Translate this page into:
Immunomodulatory effects of cardiotrophin-1 on in vitro cytokine production of monocytes & CD4+ T-lymphocytes
Reprint requests: Dr Michael Fritzenwanger, Department of Internal Medicine I, Division of Cardiology, Friedrich-Schiller-University Jena, Erlanger Allee 101, 07740 Jena, Germany e-mail: Michael.Fritzenwanger@med.uni-jena.de
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
In congestive heart failure (CHF), increased concentrations of several cytokines including cardiotrophin-1 (CT-1) and immunactivation are found. This study was performed to evaluate whether CT-1 can induce in vitro cytokines in monocytes and CD4+ T-lymphocytes of healthy volunteers.
Methods:
The study was performed in vitro to see whether CT-1 can modulate monocyte or CD4+ T-lymphocyte interleukin (IL)-1β, -2, -4, -5, -10, interferon γ (IFNγ), and tumour necrosis factor α (TNFα) expression by flow cytometry following stimulation with CT-1 alone or together with lipopolysaccharide (LPS) or phorbol myristate acetate (PMA)/ionomycine (iono).
Results:
CT-1 increased the number of TNFα and IL-1β positive monocytes. LPS induced IL-10, TNFα, and IL-1β in monocytes but only IL-2 in CD4+ T-lymphocytes, whereas PMA/iono induced all cytokines besides IL-5 in monocytes and IL-1β in CD4+ T-lymphocytes. In LPS activated monocytes, CT-1 induced a concentration-dependent reduction in the number of TNFα positive monocytes. After LPS activation, CT-1 decreased the number of CD4+ lymphocytes positive for IL-2, IL-4, and IL-5. In addition, following PMA/iono stimulation, CT-1 initiated a concentration-dependent decrease of CD4+ T-lymphocytes positive for TNFα, IL-4, IL-5, and IL-10.
Interpretation & conclusions:
The present data show that in vitro CT-1 can activate monocytes and modulate cytokine production of activated CD4+ T-lymphocytes. We speculate that CT-1 may at least be partly responsible for immunactivation in CHF.
Keywords
Cardiotrophin-1
heart failure
immunactivation
Congestive heart failure (CHF) is a systemic syndrome affecting both the neurohormonal and autonomic nervous systems in addition to displaying immunactivation and haemodynamic effects. The activities of several cytokines are known to be modulated in CHF1. Numerous studies have shown increased serum concentrations of proinflammatory cytokines such as tumour necrosis factor α (TNFα), interleukin-1 (IL-1), IL-6, IL-18, and cardiotrophin-1 (CT-1) in CHF2–6.
Monocytes from patients with CHF are characterized by an increased production of TNFα after stimulation with LPS compared to monocytes from healthy controls7. Similar results have been shown in another study reporting that peripheral blood mononuclear cells (PBMC) from patients with CHF produced significantly more TNFα than PBMC from control persons8. Further, they found a correlation between levels of TNFα from PBMC and severity of heart failure. However, not only monocyte function was altered in CHF, but also that of CD4+ T-lymphocytes8.
T-helper lymphocytes can differentiate into specialized effector cell subsets named Th1 and Th2. Th1 helper cells are responsible for proinflammatory cellular immunity and express IL-2, IL-12, IL-15, IL-18, and interferon γ (IFNγ) while Th2 helper cells express IL-4, IL-5, IL6, IL-10, and IL-13 as well as mediating humoral immunity910. For example, T-lymphocytes of patients with CHF showed increased expression of TNFα, IFN, Fas ligand, IL-10, IL-18, and macrophage inflammatory protein (MIP)-1α in addition to increased surface activation markers such as CD69 and CD2511. Fukunaga et al12 found Th1 activation in CHF together with a difference between ischaemic cardiomyopathy and idiopathic dilated cardiomyopathy. It is speculated that bacterial translocation and, as a consequence, increased lipopolysaccharide (LPS) concentration in blood because of an impaired gut barrier could be responsible for monocyte/macrophage activation in CHF13.
