Translate this page into:
Incidence of intestinal & extra-intestinal cancers among individuals with Crohn’s disease in northern India
Present address: *Department of Internal Medicine, University of Missouri-Kansas City, Missouri, U.S.A;
†Center of Global Health Research, Nuffield Department of Medicine, University of Oxford, United Kingdom
For correspondence: Dr Vineet Ahuja, Department of Gastroenterology and Human Nutrition, All India Institute of Medical Sciences, New Delhi 110 016, India e-mail: vineet.aiims@gmail.com
-
Received: ,
Abstract
Background & objectives
Crohn’s disease (CD) is associated with a higher risk of malignancy, which is attributed to disease behaviour and the usage of immunosuppressants. The burden of malignancy in CD is scarcely reported from Asia. We report real-world data on CD-related malignancy from a northern Indian cohort.
Methods
This retrospective analysis included individuals with CD who were followed up at the All India Institute of Medical Sciences, New Delhi, from 2005 to 2021. The standardized incidence ratio (SIR) was used to calculate the relative risk of malignancy in CD affected individuals compared to the general population.
Results
In this study, 952 study participants were included, with a mean age at diagnosis of 36.9±15.11 yr; 61.1 per cent were male. The median follow-up duration was 34 months [IQR (interquartile range): 19-73]. Most study participants received steroids (76.7%), immunomodulators (68.7%), or anti-TNF therapy (10.8%). The overall incidence of malignancy was 1.05 per cent, indicating a 10.45 times higher risk in CD [SIR: 10.45; 95% Confidence interval (CI):4.98-17.96]. Eight out of 826, 1 of 106 and 1 of 25 study participants developed malignancy in the first, second and third decades, respectively. The cumulative risk of malignancy was 2.7, 5.5, and 13.4 per cent in the first, second, and third decades, respectively. Regarding bowel malignancies, one study participant each developed ileocaecal adenocarcinoma, anorectal adenocarcinoma, malignant rectal fibrous histiocytoma, and gastric adenocarcinoma. Extraintestinal malignancies included single cases each of follicular neoplasia of the thyroid, neuroendocrine tumour of the pancreatic tail, breast cancer, hepatocellular cancer, oral cancer, and prostate cancer. No cases of lymphoma or skin malignancy were reported.
Interpretation & conclusions
At 30 yr, the cumulative risk of malignancy among Indian CD-affected individuals was 13.4 per cent, with a SIR of 10.45 (95% CI: 4.98- 17.96). The risk increased with increasing age at disease onset and duration.
Keywords
Malignancy
Crohn’s disease
intestinal cancer
extra-intestinal cancer
Rudolf Virchow, in 1863, linked inflammation with cancer when he observed leukocytes in the cancer tissue1. Following this astute observation, numerous inflammatory conditions have been causally associated with cancers such as Helicobacter pylori associated gastritis (gastric cancer), viral hepatitis (hepatocellular cancer), and chronic pancreatitis (carcinoma pancreas)2. Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic inflammatory condition of the gastrointestinal tract. In India and other nations in the Asia-Pacific areas, the prevalence of IBD is rapidly rising3,4. As the burden increases and more efficient treatment alternatives become available, clinicians treating these patients are increasingly encountering complications of long-standing IBD, such as intestinal as well as extra-intestinal cancers, which contribute substantially to this disease’s morbidity and mortality.
CD is more heterogeneous in terms of the extent and depth of intestinal involvement between CD and UC. Hence, the malignant complications in CD are also more heterogeneous. Individuals with CD are at an increased risk of intestinal malignancies other than colitis-associated cancers, such as fistula-associated cancers and small bowel adenocarcinoma/ lymphoma, which are related to the extent of inflammation, severity, and course of the disease5,6. The risk of extra-intestinal cancers such as of haematological system, skin, hepatobiliary system and urogenital cancers is also supposed to be elevated and is attributed to long-term immunosuppressant use, especially thiopurines and anti-TNF agents. Patients with CD have also been shown to have higher risks of lymphomas and malignancies of the stomach, lungs, breast, bladder, kidney, brain, non-melanoma skin, thyroid, and pancreas7,8.
