Translate this page into:
Improving completeness & reducing errors in medical certification of cause of death: The impact of electronic mortality software in a tertiary care centre in South India
For correspondence: Dr Ramadoss Ramu, Department of Medicine, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry 605 006, India e-mail: ramadoss2912@gmail.com
-
Received: ,
Accepted: ,
Abstract
Background & objectives
Mortality statistics are crucial for understanding public health. Accurate medical certification of cause of death (MCCD) is essential for good mortality statistics. However, the quality of MCCD form-filling remains a concern. Based on the learnings from the ICMR-National Centre for Disease Informatics and Research (ICMR-NCDIR), e-Mortality software implementation project, our institute developed and used a new in-house mortality software for MCCD from January 2021. This study compared MCCD forms before and after implementation of the mortality software.
Methods
The study was conducted from March 2024 to July 2024 in the department of Medicine at a tertiary care teaching institute in Puducherry. We analysed 105 hand-written forms from the year 2020 and 105 software-generated forms from the year 2021, focusing on completeness, errors, and International Classification of Diseases-10 (ICD-10) compatibility. We checked 13 items for completeness. Errors were categorised as major or minor, depending on how they affected ICD-10 coding.
Results
The proportion of completeness improved from 4 to 19 per cent after software introduction (P<0.001). Minor errors significantly decreased from 96 to 81 per cent (P<0.002). About 88 per cent of hand-written forms had major errors, which was significantly reduced to 42 per cent in software-generated forms (P<0.001). Compatibility of the underlying cause of death for generating ICD-10 coding improved from 73 to 96 per cent (P<0.001).
Interpretation & conclusions
The findings of this study suggest that our mortality software significantly improved completeness and modestly reduced errors. Other institutions may consider adopting an electronic format for MCCD to improve completeness and accuracy. We emphasise regular training of doctors and auditing of MCCD forms to further improve the quality of death certification.
Keywords
Completeness
errors
ICD-10 coding
ICMR-NCDIR e-MOR
MCCD
Mortality statistics are crucial for understanding disease magnitude, mortality trends, health service planning, and evaluating health indicators1. Medical Certification of Cause of Death (MCCD) is crucial for accurate mortality statistics. In 2020, only 22.5 per cent of deaths in India were medically certified2. Additionally, the quality of completed MCCD forms remains a concern.
Form-4 is used for MCCD, which requires two types of information: clerical and technical. Clerical details include patient demographics, basic death information, and medical officer details. Technical details are the information about causes of death, and they focus on identifying the ‘underlying cause of death’ (UCoD), which is the disease or injury initiating the sequence of events leading to death. This UCoD is used for International Classification of Diseases (ICD)-10 coding1. Errors in filling these details can affect MCCD accuracy and are classified as major or minor. Major errors impact the selection of the correct UCoD and ICD-10 coding, while minor errors have a lesser effect on ICD coding1. Patil et al3 analysed 729 MCCD forms and found that 642 forms (88.06%) contained at least one major error, while 671 forms (92.04%) had at least one minor error. These errors can have adverse implications on reporting the mortality data, misrepresenting the disease trends, and impair disease surveillance, resulting in poorly informed health policies and resource allocation.
Our institute uses an electronic health information system (HIS), but MCCD was initially completed manually on Form 4. The National Centre for Disease Informatics and Research (ICMR-NCDIR) developed an electronic mortality software to strengthen the quality of cause-of-death certification4. Our institute participated in the project on the implementation of the ICMR-NCDIR e-Mortality (ICMR-NCDIR e-Mor) software from May 2020 to November 2022. Based on the learnings from the ICMR-NCDIR e-Mor software, our institute developed an in-house mortality software and linked it to the HIS since January 2021.
A structured plan was designed to streamline the transition to the digital mode of writing MCCDs. The existing manual workflow was shared with the developer to ensure accurate digitalisation, and a trial version was tested to address potential issues. End users received training through instructional videos, and guiding tips were embedded in the software to improve data quality. Customisations based on user feedback were incorporated during on boarding to reduce their workload compared to the manual process.
Studies assessing the impact of digitalising MCCD on completeness and error reduction are limited in the literature. This study aimed to evaluate the effectiveness of mortality software in enhancing the quality of MCCD. Specifically, it compared the completeness, error rates, and ICD-10 coding compatibility of the underlying cause of death (UCoD) before and after the software’s implementation.