Cardiotrophin-1 is a member of the IL-6 cytokine family that comprises IL-6, IL-11, ciliary neurotrophic factor, cardiotrophin-1, cardiotrophin-like cytokine, leukaemia inhibitory factor (LIF), neuropoietin, oncostatin M, IL-27, and IL-3114. All these cytokines bind to a specific receptor chain (IL-6R, IL-11R, LIFR). Following cytokine binding, the cytokine/receptor complex associates with glycoprotein 130 (gp130) causing tyrosine phosphorylation of gp130 leading to signal transduction via the Janus kinase (JAK)/signal transducer and activation of transcription 3 (STAT3) pathway1516. CT-1 is expressed in a time-dependent manner during embryogenesis of organs and in the heart during life. Moreover, it induces cardiac myocyte hypertrophy and is capable of preventing myocyte apoptosis via a mitogen dependent kinase pathway15. Increased CT-1 concentrations were detected in patients with acute myocardial infarction and CHF. CT-1 plasma concentration has been shown to correlate with the severity of left ventricular dysfunction1718. However, in an animal model, CT-1 was found not only to affect myocytes but also the vasculature by decreasing systemic vascular resistance19, induction of acute phase proteins in rat hepatocytes20, attenuation of endotoxin-induced acute lung injury21, and modulation of human umbilical vein endothelial cells and human PBMC2223.
We hypothesize that CT-1 is able to influence cytokine production in human monocytes and CD4+ T-lymphocytes and, moreover, the cytokine may induce Th1 response in CD4+ T-lymphocytes in vitro. Hence, in this study we investigated the effect of CT-1 on cytokine production in monocytes and CD4+ T-lymphocytes in vitro. The outcome of CT-1 was also analysed on cytokine production of LPS or phorbol myristate acetate (PMA)/iono activated monocytes or CD4+ T-lymphocytes.
Material & Methods
Reagents: Recombinant human CT-1 was purchased from R & D Systems (Wiesbaden, Germany) and dissolved according to the manufacturer's instruction. Brefeldin A, LPS from Escherichia coli O26:B6, ionomycin and PMA were purchased from Sigma chemicals (Deisenhofen, Germany).
Subjects: All subjects (mean age 35.3 ± 7.5 yr) were recruited from the staff of the Department of Internal Medicine I, Jena University Hospital, Friedrich-Schiller-University, Jena, Germany during January - April, 2010. Twelve apparently healthy volunteers (8 females and 4 males) were enrolled in this study. Approval for this study protocol was obtained from the Ethics Committee of the Friedrich-Schiller-University, Jena, and written informed consent were obtained from each subject.
Sample collection: Blood samples (9 ml) were collected in lithium heparin tubes from an antecubital vein using a clean venipuncture under controlled venous stasis at 0800 h. The first 2 ml of blood was discharged, and the remaining blood was used for further immediate analysis.
Cell culture: Human PBMCs were obtained by Ficoll-paque (Amersham Bioscience, Uppsala, Sweden) centrifugation. The cells were washed three times with PBS, resuspended in RPMI 1640 supplemented with 10 per cent foetal calf serum (FCS) and 1 per cent penicillin and streptomycin (Biochrom AG, Berlin, Germany). All stimulants and media were without significant endotoxin levels according to the manufacturers’ instructions. Pharmacological agents, dissolved in fresh medium, were added to the cells for defined time intervals and concentrations. Fresh medium was added to the cells as a control.
Immunofluorescent flow cytometric analysis of cytokine production: For intracellular staining, peripheral blood was collected in lithium-heparin tubes. Blood (100 μl) was added to RPMI-1640 medium including brefeldin A (final concentration: 1 μg/ml) and incubated for 6 h at 37°C. Next, erythrocytes were lysed by NH4Cl. After washing with PBS/2 per cent FCS, cells were stained with monoclonal antibodies against the surface antigens CD3 (Coulter-Immunotech, Krefeld, Germany) CD4 (Caltag, Hamburg, Germany) and CD8 (BD-Pharmingen, Heidelberg, Germany) (15 min, RT), followed by a washing step and by fixation with 100 μl 2 per cent paraformaldehyde for 10 min at room temperature. After another wash, the cells were incubated in 100 μl permeabilisation solution (0,1% saponin and 0,1% NaN3 in PBS) together with 1 μl directly conjugated anti-cytokine antibodies (IL-1β, IL-2, IL-4, IL-5, IL-10, IFN, and TNFα, all from BD-Pharmingen, Heidelberg, Germany) for 15 min at room temperature. Followed by a further wash with permeabilisation solution, the cells were resuspended in PBS/2 per cent FCS and fluorescence intensity was analyzed by flow cytometry (FACSCalibur, Becton-Dickinson, Heidelberg, Germany). For analysis, regions were defined by forward scatter and side scatter as well as CD3+/CD4+- or CD3+/CD8+-lymphocyte populations. Data were analyzed with CellQuest Software.
