Translate this page into:
Impact of hepatitis B immunization among the Nicobarese tribe - antibody titres & seroprotection five years after vaccination
Reprint requests: Dr A.P. Sugunan, Scientist-E, Regional Medical Research Centre (ICMR), Post Bag No.13, Port Blair 744 101, Andaman & Nicobar Islands, India e-mail: sugunanap@icmr.org.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
A total of 237 Nicobarese subjects who had received hepatitis B vaccination as part of mass vaccination project during 2000-2001 were screened for anti-HBsAg titres by quantitative ELISA five years after vaccination.
Methods:
Anti-HBsAg antibody was estimated using quantitative ELISA. Proportion of the subjects with protective levels of antibody and geometric mean antibody titres were calculated.
Results:
Among the 237 study subjects, 213 had received three doses of vaccine, 17 had received two doses and seven had received one dose. The geometric mean titres of anti-HBs antibodies were 201.7, 31.9 and 23.1 mIU/ml among those who received three, two and one dose of vaccine, respectively. Among those who received three doses of vaccination, 85.9 per cent had anti-HBs antibody levels of 10 mIU/ml or more, indicating seroprotection. The difference in the seroprotection rates among those who received three doses of vaccination (85.9%) and those who received less than three doses (58.3%) was significant. Seroprotection rates one month after the first, second and third dose of vaccination were 49.1, 86.9 and 96.7 per cent, respectively. It then declined to 89 per cent by the end of the second year and to 85.5 per cent by the end of the third year, but there was no decline thereafter.
Interpretation & conclusions:
Seroprotection rate reached at the maximum one month after the third dose of HBV vaccine. Although about 15 per cent of the vaccinated persons lost seroprotection by the end of the third year, no further loss in seroprotection was observed between the third year and the fifth year.
Keywords
Follow up
hepatitis B
Nicobarese
seroprotection
vaccination
It is estimated that more than one third of world's population has been infected with hepatitis B virus (HBV) and it causes more than million deaths each year. About 5 per cent of the population are chronic carriers of HBV, and nearly 25 per cent of all carriers develop serious liver diseases such as chronic hepatitis, cirrhosis and primary hepatocellular carcinoma12. The exact carrier rate of HBV in India is not known with certainity, though an earlier preliminary attempt has suggested an estimated carrier rate of 4.7 per cent3. The World Health Organization recommends administering the first dose of hepatitis B vaccine within 24 h of birth followed by 2 or 3 doses with a minimal interval of 4 wk4.
Andaman and Nicobar Islands, India, are home to six primitive tribes, and is highly endemic for hepatitis B infection. The largest tribal group, the Nicobarese, had an HBsAg carrier rate of 23.3 per cent5 which is probably one of the highest reported rates in India6. Considering the high endemicity, a pilot project of mass hepatitis B vaccination using an indigenously manufactured recombinant DNA vaccine was initiated in 2000-2001 in two villages of Car Nicobar islands inhabited exclusively by Nicobarese7. All the tribal persons aged 45 yr or less and residing in these villages and negative for hepatitis B surface antigen and antibody (HBsAg and anti-HBs) were vaccinated as part of the project following the standard schedule of three doses, the second dose one month after and the third six months after the first dose78. More than 95 per cent of the vaccinated people developed anti-HBs antibody titre of more than 10 mIU/ml indicating seroprotection after the third dose of vaccination, but proportion of seroprotected dropped to 85.5 per cent three years after the vaccination8. In this communication we present the status of seroprotection among the persons of Nicobarese tribe vaccinated in 2000 for hepatitis B infection, five years after vaccination.
Material & Methods
The study was conducted in 2006 in Regional Medical Research Centre (RMRC), Port Blair, Andaman & Nicobar Islands, India. All subjects who had received at least one dose of vaccine in 2000 as part of the vaccination project7 and could be contacted at the time of the survey were included in the study. Five ml venous blood sample was collected from each of these subjects. Serum was separated and the titre of anti-HBs was estimated using quantitative ELISA (ANTISURASE, General Biologicals, Taiwan). The study protocol was cleared by the institutional ethical committee of RMRC, Port Blair. The difference in the proportions of seroprotected among fully vaccination subjects and others was tested by chi-square test. GM titres were log transformed and compared using t-test.