The relative risks for colorectal cancer (CRC) in CD patients vary from 1.5-39,10. CD was shown to be a risk factor for intestinal cancer in a meta-analysis involving more than 40,000 patients, and the incidence of CD-associated malignancies was 0.8/1,000 pyd (person-yr duration), with risk of colorectal and small bowel malignancy being increased by factor of 2-3, and 18.7, respectively10. However, in recent reviews and cohort studies, the risk of cancer in IBD was either of limited magnitude or similar to the general population, adding to the heterogeneity in terms of absolute and relative risk of malignancy in patients with CD2,6,11. Moreover, the reports on the cancer burden in IBD (especially CD) from India and other developing countries are relatively few as compared to the West. Therefore, more studies are required from this part of the world to provide information on the cancer risk and, thus, preventive and surveillance strategies to reduce the cancer-associated morbidity and mortality in patients with CD. Hence, through this retrospective study, we aimed to determine the burden of malignancy in affected individuals with CD, focussing on the standardized incidence ratio (SIR), incidence, and cumulative risk of developing malignancy.
Material & Methods
This retrospective cohort study was undertaken at the department of Gastroenterology and Human Nutrition, All India Institute of Medical Sciences (AIIMS), New Delhi, India, after obtaining the ethical clearance from the Institutional Review Board.
Study population
All CD affected individuals who visited the IBD clinic from July 2005 to December 2021 at the AIIMS, New Delhi, India, were included. Study participants were monitored from the time of their IBD diagnosis until the first cancer diagnosis, their death, or December 2021, whichever came first. Study participants lost to follow up were censored at their last follow up, and their follow up duration was considered only until the last follow up.
Study design and sample evaluation
This study was a retrospective cohort study of a database maintained prospectively. Information was gathered from the IBD clinic’s records, and the study participants were also personally interviewed during their clinic visits. The IBD clinic maintains a longitudinal database comprising all data pertaining to the individual’s condition, including a thorough history, clinical examination, pertinent test results, and follow-up symptom evaluation. To assess the prevalence of malignancy in long-standing CD, study participants’ data were collected on demographic parameters, disease phenotype, age at onset of CD, age at onset of malignancy, duration of disease, duration of follow-up, and endoscopic or histological evidence of malignancy, type of malignancy and their outcomes.
Outcome measures
The primary outcome of the study was to estimate the SIR and incidence of malignancy in affected individuals with CD. Secondary outcomes included types of malignancy and their outcomes, as well as the cumulative probability of developing malignancy.
Statistical analysis
For each variable, descriptive statistics, including mean, standard deviation (SD), median, interquartile range (IQR), and frequency distribution were calculated. Data were presented as mean SD or median (IQR) when appropriate. Depending on whether the quantitative variables had a normal or non-normal distribution, the Mann-Whitney U test or Student’s t-test was used for comparing the two groups, and Fischer’s exact test or chi-square test was used for comparing categorical data. Using Kaplan-Meier analysis, the cumulative incidence risk was estimated using the starting point as the time of symptom onset and the ending point as the time of malignancy identification or last follow-up for study participants without cancer; STATA-14 was used for the analysis. The SIR, which is a ratio of the observed to the expected cases, was used to calculate the relative risk of malignancy in CD affected individuals. The confidence intervals (CIs) of the SIR were calculated using standard equation12.
The calculation of SIR was done using the following formula:
Step 1: Incidence rate = (Number of new cancer cases among Crohn’s affected individuals) / (Total number of Crohn’s affected individuals)*100
Step 2: Convert the incidence rate to the incidence per lakh population. Incidence per lakh population = Incidence rate * 1000 Incidence per lakh population
SIR = (Observed incidence in Crohn’s disease) / (Expected incidence in the general population)
The following data was included to calculate the SIR: In a recent study, the research estimated ⁓1,461,427 incident cases of cancer in India during the yr 2022, with a crude rate of 100.4 per 100,000 population13.
Results
Between July 2005 and December 2021, 952 CD affected individuals were registered at the Inflammatory bowel disease (IBD) clinic under the department of Gastroenterology and Human Nutrition, AIIMS, New Delhi. The database of the cohort of CD affected individuals was used to calculate the likelihood of malignancy.
Baseline demographics of the cohort
The mean age of the study population at the onset and diagnosis of CD was 33.4±14.65 and 36.9 ± 15.11 yr, respectively. Of the study participants, 582 (61.1%) were males, and the median follow up duration was 34 (IQR: 16-73) months.