Statistical analysis: Because results were not normally distributed, Wilcoxon test was used to examine the effects of CT-1 and stimulation on cytokine production. Data were analyzed with WinSTAT for Excel. All volunteers were examined only once.
Results
Effect of CT-1 on cytokine expression in PBMC: Stimulation of PBMC with CT-1 revealed a concentration-dependent increase of intracellular TNFα and IL-1β positive staining monocytes (14.8±3.4% positive staining monocytes for TNFα without CT-1 vs. 22.4±4.6% with 100 ng/ml CT-1 equivalent to an increase of 51%, 7.4±2.4% positive staining monocytes for IL-1β without CT-1 vs. 10.6±3.0% with 100 ng/ml CT-1 equivalent to an increase of 43%, Fig. 1). CT-1 did not affect the expression of the other tested cytokines in monocytes and no modulatory effect of CT-1 was observed on the expression of the tested cytokines in CD4+ T-lymphocytes.

- Effect of various CT-1 concentrations on the number of monocytes staining positive for TNFα and IL-1β determined by flow cytometry after 6-h incubation period (n=11). Data are expressed as mean ± SEM, *P<0.05 vs. unstimulated monocytes.
Effect of CT-1 in co-stimulation with LPS on cytokine expression in PBMC: Because in severe decompensated heart failure, increased concentrations of endotoxin are found24, we tried to imitate this situation in part and stimulated PBMC with LPS (50 ng/ml) together with various concentrations of CT-1 for six hours. This LPS concentration was used based on earlier findings25.
LPS significantly induced TNFα, IL-1β, and IL-10 in monocytes but only IL-2 in CD4+ T-lymphocytes. Application of LPS together with CT-1 resulted in a CT-1 concentration-dependent decrease of the number TNFα positive staining monocytes whereas CT-1 had no significant effect on the other tested cytokines under these experimental conditions in monocytes (Fig. 2A). The stimulatory effect of CT-1 alone on TNFα concentration was much weaker than the effect of LPS on TNFα expression indicating that CT-1 is only a weak stimulus for the TNFα expression in monocytes. In CD4+ T-lymphocytes, LPS stimulation together with CT-1 resulted in a CT-1 concentration-dependent decrease of IL-2, IL-4, and IL-5 (Fig. 2B).
