Translate this page into:
In vitro evaluation of antibiotics for methicillin-resistant Staphylococcus aureus from north India
*For correspondence: r_vg@yahoo.co.uk
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Serious infections due to Staphylococcus aureus, especially those due to methicillin-resistant S. aureus (MRSA), have become a major clinical challenge. In India, the prevalence of MRSA has been reported to be 29 to 46 per cent in hospital settings12, and in community, MRSA nasal colonization has been reported to be 3.89 per cent in children of 5-15 yr age group3, to 16.3 per cent in children one month to five years of age4. Vancomycin has long been the drug of choice for treatment of infections with MRSA, however, there are concerns of decreased efficacy of vancomycin considering the accumulating evidence of poor outcome, increasing resistance and unachievable pharmacokinetic/pharmacodynamic (PK/PD) targets5. The newer anti-MRSA drugs from various classes viz. lipopeptides (daptomycin), glycopeptides (teicoplanin, oritavancin, telavancin), lipoglycopeptides (dalbavancin), oxazolidinones (linezolid), fifth generation cephalosporins (ceftaroline, ceftobiprole), diaminopyrimidines (iclaprim), streptogramins (quinupristin-dalfopristin), glycylcyclines (tigecycline), and the macrolide-lincosamide-streptogramin B (MLSB) antibiotics hold the promise to be useful alternatives in such cases where vancomycin is clinically ineffective. The use of vancomycin or an alternate anti-MRSA drug, however, should be determined based on the MIC (minimum inhibitory concentration) of the isolate for vancomycin6.
This study was conducted to evaluate the susceptibility of MRSA isolates from blood stream infections (BSI) and complicated skin and soft tissue infections (cSSTI) to vancomycin, daptomycin, teicoplanin, and linezolid, and to determine the MIC50 and MIC90 values of the MRSA isolates for vancomycin and daptomycin at the Postgraduate Institute of Medical Education and Research (PGIMER), a tertiary care center at Chandigarh in north India. From March to June, 2011, 98 consecutive clinical MRSA isolates from BSIs (14/98; 14.3%) and pus specimens of patients suffering from cSSTIs (84/98; 87.5%) defined as deeper soft-tissue infections, surgical/traumatic wound infections, major abscesses, cellulitis, and infected ulcers and burns, were evaluated for susceptibility to anti-MRSA drugs. The identification was done using standard biochemical tests7 and methicillin resistance was detected using oxacillin (6 mg/l) screen agar and cefoxitin (30 μg) disc diffusion test, and resistance was reported if either was found resistant as per the Clinical and Laboratory Standards Institute (CLSI) guidelines8. All 98 isolates were resistant by both oxacillin screen agar and cefoxitin disc diffusion test. These MRSA isolates were further tested for susceptibility to teicoplanin (30 μg) and linezolid (30 μg) by Kirby-Bauer disc diffusion method8 and for determination of MIC for vancomycin and daptomycin by E test (bioMérieux India Pvt. Ltd., New Delhi, India) following the manufacturer's instructions. All isolates were also screened with vancomycin (6 mg/l) screen agar according to CLSI guidelines8. The MIC for daptomycin was determined on Mueller-Hinton agar supplemented with 50 mg/l calcium (Difco, USA). The MIC50 and MIC90 of the MRSA isolates for vancomycin and daptomycin were calculated. All the disk diffusion tests were performed according to CLSI guidelines and S. aureus ATCC 29213 was used for quality control.