Among the overall cohort, in the majority [565 (59.3%)], the disease was diagnosed between 17–40 yr, with an almost equal number of study participants with inflammatory (441; 46.3%) and stricture (442; 46.4%) phenotypes. Sixty nine (7.2%) study participants had a fistulizing phenotype. Perianal fistula was present in 92 (9.6%) study participants. Terminal ileal disease was documented in 273 (28.7%), isolated colonic disease in 212 (22.2%), ileocolonic disease in 253 (26.6%), and isolated proximal small bowel disease (L4 disease) in 48 study participants (5.04%). A history of alcohol use was documented in 53 (5.5%) study participants. One hundred and twelve (11.7%) study participants were smokers, and 68 (7.1%) study participants reported using chewable tobacco (Table I).
| Study parameters | Outcome value |
|---|---|
| Mean age of onset ±SD (yr) | 33.4±14.65 |
| Mean age of diagnosis ± SD (yr) | 36.9 ± 15.11 |
| Males/Females | 582/370 |
| Montreal classification | |
| Age, n (%) | |
|
A1 A2 A3 |
92 (9.6) 565 (59.3) 295 (30.9) |
| Behaviour, n (%) | |
|
B1 B2 B3 |
441(46.3) 442 (46.4) 69 (7.2) |
| Location, n (%) | |
|
L1 L2 L3 L4 L1+4 L2+4 L3+4 |
273 (28.7) 212 (22.2) 253 (26.6) 48 (5.04) 112 (11.7) 20 (2.1) 34 (3.5) |
|
Perianal disease, n (%) Alcohol intake, n (%) Smoking, n (%) Chewable tobacco, n (%) |
92 (9.6) 53 (5.5) 112 (11.7) 68 (7.1) |
| Total follow up disease duration (months) median (IQR) | 34(16-73) |
| EIM, n (%) | 279 (29.2) |
| Medication use, n (%) | |
|
Steroids Immunomodulators Biologicals |
731 (76.7) 654 (68.6) 103 (10.8) |
| History of hospitalization, n (%) | 201 (21.1) |
| Prior history of surgery, n (%) | 154 (16.1) |
SD, standard deviation; EIM, extra-intestinal manifestation; IQR, interquartile range
Two hundred one (21.1%) study participants had a prior history of hospitalization, and 154 (16.1%) had a history of surgery in the past. Among the overall cohort, 731 (76.7%) study participants received steroids; immunomodulator use was documented in 654 (68.6%) and 103 (10.8%) received anti-TNF therapy (Table I).
Incidence of malignancy in study participants with CD
Ten study participants developed malignancy during the follow up, with an overall incidence of 1.05 per cent. Of these, four and six study participants developed intestinal and extraintestinal malignancy, respectively. Hence, the incidence of intestinal and extra-intestinal malignancy was 0.42 per cent and 0.63 per cent, respectively. Eight study participants developed malignancy in the first decade; one developed during the second decade and another during the third decade of disease diagnosis. The incidence density was 2.16/1000 person-year follow up (95% CI: 1.16–4.02). Incidence density for intestinal and extraintestinal malignancies were 0.86/1000 person-year (95% CI: 0.32-2.3) and 1.29/1000 person-year (95% CI: 0.58-2.89), respectively. The number of study participants with disease duration of <10, 10-20 and >20 yr was 826, 101 and 25, respectively. Hence, the incidence density was 2.05/1000 person-year for disease duration under 10 yr, 1.66/1000 person-year for disease duration between 10 and 20 yr, and 10.1/1000 person-year for disease lasting more than 20 yr.
There was 10.45 times greater risk of malignancy in CD (SIR, 10.45; 95% CI: 4.98- 17.96). Kaplan-Meier analysis revealed that the cumulative risk of acquiring malignancy was 2.7 per cent in the first decade, 5.5 per cent in the second, and 13.4 per cent in the third (Figure).