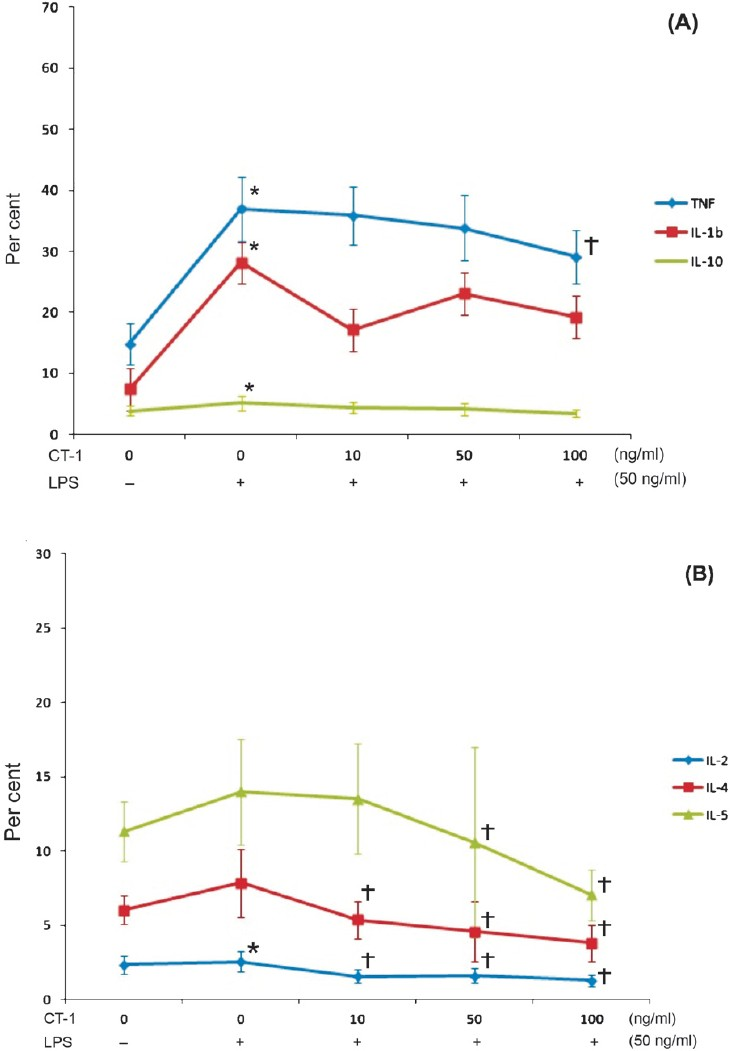
-
A. Effect of LPS and various CT-1 concentrations on monocyte TNFα, IL-5, and IL-10 production determined by flow cytometry after 6-h incubation period (n=11). Data are expressed as mean ± SEM, *P<0.05 vs. unstimulated monocytes, †P<0.05 vs. LPS alone. B. Effect of LPS and various CT-1 concentrations on CD4+ T-lymphocyte IL-2, IL-4, and IL-5 production determined by flow cytometry after 6-h incubation period (n=12). Data are expressed as mean ± SEM, *P<0.05 vs. unstimulated CD4+ T-lymphocytes, †P<0.05 vs. LPS alone.
Effect of CT-1 in co-stimulation with PMA/iono on cytokine expression in PBMC: In the next sets of experiments, PMA was used together with ionomycin for PBMC stimulation. PMA/iono caused a significant induction of IFN, TNFα, IL-2, IL-4, and IL-10 and a significant inhibition of IL-1β expression in monocytes. CT-1 had no significant effect on the tested cytokines in monocytes.
CD4+ T-lymphocytes activated with PMA/iono exhibited a significant increase of the measured cytokines with the exception of IL-1β (not shown) and IL-5 (Fig. 3). Increasing CT-1 concentrations caused a concentration-dependent decrease of TNFα, IL-4, IL-5, and IL-10 in activated CD4+ T-lymphocytes. The stimulatory effect of PMA/iono was more pronounced compared to the stimulatory effect of LPS on CD4+ T-lymphocytes.
