death (the competing event being discharge) in all bleeders and non-bleeders.
Survival estimates: Median time to discharge was 11 (95% CI, 9-12) days [mean time 13.85 days (95% CI, 12.1-15.7)] in the overall patients with bleeders having a mean time to discharge of 21.47 (95% CI, 17.78-25.26) days versus non-bleeders at 9.9 (95% CI, 8.6-11.2) days (Log-rank P<0.001) (Fig. 3B). All log-rank tests had a P<0.05, suggesting the probability of discharge was higher among non-bleeders.
Competing risks regression for the outcome of death: Competing-risks regression analysis with death as the dependent variable and discharge as the competing event gave subhazard ratio (SHR) for death in bleeders as 3.35 (95% CI, 1.97-5.65; P<0.001). In those with major bleeding, the SHR for death was 3.14 (95% CI, 2.1-4.69; P<0.001) and in those with CRNMB it was 3.35 (95% CI, 1.98-2.66; P<0.001) (Fig. 3C and D). These outcomes were not adjusted for each other. The models used in these regression analyses were all significantly better than the null at P<0.001.
Thrombotic events and event rates: Eight of 155 (5.16%) patients had thrombotic events. Six of these patients did not have any concomitant bleeding event, one had CRNMB and one had a major bleeding event. Both these patients who had a thrombotic event, a form of bleeding event had expired. Of those eight patients with thrombosis, four (50%) had pulmonary embolism, two each (25%) had cerebrovascular accidents and two myocardial infarction. None of the patients with thrombosis had DIC at the time of the thrombotic event. Mortality was 6/8 (75%) in those with thrombosis versus 53/147 (36.1%) in those without thrombosis (Chi-square test, P=0.027).
Predictors of thrombotic events: As thrombotic events were not the main focus of this paper, the frequency of events and adjusted OR are depicted in Figure 4 A and B.
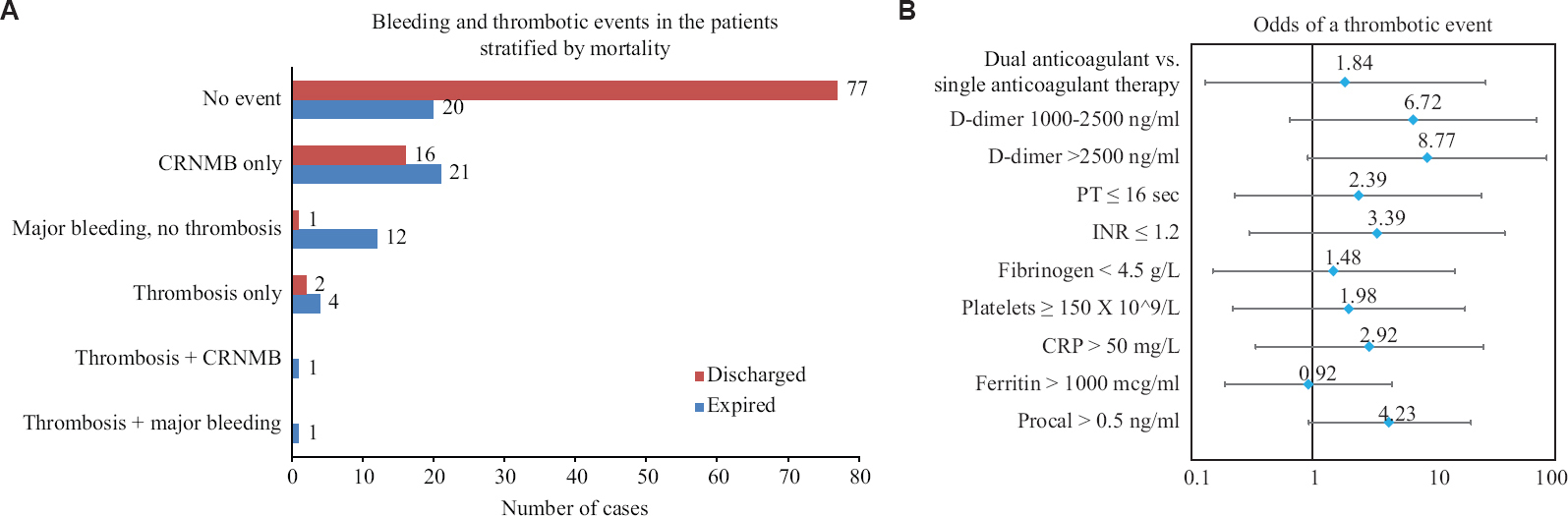
- (A) Bar chart showing outcomes (discharged vs. expired) with respect to bleeding and thrombotic events (B) Forest plot showing adjusted odds (95% CIs) of developing a thrombotic event. Odds ratios have been obtained from binary logistic regression, adjusted for age, body mass index, comorbidities, PaO2/FiO2 ratio on day one and status of being intubated on day one. The markers were not adjusted for each other but were adjusted for the number of anticoagulants at baseline and the above-mentioned variables; Dual anticoagulant - heparin + aspirin or heparin + aspirin and clopidogrel. Single anticoagulant - enoxaparin or unfractionated heparin (all at therapeutic doses). CI, confidence interval.