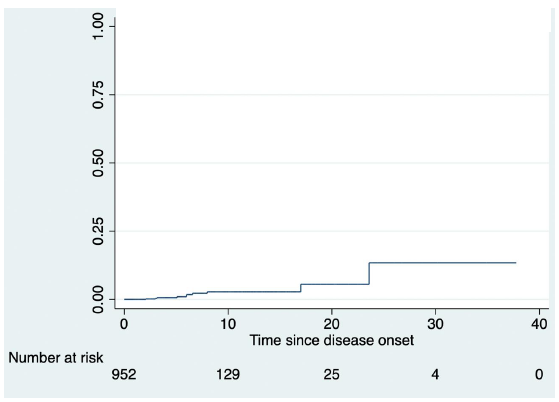
- Kaplan-Meier curve showing increasing incidence of malignancy with increasing duration of disease.
Types of malignancy
Intestinal cancer
Of the four study participants with bowel malignancy, one study participant had adenocarcinoma of the ileocaecal area, one study participant had anorectal adenocarcinoma, one study participant developed malignant rectal fibrous histiocytoma and one study participant developed gastric adenocarcinoma. All study participants were males, and the disease duration at which malignancy developed ranged from 3 to 28 yr. All except one study participant had a complicated phenotype (stricture or penetrating), and 50 per cent of study participants had associated peri-anal disease (Table II). Only one study participant had a history of smoking. All study participants were exposed to steroids and a history of immunomodulator and biologic use was present in 75 per cent of study participants. All study participants were symptomatic; none had any family history of cancer, and 2/4 had metastatic disease (Table II). Two of these four study participants (one with ileocaecal adenocarcinoma and the other with the anorectal adenocarcinoma) succumbed to their illness.
| Gender | BMI | Age at diagnosis (yr) | Age at development of malignancy (yr) | Montreal classification | Duration of follow up (month) | EIM | Symptomatic at presentation | Family history of malignancy | Smoking | Tobacco | Alcohol | Immunomodulators/duration (months) | Steroids | Biologicals/ duration (Months) | Stage of cancer at diagnosis | Hospitalization | Surgery | Death | Type of malignancy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | 24.61 | 40 | 68 | A3B2L3 | 283 | No | Yes, Pain abdomen | No | No | No | No | Yes / 1 | Yes | Yes / 2 | Non resectable, metastatic | Yes | No | Yes | Adenocarcinoma of Ileocaecal area |
| Male | 21.31 | 31 | 36 | A2B2L2P | 79 | Episcleritis | Yes, bleeding PR | No | Yes | No | Yes | Yes/ 15 | Yes | Yes / 5 | Localised to rectum | Yes | No | No | Malignant Rectal fibrous histiocytoma |
| Male | 17.63 | 44 | 49 | A3B3L2P | 23 | No | Yes, fever & upper respiratory symptoms | No | No | Yes | Yes | Yes / 8 | Yes | Yes / 3 | Non resectable with distant metastasis | Yes | Yes | Yes | Anorectal Adenocarcinoma |
| Male | 21.57 | 60 | 63 | A3B1L1 | 24 | No | Yes, Pain abdomen & fullness | No | No | No | Yes | No / - | Yes | No / - | Resectable early gastric antral cancer | No | Yes | No | Adenocarcinoma stomach |
| Female | 23.1 | 54 | 58 | A3B2L1+4 | 61 | No | Yes, Pain abdomen | No | No | No | No | No /- | No | No / - | Resectable thyroid nodule with pelvic bone & right lung metastasis | No | Yes | No | Follicular neoplasia of thyroid with right lung & pelvic bone metastasis |
| Male | 24.2 | 68 | 71 | A3B2L1 | 60 | No | Asymptomatic | No | No | No | No |
Yes / 10 (2-month Azathioprine and 8-month Methotrexate) |
Yes | No / - | Resectable | No | No | No | Neuroendocrine tumour of tail of pancreas. |
| Female | 29.49 | 50 | 51 | A3B1L2 | 47 | No | Yes, breast lump | No | No | No | No | No / - | Yes | No / - | Resectable disease, underwent Mastectomy, Chemotherapy & radiotherapy | No | Yes | No | Right breast cancer |
| Female | 21.7 | 59 | 67 | A3B2L1 | 23 | No | Yes, Progressive weakness | No | No | No | No | Yes / 3 | Yes | No / - | Metastatic HCC | Yes | No | Yes | Hepatocellular cancer |
| Male | 22.6 | 64 | 69 | A3B2L1+4P | 60 | No | Yes, Thin urine stream | No | No | No | No | Yes /2 | Yes | No / - | Resectable disease | No | No | No | Prostrate cancer |
| Female | 19.8 | 19 | 36 | A2B1L2 | 204 | Yes | Yes, Oral non healing ulcer | No | No | No | No | Yes / 3 | No | No / - | Resectable disease | No | No | No | Oral cancer |
HCC, hepatocellular cancer; BMI, body mass index
Extra-intestinal cancer
Among the six study participants with extraintestinal malignancies, each one developed follicular neoplasia of the thyroid, neuroendocrine tumour of the tail of pancreas, breast cancer, hepatocellular cancer (HCC), squamous cell carcinoma of the oral cavity and prostate cancer. No study participant developed lymphoma or skin malignancy. Thirty-three per cent of study participants were males, and the disease duration at which malignancy developed ranged from 1 to 17 yr (Table II). All except two study participants had a complicated phenotype, and 16 per cent of study participants had isolated perianal disease. All except one study participant were symptomatic at presentation, none had any family history of cancer, and 2/6 had metastatic disease (Table II). The study participant who developed HCC succumbed to her illness.