Materials & Methods
After ethics committee approval, a retrospective study was undertaken on MCCD forms at the department of Medicine, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, a tertiary care centre in India. Approximately 3,000 to 3,500 deaths are reported annually from the institute, with 50 to 60 per cent occurring in the department of Medicine. We analysed 210 forms: 105 hand-written in 2020 and 105 software-generated in 2021, after implementing the mortality software. The forms were selected consecutively. Medico-legal forms were excluded. These forms were filled by the medicine residents treating the patients. The residents were trained to fill the MCCD process digitally through a screen-recording video with a voice-over, which was provided at the time of the software implementation. This study was conducted from March to July 2024.
Form-4, shown in supplementary figure 1, required clerical details (patient information, death event, certifying doctor) and technical details (causes of death). Technical details were divided into: Part I: Direct events leading to death (three lines: Ia, Ib, Ic) and Part II: Indirectly contributing conditions, like co-morbidities.
The feasible variables mentioned in the MCCD audit framework were used to assess the completeness and errors in both types of forms1. The completeness and errors were assessed by the study investigators who were trained in MCCD.
Completeness
The following 13 items were checked for completeness of MCCD namely, name of the hospital, ward number, date of death, time of death, name of the deceased, sex, age, at least one line filled in part I of the cause of death, the time interval between the onset of the condition and death, manner of death, pregnancy details if deceased was a female, name and signature of the medical officer certified the death.
Errors
Minor errors included illegible handwriting, abbreviations, and missing any of the above completeness items. Major errors included mechanism of death written in Part I (e.g., cardiac arrest), ambiguous/vague/ill-defined causes of death entered in Part I (e.g., Fever, back pain), multiple causes written in one line in any of the lines in Part I, improper sequence of events leading to death and contributing causes written in part I instead of part II (e.g., mentioning hypertension in part I in a death occurred due to viral pneumonia associated acute respiratory distress syndrome).
ICD-10 coding compatibility
The lowermost cause mentioned in Part I (Supplementary Fig. 1) is considered the UCoD, and it is used for generating ICD-10 coding. The UCoD was checked on the WHO’s ICD-10 coding website to determine its compatibility with the generation of the ICD-10 code5.
Sample size estimation
In a previous study6, the proportion of major error was 82 per cent. Assuming a similar percentage of major errors among the forms before the introduction of eMor software, expecting an absolute reduction of 12 per cent after the introduction of the software (reducing major error from 82 to 70%), with a correlation of 0.5, power of 80 per cent and alpha error of five per cent, the estimated sample size was 105 pairs. The total sample was 210. The sample size is calculated using STATA version 17 software, assuming a difference in proportions between pre- and post-samples (McNemar test).
Statistical analysis
Categorical variables were mentioned in percentages. The McNemar test was used to analyse changes in completeness and error rates of data before and after implementing the mortality software. All statistical analyses were conducted at a 5 per cent significance level, with a P value <0.05 considered statistically significant. Data analysis was performed using Stata Statistical Software: Release 12 (College Station, TX: StataCorp LP, USA). Microsoft Excel was used to create a graph.
Results
Of thirteen completeness items (Table I), only three were filled in all hand-written forms, compared to eleven in all software-generated forms. Notable improvement in completeness was observed in two items, namely, the time interval between disease onset and death (rising from 7 to 20%) and pregnancy details (increasing from 70 to 100%).The doctor’s name was included in all software-generated forms, up from 94 per cent in hand-written forms. Fully completed forms (13/13 items) rose from 4 to 19 per cent.