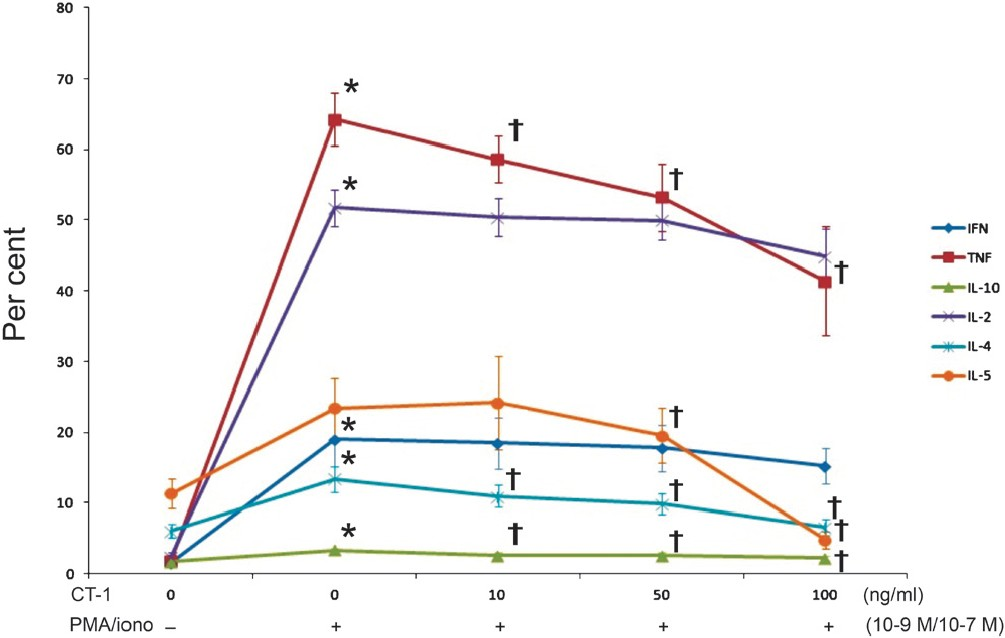
- Effect of PMA/iono and various CT-1 concentrations on CD4+ T-lymphocyte IFN, TNFα, IL-1β, IL-2, IL-4, IL-5, and IL-10 production determined by flow cytometry after 6-h incubation period (n=12). Data are expressed as mean ± SEM, *P<0.05 vs. unstimulated CD4+ T-lymphocytes, †P<0.05 vs. PMA/iono alone.
Modulation of Th1/Th2 cytokines by CT-1 in vitro: The capability of CT-1 to modulate the ratio of IFN/IL-4 in activated CD4+ T-lymphocytes in vitro was investigated. Both CT-1 alone and LPS together with CT-1 did not systematically influence the ratio of IFN/IL-4 in CD4+ T-lymphocytes (data not shown). PMA/iono caused an increase of the IFN/IL-4 ratio. In PMA/iono activated CD4+ T-lymphocytes CT-1 caused a concentration-dependent increase of the ratio IFN/IL-4 (Fig. 4). This increase was due to a significant reduction in the number of IL-4+ CD4+ T-lymphocytes after CT-1 application as CT-1 only slightly and insignificantly decreased the number of IFN+ CD4+ T-lymphocytes.
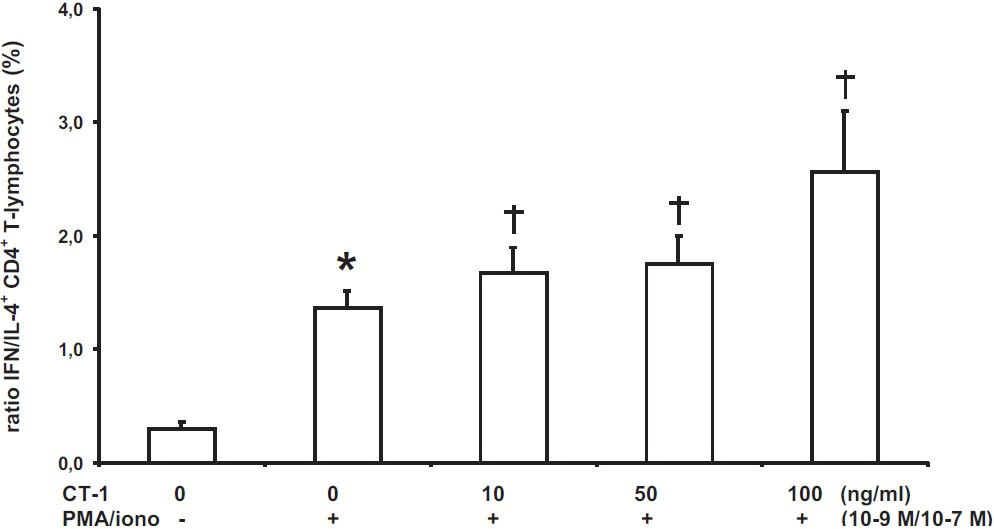
- Ratio of IFN/IL-4 expressing CD4+ T-lymphocytes after PMA/iono stimulation and with various CT-1 concentrations determined by flow cytometry after a 6-h period (n=12). Data are expressed as mean ± SEM, *P<0.05 vs. unstimulated CD4+ T-lymphocytes, †P<0.05 vs. PMA/iono alone.