Comparison of disease characteristics between study participants with malignancy vs. those without malignancy
The mean age in year at diagnosis was significantly higher in patients who developed malignancy vs. those who did not (49.5±16.07 vs. 36.8±15.06, P=0.0083). The gender distribution was similar between these two cohorts. More study participants with malignancy had complicated disease behaviour and perianal disease, though the difference was not significant (70% vs. 53.4% and 20% vs. 9.5%). The disease location was not significantly different between the two cohorts (Table III).
| Parameters | Study participants who developed malignancy (n=10) | Study participants without malignancy (n=942) | P value |
|---|---|---|---|
| Mean age of diagnosis ± SD (yr) | 49.5±16.07 | 36.8 ± 15.06 | 0.0083 |
| Gender, Male; n (%) | 5 (50) | 577 (61.2) | 0.52 |
| Disease behaviour, n(%) | |||
|
B1 B2 B3 |
3 (30) 6 (60) 1 (10) |
438(46.4) 436(46.2) 68(7.2) |
0.35 0.52 0.53 |
| Location of disease, n (%) | |||
|
L1 L2 L3 L4 L1+4 L2+4 L3+4 |
4 (40) 4(40) 1(10) - 1(10) - - |
269(28.5) 208(22.08) 252(26.7) 48(5.09) 111(11.7) 20(2.12) 34 (3.6) |
0.48 0.242 0.306 - 1 - - |
| Perianal disease, n (%) | 2 (20) | 90(9.5) | 0.251 |
| Smoking, n (%) | 2 (20) | 110(11.6) | 0.33 |
| Alcohol, n (%) | 2 (20) | 52 (5.5) | 0.106 |
| Tobacco, n (%) | 2 (20) | 67 (7.11) | 0.16 |
| Steroid, n (%) | 6(60) | 725(77) | 0.206 |
| Anti- TNF, n (%) | 3(30) | 100(10.6) | 0.084 |
| Immunomodulator use, n (%) | 6 (60) | 648 (68.7) | 0.514 |
| History of surgery, n (%) | 2 (20) | 152 (16.1) | 0.66 |
| Duration of symptoms before diagnosis of cancer [Median (IQR)], Months | 48(14-97.5) | - | - |
| Duration last follow up in those without cancer [Median (IQR)], Months | - | 33(19-72.7) | - |
TNF, tumour necrosis factor
History of smoking, alcohol consumption, tobacco and anti-TNF use though was higher in study participants who developed malignancy, and the difference was again not significant. History of surgery, steroid and immunomodulator use was comparable in the two cohorts (Table III).
Discussion
We report the incidence of malignancy in a reasonably large cohort of study participants with CD from northern India. The incidence of intestinal malignancy was 0.42 per cent, and the overall incidence (both intestinal and extra-intestinal) was 1.05 per cent. Most of these study participants had the onset of CD beyond the fifth decade and had a complicated phenotype, and among study participants with intestinal malignancy, most were exposed to immunomodulators and biologics. However, we could not determine the risk factors for malignancy due to our cohort’s low number of study participants with malignancy. Interestingly, no study participant developed lymphoma or skin cancer, the two most frequent malignancies reported with azathioprine and anti-TNF use in IBD14.