Results & Discussion
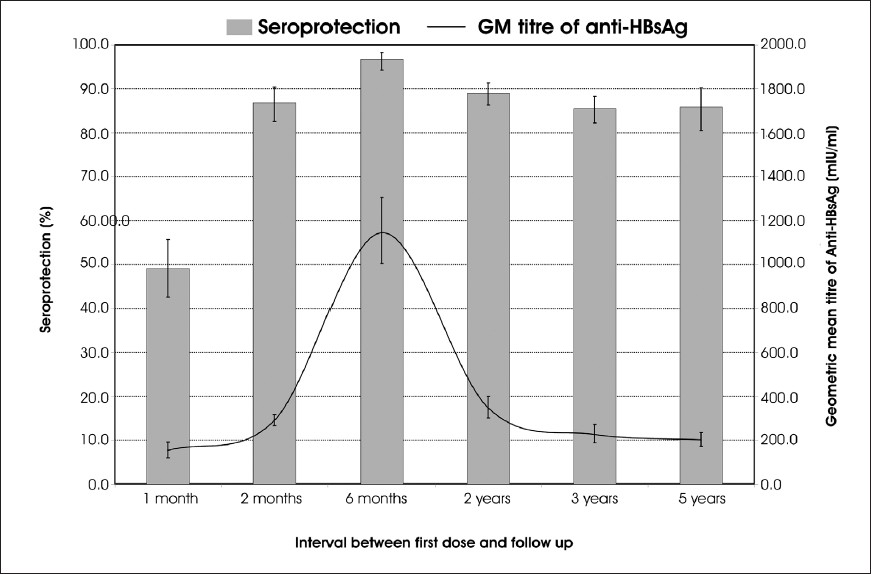
A total of 237 persons who were part of the original cohort for vaccination, were included in the study. Among them, 213 had received three doses of vaccine, 17 had received two doses and the remaining seven persons had received only one dose of vaccine. The geometric mean (GM) titres of anti-HBs antibodies were 201.7, 31.9 and 23.1 mIU/ml among those who received three, two and one dose of vaccine, respectively (Table). The GM titre of anti-HBs antibodies for those who received less than three doses of vaccine (29.0 mIU/ml) was significantly less than that for those who received three doses of the vaccine (P<0.0001). After the vaccination, the GM titre reached a peak level of about 1150 mIU/ml by six month of vaccination and then started declining slowly over the next years reaching a level of 200 mIU/ml by the end of five years (Fig.).
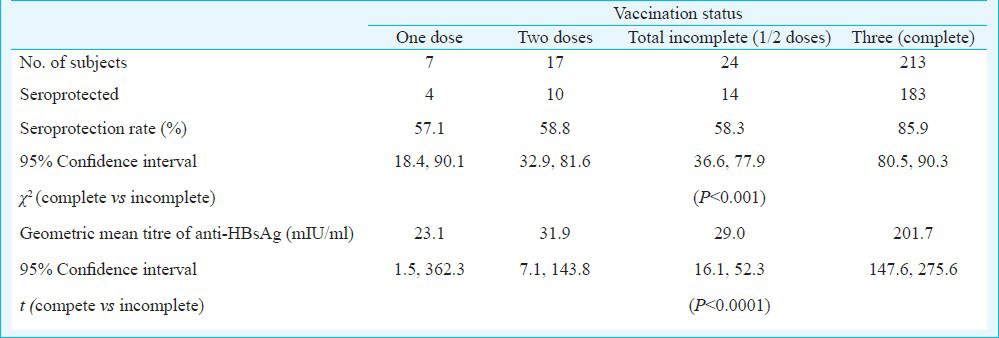
Among the 213 persons, who received three doses of vaccination, 85.9 per cent had anti-HBs antibody levels of 10 mIU/ml or more, indicating seroprotection9 (Table). Seroprotection rate among the subjects who received two doses of vaccine was 58.8 per cent and that among those who received only one dose of vaccine was 57.1 per cent (Table). The difference in the seroprotection rates among those who received three doses of vaccination (85.9%) and those who received less than three doses (58.3%) was significant (P<0.001).
Among the persons who received three doses of vaccination, 24 (11.3%, 95% CI: 7.4, 16.3) did not have any detectable levels of antibody at the end of five years and six persons (2.8%, 95% CI: 1.0, 6.0) had low levels of antibodies (<10 mIU/ml). While about half of the vaccinated persons (n=103 48.4%, 95% CI: 41.5, 55.3) had antibody titre in the range of 100-999.99 mIU/ml, 9.4 per cent (n=20) (95% CI: 5.8, 14.1) had antibody titres in the range of 10-99.99 mIU/ml and 28.2 per cent (n=60) (95% CI: 22.2, 34.7) and titres of 1000 mIU/ml or more.
Seroprotection rate one month after the first dose of vaccination was 49.1 per cent (95% CI: 42.6, 55.7). This increased to 86.9 per cent (95% CI: 82.6, 90.4) after the second dose and reached the peak of 96.7 (95% CI: 94.3, 98.3) one month after the third dose7. By second year the seroprotection rate declined to 89 per cent (95% CI: 86.3, 91.4) and by the end of the third year it declined further to 85.5 per cent (95% CI: 82.3, 88.3)78. The geometric mean titre of anti-HBs antibodies recorded a rise from 154 mIU/ml one month after vaccination to 291.1 after second dose and to the peak of 1145.7 after the third dose. It declined rapidly to 347.2 mIU/ml at the end of the second year78, but thereafter the decline was less drastic (Fig.).
Hepatitis B infection is a major public health problem among the Nicobarese6. The pilot project on vaccinating eligible persons in two villages of Car Nicobar was initiated as a first step of a hepatitis B control programme7. Hepatitis B vaccination has since been included in the primary immunization schedule of newborn children both in Andaman and Nicobar Districts10.