Discussion
The presence of COVID-19-associated coagulopathy11 and the high incidence of thromboembolism12 despite being on prophylactic anticoagulation has forced the practice of therapeutic anticoagulant dosing by many of institutions across the world13. Although there are studies proving the benefit of this therapeutic regimen in a few subsets of COVID-19 patients1415 like the elderly and those on mechanical ventilation, there appears to be an increased risk of bleeding in patients who were given this regimen16.
This study was aimed to determine the incidence and factors associated with such bleeding events in patients with moderate-to-severe COVID-19 disease who were receiving therapeutic dose of anticoagulants. In our 155 total patients, the incidence was 33.54 per cent for any form of bleeding, CRNMB in 33.5 per cent and major bleeding of 9.03 per cent. These were higher than most of the other studies reported17; however, it would be prudent to note that majority (71% at admission) of our patients were those with severe COVID-19, were critically ill and on therapeutic anticoagulation. Several studies have demonstrated an association between increased incidence of bleeding events and higher intensity of anticoagulation18-21 as well as higher D-dimer and ferritin levels192122. This was also in line with our study findings although the CIs were wide. Based on our results, the suggestion would be to avoid the use of therapeutic doses of a heparin-based anticoagulant in combination with antiplatelet therapy.
The high proportion of gastrointestinal bleeding (8.3%, 13/155 patients, 19 events) may be correlated with the severity of illness rather than anticoagulant use per se. This was difficult to comment on from our dataset as we did not compare with those not on anticoagulants. We would suggest the use of aggressive stress ulcer prophylaxis as many of those with COVID-acute respiratory distress syndrome (ARDS) would also be on high dose or prolonged steroid therapy.
There was a poor association of conventional coagulation parameters such as PT, aPTT and INR with bleeding while there were elevations in the inflammatory markers D-dimer, ferritin, procalcitonin and the incidence of mechanical ventilation among the bleeders. K-fold cross-validation also revealed that platelet count [correlation coefficient −0.44 (SE −2.08), P=0.04] and P/F ratio [correlation coefficient −0.65 (SE 0.32), P=0.04] at admission to the ICU were the best predictors of having a bleeding event while CCI, SOFA score and D-dimer had a non-significant association in decreasing order of importance. The intensity and type of anticoagulation did not have any predictive value on the incidence of bleeding.
This had indicated that the bleeding associated with COVID-19 is a function of severe disease and inflammation rather than being related to only the use of therapeutic anticoagulation. Similar to the thromboinflammation theory23 in COVID-19, it is likely that this higher incidence of bleeding noted in our patients could be due to an inflammation-mediated qualitative dysfunction in coagulation. Viscoelastic methods such as thromboelastography might be better at detecting those at risk of bleeding and consequently help titrate the anticoagulation intensity.
In our study, the bleeders had higher all-cause mortality of 67.31 per cent against 23.3 per cent in non-bleeders. When adjusted for comorbidities, age, BMI and inflammatory markers at the time of bleed, any bleeding event had an odds of death of 4.13 (95% CI, 1.39-12.29, P=0.011) on binary logistic regression. When the status of being on invasive ventilation, P/F ratio at the time of bleeding was added to the model, this association became non-significant 4.81 (0.06-382.5, P=0.482). When adjusted for the severity of COVID-19 ARDS, the presence of a major bleed, presence of only CRNMB and the presence of a thrombotic event did not significantly elevate the odds of death. This would suggest that the severity of hypoxia and inflammation associated with COVID-ARDS may play a major role in the incidence of bleeding and may influence the mortality more than the isolated presence of a bleeding or thrombotic event.