| S. No. | Variable written in forms | Hand-written form (n -105), n (%) | Software-generated form (n -105), n (%) | P value |
|---|---|---|---|---|
| 1 | Name of the hospital | 105 (100) | 105 (100) | 1 |
| 2 | Ward number | 105 (100) | 105 (100) | 1 |
| 3 | Date of death | 105 (100) | 105 (100) | 1 |
| 4 | Time of death | 102 (97) | 105 (100) | 0.25 |
| 5 | Name | 104 (99) | 105 (100) | 1 |
| 6 | Age | 104 (99) | 105 (100) | 1 |
| 7 | Gender | 104 (99) | 105 (100) | 1 |
| 8 | At least one line filled in part I of cause of death | 104 (99) | 105 (100) | 1 |
| 9 | Time interval between the onset of the condition and death | 7 (7) | 21 (20) | 0.009 |
| 10 | Manner of death | 104 (99) | 105 (100) | 1 |
| 11 | Pregnancy details if deceased is a female* | 25/36 (70) | 40/40 (100) | 0.001 |
| 12 | Signature of the medical officer | 102 (97) | 104 (99) | 0.62 |
| 13 | Name of the medical officer | 99 (94) | 105 (100) | 0.03 |
| 14 | Number of missing variables out of 13 variables analysed | |||
| 0 (fully completed) | 4 (4) | 20 (19) | 0.001 | |
| 1 | 93 (88) | 85 (81) | 0.18 | |
| 2 | 7 (7) | 0 | - | |
| 5 | 1 (1) | 0 | - | |
As shown in table II, Minor errors decreased from 96 to 81 per cent, while the use of abbreviations remained almost the same (5 vs. 4%). Major errors dropped significantly, with forms free of major errors increasing from 12 to 58 per cent. The compatibility of the UCoD for ICD-10 coding improved from 73 to 96 per cent (P<0.001) as depicted in figure.
| S. No. | Errors | Hand-written form (n-105), n (%) | Software generated form (n-105), n (%) | P value |
|---|---|---|---|---|
| I | Minor errors | |||
| 1 | Illegible handwriting | 2 (2) | 0 (0) | 0.5 |
| 2 | Abbreviations used in cause of death | 5 (5) | 4 (4) | 1 |
| 3 | Any error in clerical details | 101 (96) | 85 (81) | 0.002 |
| II | Major errors | |||
| 1 | Mechanism of death written in part I | 19 (18) | 8 (7.6) | 0.02 |
| 2 | Ambiguous/ Vague/ ill-defined cause of death in part I | 6 (6) | 0 (0) | 0.03 |
| 3 | Multiple causes written in one line in part I | 81 (77) | 27 (26) | <0.001 |
| 4 | Improper sequencing of events leading to death | 42 (40) | 12 (11) | <0.001 |
| 5 | Contributing causes written in Part I instead of Part II | 19 (18) | 7 (7) | 0.02 |
| 6 | Forms with any major error | 92 (88) | 44 (42) | <0.001 |
P<0.05 was considered significant. MCCD, medical certification of cause of death
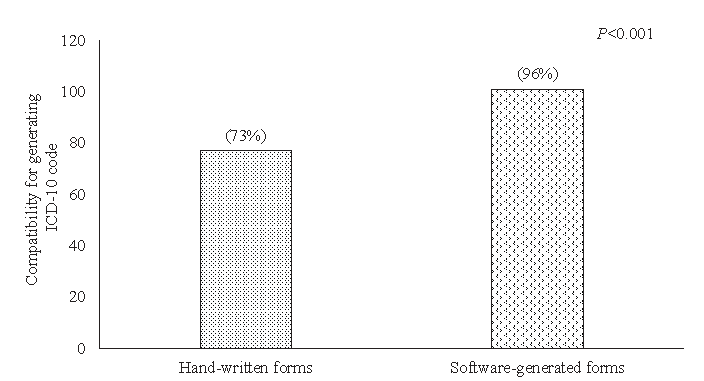
- Compatibility of UCoD for generating ICD-10 coding. UCoD, underlying cause of death; ICD-10, International Classification of Diseases-10.
Discussion
The introduction of mortality software significantly improved MCCD form completeness and reduced both minor and major errors, enhancing ICD-10 coding compatibility.
Patil et al3 reported only 4.4 per cent completeness in MCCD forms. In our study, completeness improved from 4 per cent in hand-written forms to 19 per cent with software use. The main deficiency in software-generated forms was the ‘time interval between disease onset and death,’ present in only 20 per cent of forms. Uplap et al7 reported similar issues, with only 0.5 per cent completeness for this detail7. Improved completion of this item could further enhance the completeness and accuracy of MCCD, as it is crucial to determine the sequence of events leading to death.