IBD involves persistent inflammation and intestinal damage, potentially leading to cancer through various pathways, although the precise mechanism remains incompletely understood15. Intestinal mucosa in IBD has higher levels of pro-inflammatory cytokines like interleukin-6 (IL-6) and tumour necrosis factor- α (TNF- α). IL-6, via activation of STAT3, can promote the carcinogenesis of colorectal cancer by facilitating proliferation and inhibiting the apoptosis of epithelial cells. Furthermore, TNF-α could also promote tumorigenesis by activating the NF-κB pathway16. In active CD or UC, inflammatory cells expressing COX-2 and NOS-2 produce reactive oxygen and nitrogen species, accelerating intestinal ageing and increasing susceptibility to genetic and epigenetic changes promoting carcinogenesis15. In conclusion, the primary risk factor for triggering local tumorigenesis is the persistent inflammation of intestinal tissue in IBD.
A recent study in India estimated 1,461,427 incident cancer cases in 2022, with a crude rate of 100.4 per 1,00,000 population13. In our study of 952 CD affected study participants, 10 developed malignancy, yielding an incidence rate of 1050 per 1,00,000 individuals with CD. The SIR was 10.45 (95% CI: 4.98-17.96), indicating a heightened risk of malignancy compared to the general population. The overall incidence density of malignancy was 2.1/1000 person-yr of follow up. For intestinal and extra-intestinal malignancy, it was 0.86/1000 person-yr and 1.29/ 1000 person-yr, respectively.
The incidence rate of colorectal cancer in CD was 53.3 per 1,00,000 person-yr worldwide, according to a meta-analysis17. In a recent cohort study from Sweden and Denmark, the incidence rate of small bowel cancer in CD was 24.4 per 100,000 person-yr18, and the prevalence of small bowel cancers, according to another meta-analysis, was 1.15/1000 patients in CD19. One study participant each in our study developed ileocaecal and anorectal adenocarcinoma. Hence, the incidence and prevalence rates of small bowel and colorectal cancer in the present study were similar to the earlier reported investigations. The risk of CRC in UC increases with increasing disease duration, as shown by a recent Asian meta-analysis and a cohort study from northern India20,21. In the meta-analysis of Asian patients with ulcerative colitis, involving 31,287 patients, 293 colorectal cancer cases were identified. The risk of colorectal cancer was 0.02 per cent (95% CI: 0-0.04) at 10 yr, 4.81 per cent (3.26-6.36) at 20 yr, and 13.91 per cent (7.09-20.72) at 30 yr20. Similarly, in the present study the risk of malignancy increased with increasing disease duration, and the cumulative risk of developing malignancy was 2.7 per cent in the first, 5.5 per cent during the second, and 13.4 per cent in the third decade.
Two study participants with colonic disease had malignancy affecting the rectum. Both study participants were undergoing routine surveillance for colorectal cancer. The first study participant, diagnosed with anorectal adenocarcinoma, was discovered to have metastatic disease at diagnosis despite undergoing endoscopic surveillance where no dysplastic lesions were detected. The second study participant was also identified to have a rectal polyp during surveillance, which, upon polypectomy, revealed malignant fibrous histiocytoma.
Regarding the risk of intestinal malignancy in CD, prior studies identified stricture formation/ penetrating disease as risk factors22. In our study, two of the four study participants with bowel malignancy had stricturing and one study participant had a fistulizing phenotype, and two of these study participants also had perianal fistulizing disease. Therefore, study participants with stricturing and fistulizing diseases are probably at higher risk and should remain under surveillance. The evidence on perianal fistula related cancer in CD is limited to case series, and possible risk factors include associated colonic disease and chronic perianal disease, similar to our study participant23.