Although follow up studies conducted earlier showed good immunological response of the vaccinated persons soon after the completion of the vaccination schedule, the antibody levels in 15 per cent of the vaccinated persons dropped below protective levels by the end of three years8. However, neither the mean antibody titre nor the seroprotection rate decreased significantly between the third year and fifth year after vaccination. The trend in seroprotection rate was a rapid increase between first and second dose followed by a less marked rise and then a decline over the next 18 months to stabilize at around 85 per cent. In contrast, the GM titre showed a remarkable rise of more than three-fold between second and third dose and an equally steep decline the next 18 months to reach levels prior to the third dose. Although the trend indicates that the effect of the third dose of vaccination was to push up the antibody titre to high levels for a short period of time, it might also have contributed in retarding the drop of seroprotection rate after the third dose of vaccination, because the seroprotection rate among those who did not receive the third dose was significantly lower (58.3%) as compared to that among those who received full dose of vaccination (85.9%).
The trends in seroprotection rate and geometric mean titre of anti-HBs antibodies after vaccination reported by various studies showed considerable variations. While the seroprotection rate five years after immunization among under-5 children in South Africa11 was comparable to that observed in the present study (87%), in Belgium, where a booster dose was administered at 12 months, a higher seroprotection rate (92.5%) was observed12. In Taiwan, where more than 90 per cent of children below eight years of age were vaccinated, 88 per cent of children in the age group of 3-4 yr and 79.9 per cent of the children aged 5-6 yr were seroprotected after 15 yr of vaccination13.
The hepatitis B epidemiology among the Nicobarese is unique with very high carrier rate of HBsAg and both vertical and horizontal transmission of infection playing a role in maintaining the infection in the community6. There is some evidence to suggest that vertical transmission is more likely to lead to long-term carrier state of hepatitis B virus1415. Because of the high prevalence of hepatitis B in the Nicobarese community, the chance of repeated exposures to the virus is high. The impact of these factors might affect the eventual success of the universal vaccination programme.
In conclusion, seroprotection rate after HBV vaccination among the Nicobarese tribe peaked at 96.7 per cent one month after the third dose and then declined to 85.5 per cent by the end of the third year. However, there was no further significant decline in the seroprotection rate until the end of the fifth year.
Acknowledgment
The authors acknowledge the Indian Council of Medical Research, New Delhi, for providing financial grant for the study (Project NoTribal/9/2003-ECD II), and Lady Tata Memorial Trust for providing junior scholarship to the second author (HB).
References
- Community-based epidemiology of hepatitis B virus infection in West Bengal, India: prevalence of hepatitis B e antigen-negative infection and associated viral variants. J Gastroenterol Hepatol. 2005;20:1712-20.
- [Google Scholar]
- Prevalence of HBV in the general population of India. In: Sarin SK, Singhal AK, eds. Hepatitis B in India: problems and prevention. New Delhi: CBS Press; 1996. p. :5-16.
- [Google Scholar]
- World Health Organization. Hepatitis B vaccines. Wkly Epidemiol Rec. 2009;84:405-20.
- [Google Scholar]
- Prevalence of hepatitis B infection among the primitive tribes of Andaman & Nicobar islands. Indian J Med Res. 2000;111:199-203.
- [Google Scholar]
- Epidemiology of hepatitis B infection among the Nicobarese - a mongoloid tribe of the Andaman and Nicobar Islands, India. Epidemiol Infect. 2002;128:465-71.
- [Google Scholar]
- Immune response to an indigenously developed hepatitis-B (Shanvac B) vaccine in a tribal community of India. Vaccine. 2002;20:3431-5.
- [Google Scholar]
- Hepatitis B vaccination in a hyper endemic tribal community from India: assessment after three years. Vaccine. 2004;23:399-403.
- [Google Scholar]
- Hepatitis B vaccines: assessment of the seroprotective efficacy of two recombinant DNA vaccines. Clin Ther. 2001;23:392-403.
- [Google Scholar]
- Epidemiology of hepatitis B virus infection among the tribes of Andaman and Nicobar Islands, India. Trans R Soc Trop Med Hyg. 2008;102:729-34.
- [Google Scholar]
- The first five years of universal hepatitis B vaccination in South Africa: evidence for elimination of HBsAg carriage in under 5-year-olds. Vaccine. 2001;19:3919-26.
- [Google Scholar]
- Long-term persistence of anti-HBs after vaccination with a recombinant DNA yeast-derived hepatitis B vaccine: 8-year results. Vaccine. 1998;16:1933-5.
- [Google Scholar]
- Hepatitis B virus infection in children and adolescents in a hyperendemic area: 15 years after mass hepatitis B vaccination. Ann Intern Med. 2001;135:796-800.
- [Google Scholar]
- Risks of chronicity following acute hepatitis B virus infection: a review. Clin Infect Dis. 1995;20:992-1000.
- [Google Scholar]
- Epidemiology and prevention of hepatitis B virus infection. Int J Med Sci. 2005;2:50-7.
- [Google Scholar]