Our study was prone to the inherent limitations of a retrospective study. We did not perform any sensitivity analysis to confirm the robustness of the association between bleeding and inflammatory markers. Not all patients who experienced mortality were imaged for embolic events. There may be over or under-reporting of events. Pulmonary bleeding might have been underdiagnosed as not all patients underwent bronchoscopy and this haemorrhage might have been misinterpreted as worsening infection or ARDS. This was a single-centre study in critically ill COVID-19 patients and the results may not be generalizable to all subsets of COVID-19 severity.
Our findings suggest avoiding the use of therapeutic doses of heparin-based anticoagulants in combination with oral antiplatelet agents. Our findings also suggest that bleeding in critically ill COVID-19 is probably associated more with the severity of illness (ARDS/hypoxia) and inflammation, as suggested by K-fold cross-validation, P/F ratio at admission and CRP trends, rather than purely anticoagulant use. Monitoring qualitative coagulation parameters using techniques such as thromboelastography will probably provide more insight into the risk of bleeding than conventional coagulation parameters.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- COVID-19-associated hyperviscosity:A link between inflammation and thrombophilia? Lancet. 2020;395:1758-9.
- [Google Scholar]
- Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine. 2020;61:103104.
- [Google Scholar]
- Incidence of thromboembolism in patients with COVID-19:A systematic review and meta-analysis. Thromb J. 2020;18:34.
- [Google Scholar]
- Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19:A propensity score-matched analysis. Am J Hematol. 2021;96:471-9.
- [Google Scholar]
- Effect of intermediate-dose vs. standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the Intensive Care Unit:The INSPIRATION randomized clinical trial. JAMA. 2021;325:1620-30.
- [Google Scholar]
- Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019:A systematic review and meta-analysis. Chest. 2021;159:1182-96.
- [Google Scholar]
- Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692-4.
- [Google Scholar]
- Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients:Communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119-26.
- [Google Scholar]
- Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327-30.
- [Google Scholar]
- COVID-19-associated coagulopathy:An exacerbated immunothrombosis response. Clin Appl Thromb Hemost. 2020;26:1076029620943293.
- [Google Scholar]
- High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18:1743-6.
- [Google Scholar]
- The use of therapeutic-dose anticoagulation and its effect on mortality in patients with COVID-19:A systematic review. Clin Appl Thromb Hemost. 2020;26:1076029620960797.
- [Google Scholar]
- Therapeutic anticoagulation is associated with decreased mortality in mechanically ventilated COVID-19 patients. medRxiv 2020 Doi: https://doi.org/10.1101/2020.05.30.20117929
- [Google Scholar]
- Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:122-4.
- [Google Scholar]
- Impact of anticoagulation prior to COVID-19 infection:A propensity score-matched cohort study. Blood. 2020;136:144-7.
- [Google Scholar]
- Thrombosis, bleeding, and the observational effect of early therapeutic anticoagulation on survival in critically ill patients with COVID-19. Ann Intern Med. 2021;174:622-32.
- [Google Scholar]
- Impact of thromboprophylactic strategy on bleeding risk among in- and outpatients with COVID-19. J Am Coll Cardiol. 2021;77:3111.
- [Google Scholar]
- Bleeding risk in hospitalized patients with COVID-19 receiving intermediate- or therapeutic doses of thromboprophylaxis. J Thromb Haemost. 2021;19:1981-9.
- [Google Scholar]
- The hazard of (sub)therapeutic doses of anticoagulants in non-critically ill patients with COVID-19:The Padua province experience. J Thromb Haemost. 2020;18:2629-35.
- [Google Scholar]
- Empiric use of anticoagulation in hospitalized patients with COVID-19:A propensity score-matched study of risks and benefits. Biomark Res. 2021;9:29.
- [Google Scholar]
- Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION):An open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253-63.
- [Google Scholar]
- Thromboinflammation:Challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133:906-18.
- [Google Scholar]