Patients with IBD are also at increased risk of extra-intestinal cancers24, with the extent of risk depending on the cancer type and IBD type. Immunosuppressive treatments may promote extra-intestinal malignancies by hampering immune surveillance or inducing DNA damage25. Thiopurine use has been linked to a significant increase in the incidence of lymphomas and non-melanoma skin malignancies among IBD patients25. However, our investigation did not show that study participants receiving immunosuppressive treatments had an increased incidence of lymphoproliferative malignancy14. In a Chinese cohort study, haematological and urinary tract cancers were more common in elderly onset IBD, while thyroid cancer was higher in the adult-onset group26. Although Hashimoto’s thyroiditis and Grave’s disease, two autoimmune thyroid illnesses, have been linked to IBD and specifically CD, there is no data on the association between IBD and thyroid cancer27. One study participant each in our study did develop follicular thyroid cancer and prostate cancer; the study participant who developed thyroid cancer had no exposure to any immune suppressant, while the one with prostate cancer was exposed to thiopurines. Other extra-intestinal malignancies included neuroendocrine tumour of the pancreatic tail, right breast cancer, oral squamous cell carcinoma and HCC. The evidence of association of these malignancies with CD is scant, and it is possible that these patients might have developed cancer independent of CD.
In the present study, the crude incidence of malignancy in study participants with CD was 1050 per 1,00,000 population, which is higher than the national crude incidence of 100.4 per 100,000 in the general population13. Several factors, including ethnicity, genetics, nutrition and variations in colonoscopy surveillance, which are known to affect the risk of IBD-related malignancy, could account for the greater malignancy prevalence in our study cohort. However, these data are only from a single tertiary care hospital that deals with more severe and complicated cases of IBD, which could lead to referral bias and overestimation of the risk of cancer. Unlike the present study, in a recent survey conducted by European experts, a pan-European study and analysis of the Swiss IBD cohort, the risk of cancer in IBD was similar to that in the general population28,29. Similar to overall malignancies, the risk of CRC in IBD has also been questioned recently, probably related to better control of inflammation and improved surveillance strategies28. Among the Asian studies, in a recent population-based cohort study from China, there were no significant differences in cancer risk between CD patients and the general population30; however, in another study, the risk of colorectal cancer was higher in patients with elderly onset disease, similar to our study, where 2/3rd of study participants had age of onset >50 yr31. Recent studies from Italy and Denmark also confirm our observation of an increased risk of malignancy in CD32,33. However, in a study from our centre, the prevalence of CRC in UC was 1.97 per cent as compared to 0.4 per cent prevalence of intestinal malignancy in CD in the present investigation, suggesting a lower risk of malignancy in CD as compared to UC21.
However, our study had some limitations. First, this was a retrospective analysis, though the records were maintained prospectively. Because of the retrospective design, some study participants were lost to follow up. However, we accounted for them by censoring them at the last follow up, and the follow up duration was considered only until their last follow up. Secondly, though we could estimate the burden of malignancy compared to that in the general population, the predictors could not be estimated because of very low numbers of study participants with malignancy. Thirdly, these data reflected a referral bias and possibly overestimated the risk of malignancy. Fourth, the median duration of follow up was quite short (34 months), and we had a very small number of study participants with a 30 yr follow up. Lastly, we did not have a comparison group without a CD. However, we could circumvent this by comparing the risk with that of the general population in the study published by Sathishkumar et al13.
Overall, to conclude, we observed a noticeable occurrence of both intestinal and extra-intestinal malignancies among affected individuals with CD from India. Elderly and patients with complicated diseases might be at a higher risk and require close surveillance.
Financial support & sponsorship
None.
Conflicts of Interest
None.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Epidemiology of inflammatory bowel disease in India: The great shift east. Inflamm Intest Dis. 2017;2:102-115.
- [Google Scholar]
- Evolving trends and burden of inflammatory bowel disease in Asia, 1990-2019: A comprehensive analysis based on the global burden of disease study. J Epidemiol Glob Health. 2023;13:725-39.
- [Google Scholar]
- Colitis-associated cancer: The dark side of inflammatory bowel disease. Gut. 2011;60:1609-10.
- [Google Scholar]
- Risk of malignant cancers in inflammatory bowel disease. J Crohns Colitis. 2019;13:1302-10.
- [Google Scholar]
- Malignancies in patients with inflammatory bowel disease: Results from 20 years of follow-up in the IBSEN study. J Crohns Colitis. 2017;11:571-7.
- [Google Scholar]
- Risk of cancer in patients with inflammatory bowel diseases: A nationwide population-based cohort study with 30 years of follow-up evaluation. Clin Gastroenterol Hepatol. 2014;12:265-73.e1.