Discussion
Our study examined in vitro cytokine expression of PBMC in healthy volunteers following stimulation with CT-1, a cytokine found increased in CHF which is mainly produced by the failing ventricle26. CT-1 caused a concentration-dependent increase in TNFα and IL-1β in monocytes without affecting cytokine production in CD4+ T-lymphocytes. Our in vitro data are in good agreement with the observation of Conraads et al27 who demonstrated that native monocytes from CHF patients produced more IL-1β and TNFα than monocytes from healthy controls. Further, cytokine expression increased with disease activity in this study27. Although the present results were obtained in vitro using PBMC of healthy volunteers, CT-1 caused monocyte activation similar to that found in CHF. Further, in vitro CT-1 caused monocyte activation without impairment of the physiologic barrier function of the gut and without bacterial translocation, as well as in the absence of increased serum endotoxin concentrations thought to be responsible for monocyte activation in CHF2427. Not only monocyte function is modulated in CHF, but enhanced expression of proinflammatory cytokines and activation markers of T-cells have also been reported11. Fukunaga et al28 demonstrated that in CHF more CD4+ T-lymphocytes produce IFN and that the number of CD4+ IFN producing T-lymphocytes increases with disease activity. The quantity of CD4+ T-lymphocytes staining for IL-4, however, was not affected significantly28. This group also showed that CHF patients with ischaemic heart disease display a higher proportion of IFN positive CD4+ T-lyphocytes than patients with idiopatic dilated cardiomyopathy12. In contrast to monocytes, CT-1 alone did not influence cytokine expression of CD4+ T-lymphocytes. This indicates that CT-1 alone cannot be responsible for CD4+ T-lymphocyte activation in vitro. The exact mechanism of CD4+ T-lymphocyte activation in CHF is not fully understood and remains to be further elucidated.
Our study also revealed that CT-1 caused a downregulation of several cytokines in activated monocytes and CD4+ T-lymphocytes. In an animal model of endotoxin-induced lung injury, CT-1 abrogated endotoxin-induced neutrophil accumulation and lung oedema21. Further, CT-1 attenuated endotoxin-induced endothelium-dependent and -independent impaired pulmonary vasorelaxation21. Despite decreasing cytokine production in activated monocytes and CD4+ T-lymphocytes, CT-1 caused a shift to a Th-1 inflammatory pattern in activated CD4+ T-lymphocytes which could be proven by an increasing IFN/IL-4 ratio. The shift towards a Th-1 inflammatory state in CHF has been previously described12. However, the shift of IFN/IL-4 ratio in vivo was driven by the increasing number of IFN producing CD4+ T-cells whereas in our in vitro study, the higher IFN/IL-4 ratio in CD4+ T-lymphocytes was due to a pronounced decrease in the number of IL-4 producing CD4+ T-lymphocytes rather than because of an increase of IFN producing T-lymphocytes compared to the quantity of IFN producing CD4+ T-lymphocytes. Our data suggested that in vivo CT-1 might not be responsible for the Th-1 inflammatory shift in CHF.
Regarding study limitations, one constraint of the current study could be the high CT-1 concentrations used compared to concentrations reported in patients with CHF17. Natal et al29 found serum CT-1 concentrations of about 100 ng/ml in healthy controls and in patients with metabolic syndrome. Hence, CT-1 serum concentrations in both healthy persons and patients are still a matter of discussion. Yet, independent of the reported CT-1 serum concentration, the amount of CT-1 is likely to be much higher in the myocardium as it is the source of CT-1 in CHF26. Although precise intramyocardial CT-1 concentrations are not known to date, mRNA and immunhistochemical studies showed increased CT-1 expression in hearts of patients with CHF30. Since several proinflammatory cytokines are elevated and PBMC are activated in CHF, it is difficult to study the effect of a single cytokine in PBMC of patients with CHF. For this reason, we employed PBMC from healthy volunteers in culture and stimulated these with recombinant human CT-1. This simplification may explain why all reported changes of cytokine expression in monocytes and CD4+ T-lymphocytes in CHF could not be determined after stimulation with CT-1. Another reason may be the fact that the expression of cytokines was determined following an incubation period of 6 h. However, there are no reported data for longer incubation periods.