The use of ICMR-NCDIR e-MOR software simplified the process of MCCD writing and reduced the burden of doctors. The user interface framework of fields in our in-house software was developed based on the learnings from ICMR-NCDIR eMOR software. Our software is designed in such a way that doctors certifying death must enter their credentials to log in and enter the patient’s hospital number to begin filling the form. The software then automatically retrieves the patient’s demographic details and the doctor’s information. Mandatory fields include the date, time, place, manner of death, address, mode of dying, Immediate cause of death (I-a), a short description of circumstances of injury in unnatural death, type of medical attention received before death, details of substance abuse, and pregnancy details if applicable. The form cannot be submitted without completing these fields. Free text writing is allowed to mention the causes of death in part I and part II. However, the time interval between disease onset and death is not mandatory. Additionally, the signature is added after the form is printed, resulting in persisting deficiencies in the above two areas. The software had the limitation of not having in-built validation for ensuring the completeness of the form. Making time intervals mandatory and using digital signatures will help reduce these errors. The ICMR-NCDIR eMOR software had some advantages over our mortality software, including detailed capture of neoplasm classification and staging, validation checks to prevent gender-based diagnosis mismatches, and in-built ICD-10 coding functionality for improved accuracy.
Before software implementation, 96 per cent of hand-written forms had minor errors, which decreased to 81 per cent post-implementation (P<0.002). Illegible handwriting was found in only two per cent of hand-written forms, compared to 26 per cent in the study by Patil et al3. The software eliminated illegibility by digital entry. Major errors decreased from 88 per cent in hand-written forms to 42 per cent in software-generated forms. A retrospective study of 104 hand-written forms conducted in Karnataka revealed that major errors were present in 82 per cent of forms6.
As shown in supplementary figure 2, our software includes a drop-down menu for selecting the mode of death to prevent its misuse in Part I, a key feature adopted from the ICMR-NCDIR e-Mor software. It highlights the description of immediate and antecedent causes-of-death in red to guide accurate entries. Unlike hand-written forms with just two lines for contributing causes, the software lists a few common co-morbid conditions and allows additional entries (e.g., cancer site and histology), which helps reduce major errors. Major errors remained high despite the introduction of the software, likely due to the lack of continuous training and feedback for residents.
This study demonstrated the use of mortality software for recording MCCD, which impacts the quality of information in terms of completeness and accuracy. The study also highlights the use of the framework for assessing MCCD audits to evaluate of the quality of MCCD in the hospital.
The study’s limitations include its focus on the department of Medicine only, the analysis of MCCD forms for deaths due to natural causes only, and the absence of cross-verification of these forms with case files, which may impact the generalisability of the findings.
Future studies should aim to include other departments and extend to MCCD forms for medicolegal deaths. This will enhance the applicability of the findings. Mortality software is not yet widely adopted, and further research is needed to explore effective implementation strategies.
Overall, this study found that our electronic Mortality software significantly improved completeness and modestly reduced errors. The study demonstrated that the use of technology solutions can improve the quality of data largely. Other institutions may consider adopting an electronic format for MCCD to improve completeness and accuracy. We emphasise regular training of doctors and auditing of MCCD forms to further improve the quality of death certification.
Acknowledgment
Authors acknowledge receiving funding for standardising the MCCD system by implementation of ICMR-NCDIR e-Mor software vide grant number: NCDIR/e-mor-MCCD/22/2020, to strengthen MCCD in a project mode. The expertise and guidance received during this project enabled the integration of several features of ICMR-NCDIR e-Mor software in our in-house mortality software system.
Financial support & sponsorship
The first author (AT) received funding under JIPMER’s Golden Jubilee Short-Term Research Award for Undergraduate Students (GJ STRAUS 2024; JIP/UGRMC/GJSTRAUS/2024/1).
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Framework for audit of medical certification of cause of death at health facility. 2022. Available from: https://ncdirindia.org/All_Reports/MCCD_Frmwrk/Framework_MCCD_Audit.pdf, accessed on December 27, 2024.
- Annual report on Medical Certification of Cause of Death-2020. Available from: https://censusindia.gov.in/nada/index.php/catalog/42681, accessed on December 27, 2024.
- Death certification errors in medical certificates of cause of death related to COVID-19 disease. Int J Community Med Public Health. 2022;9:3746-52.
- [Google Scholar]
- ICMR-NCDIR electronic Mortality software (ṈCDIR e-Mor) (internet).. Available from: https://ncdirindia.org/e-mor/test_emor/default.aspx, accessed on December 27, 2024.
- ICD-10 Version: 2019 [homepage on the internet]. Available at: https://icd.who.int/browse10/2019/en, accessed on December 27, 2024.
- Critical analysis of errors in medical certification of cause of death [MCCD] in a teaching hospital. Int J Forens Sci. 2019;4:000157.
- [Google Scholar]
- Assessment of medical certificate of cause of death at a tertiary care centre in mumbai, India. Indian J Forensic Community Med. 2019;6:70-4.
- [Google Scholar]