- [Google Scholar]
- Cancer risk in patients with inflammatory bowel disease: A population-based study. Cancer. 2001;91:854-62.
- [Google Scholar]
- Intestinal cancer risk in Crohn’s disease: A meta-analysis. J Gastrointest Surg. 2011;15:576-83.
- [Google Scholar]
- Declining risk of colorectal cancer in inflammatory bowel disease: An updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19:789-99.
- [Google Scholar]
- Guidelines for examining unusual patterns of cancer and environmental concerns. Available from: https://www.cdc.gov/cancer-environment/php/guidelines/index.html, accessed on March 6, 2024.
- Cancer incidence estimates for 2022 & projection for 2025: Result from national cancer registry programme, India. Indian J Med Res. 2022;156:598-607.
- [Google Scholar]
- Minimal risk of lymphoma and non-melanoma skin cancer despite long-term use of thiopurines in patients with inflammatory bowel disease: A longitudinal cohort analysis from northern India. J Gastroenterol Hepatol. 2022;37:1544-53.
- [Google Scholar]
- Clinicopathological and molecular specificities of inflammatory bowel disease-related colorectal neoplastic lesions: The role of inflammation. J Crohns Colitis. 2018;12:1486-98.
- [Google Scholar]
- IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103-13.
- [Google Scholar]
- Worldwide incidence of colorectal cancer, leukemia, and lymphoma in inflammatory bowel disease: An updated systematic review and meta-analysis. Gastroenterol Res Pract. 2016;2016:1632439.
- [Google Scholar]
- Inflammatory bowel disease and risk of small bowel cancer: A binational population-based cohort study from Denmark and Sweden. Gut. 2021;70:297-308.
- [Google Scholar]
- Small bowel adenocarcinoma in Crohn’s disease: A systematic review and meta-analysis of the prevalence, manifestation, histopathology, and outcomes. Int J Colorectal Dis. 2022;37:239-50.
- [Google Scholar]
- Risk of colorectal cancer in Asian patients with ulcerative colitis: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:269-76.
- [Google Scholar]
- Long-term follow-up reveals high incidence of colorectal cancer in Indian patients with inflammatory bowel disease. United European Gastroenterol J. 2017;5:708-14.
- [Google Scholar]
- Cancer risk in inflammatory bowel disease: A 6-year prospective multi-center nested case-control IG-IBD study. Inflamm Bowel Dis. 2020;26:450-9.
- [Google Scholar]
- Fistula-related cancer in Crohn’s disease: A systematic review. Cancers (Basel). 2021;13:1445.
- [Google Scholar]
- The risk of extraintestinal cancer in inflammatory bowel disease: A systematic review and meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2021;19:1117-1138.e19.
- [Google Scholar]
- Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143:390-399.e1.
- [Google Scholar]
- The incidence rate and risk factors of malignancy in elderly-onset inflammatory bowel disease: A Chinese cohort study from 1998 to 2020. Front Oncol. 2021;11:788980.
- [Google Scholar]
- Concomitant thyroid disorders and inflammatory bowel disease: A literature review. Biomed Res Int. 2016;2016:5187061.
- [Google Scholar]
- Cancer in inflammatory bowel disease 15 years after diagnosis in a population-based European collaborative follow-up study. J Crohns Colitis. 2011;5:430-42.
- [Google Scholar]
- Malignancies in inflammatory bowel disease: Frequency, incidence and risk factors-results from the Swiss IBD cohort study. Am J Gastroenterol. 2019;114:116-26.
- [Google Scholar]
- Risk of malignancy in patients with inflammatory bowel disease: A population-based cohort study from China. Int J Cancer. 2022;150:1770-8.
- [Google Scholar]
- The incidence rate and risk factors of malignancy in elderly-onset inflammatory bowel disease: A Chinese cohort study from 1998 to 2020. Front Oncol. 2021;11:788980.
- [Google Scholar]
- Risk of intestinal and extra-intestinal cancers in patients with inflammatory bowel diseases: A population-based cohort study in northeastern Italy. PLoS One. 2020;15:e0235142.
- [Google Scholar]
- Cancer risk in inflammatory bowel disease according to patient phenotype and treatment: A Danish population-based cohort study. Am J Gastroenterol. 2013;108:1869-76.
- [Google Scholar]