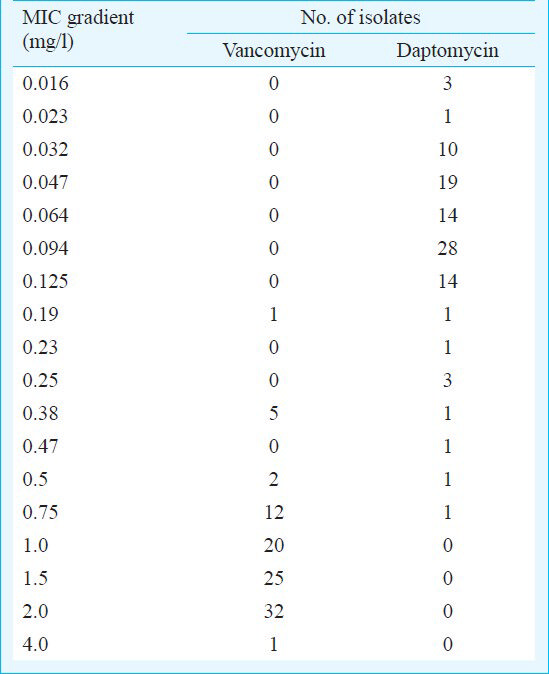
Of the 98 MRSA isolates, 59.2 per cent (58/98) were from males and 40.8 per cent (40/98) from female patients. A majority of the specimens i.e. 79.6 per cent (78/98) were received from patients from ICUs, 14.3 per cent (14/98) from emergency OPDs and wards, and 6.1 per cent (6/98) from general wards. All MRSA isolates were found to be susceptible to daptomycin, teicoplanin, and linezolid. The MIC50 and MIC90 values for vancomycin and daptomycin were found to be 1.5 and 2 mg/l, and 0.094 and 0.125 mg/l, respectively. Of all the isolates, 32.6 per cent (32/98) had vancomycin MIC 2 mg/l, 20.4 per cent (20/98) had MIC 1 mg/l, and 20.4 per cent (20/98) had a MIC <1 mg/l. The geometric mean MIC was 1.26 mg/l for vancomycin, and 0.076 mg/l for daptomycin. One vancomycin-intermediate S. aureus (VISA) isolate was found with an MIC of 4 mg/l from a patient with blood stream infection. This isolate, although sensitive to daptomycin, had a high MIC (0.25 mg/l) i.e. more than the MIC90(0.125 mg/l) for daptomycin. No vancomycin-resistant S. aureus (VRSA) was found by either E-test or vancomycin screen agar.
In this study, we report high MIC50(1.5 mg/l) and MIC90(2 mg/l) of MRSA isolates for vancomycin, and emergence of VISA (MIC 4 mg/l) in our region, though none of the isolates was resistant to vancomycin, daptomycin, teicoplanin, and linezolid. The Infectious Disease Society of America (IDSA) guidelines, 2011, state that for isolates with a vancomycin MIC >2 mg/l, an alternate to vancomycin should be used, while for isolates with MIC <2 mg/l the patient's clinical response should determine the continued use of vancomycin, independent of the MIC6. Vancomycin MIC >1.0 mg/l has been shown to be associated with poor outcome in patients with MRSA infection9, and this has been attributed to the excessive use of vancomycin10. Sakoulas et al9 observed that efficacy of vancomycin in the treatment of S. aureus infections decreased for isolates with vancomycin MICs of 1 mg/l, and the number of clinical failures for patients treated with vancomycin may rise as vancomycin MICs increase to 2 mg/l.
The emergence of VISA/VRSA is now a global issue, with reports from Japan, United States of America, Brazil, France, United Kingdom, Germany, Jordan, and Belgium1112. From India, only a few studies have reported VISA/VRSA from different regions. Menezes et al13 tested 102 clinical isolates of MRSA from Puducherry in southern India and found one VISA isolate with MIC 5 mg/l. In another study from Intensive Care Units (ICU) of Hyderabad, of the 358 S. aureus isolates tested, there were 16 VISA showing an MIC range between 4-8 mg/l, and seven VRSA with an MIC in the range of 16-64 mg/l11. Another study from Varanasi reported nasal colonization of VISA in ICU patients14. Shifting trends of vancomycin susceptibility patterns in S. aureus have been reported from a tertiary care hospital in north India, wherein the authors noted a gradual rise in MIC for vancomycin from 1 to 2 mg/l over a five year period from 2004-2008, however, they did not find any VISA/VRSA isolate in their study15. Further, the clinical failures of vancomycin due to its slow bactericidal activity coupled with increasing MICs have necessitated a search for new, more effective agents16.
The MRSA isolates were 100 per cent susceptible to the commonly available anti-MRSA drugs (daptomycin, teicoplanin, and linezolid). Teicoplanin susceptibility testing, however, is still under investigation and the disc diffusion test cannot be used as standard interpretive criteria to differentiate teicoplanin-intermediate and teicoplanin-resistant staphylococci from teicoplanin-susceptible strains8. Daptomycin has been proved to be effective for infections due to MSSA and MRSA, and is approved for use in the treatment of cSSTI and S. aureus bacteraemia including right sided infective endocarditis16. The use of daptomycin to treat patients who have not responded to vancomycin requires careful consideration, as the isolates with vancomycin MIC ≥2 mg/l may have daptomycin MICs in the non susceptible range (>1 mg/l), and persistent bacteraemia and clinical failures with daptomycin have been associated with daptomycin MICs >1 mg/l617. However, none of the isolates from our region had daptomycin MIC >1 mg/l. This was in agreement with our previous assessment of daptomycin MICs, when all 63 MRSA isolates tested had MIC ≤1 mg/l18.
Teicoplanin has a limited role for severe MRSA infections5. A systematic review of the data available on 73 patients with MRSA infections from five trials yielded a relative risk of all cause mortality of 0.67 for vancomycin versus teicoplanin19. Other studies have documented an increase in teicoplanin MICs, in common with vancomycin MICs, in MRSA20.
Linezolid is preferred to vancomycin in treatment of MRSA ventilator-associated and hospital-acquired pneumonias, especially when there is a history of recent vancomycin exposure, vancomycin MIC >1 mg/l or when elevated MIC is considered likely, and in renal failure5. It has an advantage of 100 per cent oral bioavailablity and a novel mechanism of action, however, its use is limited by haematologic toxicity, thromobocytopenia, peripheral and optic neuropathy, and lactic acidosis21.
The higher MICs observed in the present study should be extrapolated with certain limitations as E test, which is reported to give higher MIC values than that reference broth microdilution method, was used6. Secondly, the VISA isolate could not be confirmed by broth microdilution method as the isolate could not be revived.
In conclusion, our study shows an emergence of S. aureus isolates with high MIC to vancomycin. There is a need to enforce the judicious use of vancomycin, and balancing the antibiotic pressure of vancomycin on the bacterial population by using alternative anti-MRSA drugs when appropriate, keeping in mind that the use of newer anti-bacterial agents needs to be carefully defined to prevent misuse and emergence of resistance to these drugs.
Acknowledgment
Authors acknowledge Novartis India Limited for providing E-strips of daptomycin and vancomycin for this study.
References
- Prevalence and antimicrobial susceptibility pattern of methicillin-resistant Staphylococcus aureus [MRSA] isolates at a tertiary care hospital in Mangalore, South India. J Lab Physicians. 2010;2:82-4.
- [Google Scholar]
- Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in a tertiary care hospital in Northern India. J Lab Physicians. 2010;2:78-81.
- [Google Scholar]
- A community-based study on nasal carriage of Staphylococcus aureus. Indian J Med Res. 2009;130:742-8.
- [Google Scholar]
- Nasal carriage and antimicrobial susceptibility of Staphylococcus aureus in healthy preschool children in Ujjain, India. BMC Pediatr. 2010;10:100.
- [Google Scholar]
- Management of serious meticillin-resistant Staphylococcus aureus infections: what are the limits? Int J Antimicrob Agents. 2011;37:202-9.
- [Google Scholar]
- Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285-92.
- [Google Scholar]
- Staphylococcus: Cluster-forming Gram-positivecocci. In: Collee JG, Fraser AG, Marmion BP, Simmons A, eds. Mackie and McCartney practical medical microbiology. London, United Kingdom: Churchill Livingstone; 1996. p. :245-61.
- [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; twenty-first informational supplement. Document M100-S21. Wayne, PA: CLSI; 2011.
- [Google Scholar]
- Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42:2398-402.
- [Google Scholar]
- Efficacy and safety of daptomycin in the treatment of Gram-positive catheter-related bloodstream infections in cancer patients. Int J Antimicrob Agents. 2010;36:182-6.
- [Google Scholar]
- Vancomycin resistance among methicillin resistant Staphylococcus aureus isolates from intensive care units of tertiary care hospitals in Hyderabad. Indian J Med Res. 2011;134:704-8.
- [Google Scholar]
- First report of vancomycin-resistant staphylococci isolated from healthy carriers in Brazil. J Clin Microbiol. 2005;43:179-85.
- [Google Scholar]
- Emergence of vancomycin-intermediate Staphylococcus species in southern India. J Med Microbiol. 2008;57:911-2.
- [Google Scholar]
- Colonization with vancomycin- intermediate Staphylococcus aureus strains containing the vanA resistance gene in a tertiary-care center, In north India. J Clin Microbiol. 2012;50:1730-2.
- [Google Scholar]
- Decreased susceptibility to vancomycin in meticillin-resistant Staphylococcus aureus: a 5 year study in an Indian tertiary hospital. J Med Microbiol. 2010;59:375-6.
- [Google Scholar]
- Clinical experience with daptomycin: bacteraemia and endocarditis. J Antimicrob Chemother. 2008;62(Suppl 3):iii35-9.
- [Google Scholar]
- Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355:653-65.
- [Google Scholar]
- Daptomycin susceptibility of methicillin resistant Staphylococcus aureus (MRSA) Indian J Med Res. 2012;136:676-7.
- [Google Scholar]
- Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis. Antimicrob Agents Chemother. 2009;53:4069-79.
- [Google Scholar]
- Staphylococcus aureus with reduced glycopeptide susceptibility in Liverpool, UK. J Antimicrob Chemother. 2010;65:721-4.
- [Google Scholar]
- Peripheral neuropathy associated with prolonged use of linezolid. Lancet Infect Dis. 2004;4:528-31.
- [Google Scholar]