In conclusion, our study demonstrates that in vitro the cytokine CT-1 can induce several cytokines in monocytes which are also found increased in CHF. Our data support a new mechanism responsible for monocyte activation in CHF without LPS translocation in the gut. CT-1 may have several modulatory effects on cytokine expression of activated monocytes and activated CD4+ T-lymphocytes. Because this effect independent of the stimulus was decreased in a concentration-dependent manner by CT-1, it is speculated that elevated CT-1 in CHF may be able to modulate monocyte and CD4+ T-lymphocyte activation found in CHF.
Acknowledgment
Authors thank Annett Schmidt for technical assistance, and Nasim Kroegel for editing the manuscript.
References
- Pathophysiological role of cytokines in congestive heart failure. Annu Rev Med. 2001;52:15-27.
- [Google Scholar]
- Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83:376-82.
- [Google Scholar]
- Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J Am Coll Cardiol. 1996;28:964-71.
- [Google Scholar]
- Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201-6.
- [Google Scholar]
- Increased circulating soluble form of Fas in patients with dilated cardiomyopathy. Jpn Circ J. 1998;62:873-6.
- [Google Scholar]
- Monocyte activation in congestive heart failure due to coronary artery disease and idiopathic dilated cardiomyopathy. Int J Cardiol. 1998;63:237-44.
- [Google Scholar]
- Elevated tumor necrosis factor alpha of blood mononuclear cells in patients with congestive heart failure. Int J Cardiol. 1999;71:257-61.
- [Google Scholar]
- Enhanced expression of inflammatory cytokines and activation markers in T-cells from patients with chronic heart failure. Cardiovasc Res. 2003;60:141-6.
- [Google Scholar]
- Relation between CD4+ T-cell activation and severity of chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2007;100:483-8.
- [Google Scholar]
- Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am J Cardiol. 1997;79:1426-30.
- [Google Scholar]
- Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227-36.
- [Google Scholar]
- Expression cloning of cardiotrophin 1, a cytokine that induces cardiac myocyte hypertrophy. Proc Natl Acad Sci USA. 1995;92:1142-6.
- [Google Scholar]
- Cardiotrophin-1. Biological activities and binding to the leukemia inhibitory factor receptor/gp130 signaling complex. J Biol Chem. 1995;270:10915-22.
- [Google Scholar]
- Non-competitive immunochemiluminometric assay for cardiotrophin-1 detects elevated plasma levels in human heart failure. Clin Sci (Lond). 2002;102:411-6.
- [Google Scholar]
- Plasma cardiotrophin-1 following acute myocardial infarction: relationship with left ventricular systolic dysfunction. Clin Sci (Lond). 2002;102:9-14.
- [Google Scholar]
- Effects of cardiotrophin-1 on haemodynamics and cardiac function in conscious rats. Cytokine. 1998;10:19-25.
- [Google Scholar]
- Murine cardiotrophin-1 stimulates the acute-phase response in rat hepatocytes and H35 hepatoma cells. J Interferon Cytokine Res. 1996;16:69-75.
- [Google Scholar]
- Cardiotrophin-1 attenuates endotoxin-induced acute lung injury. J Surg Res. 1999;84:240-6.
- [Google Scholar]
- Cardiotrophin-1 induces intercellular adhesion molecule-1 expression by nuclear factor kappaB activation in human umbilical vein endothelial cells. Chin Med J (Engl). 2008;121:2592-8.
- [Google Scholar]
- Cardiotrophin-1 induces interleukin-6 synthesis in human monocytes. Cytokine. 2007;38:137-44.
- [Google Scholar]
- Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet. 1999;353:1838-42.
- [Google Scholar]
- LPS-induced release of IL-1 beta, IL-6, IL-8, TNF-alpha and sCD14 in whole blood and PBMC from persons with high or low levels of HDL-lipoprotein. Cytokine. 1994;6:521-9.
- [Google Scholar]
- The heart is a source of circulating cardiotrophin-1 in humans. Biochem Biophys Res Commun. 2000;279:320-3.
- [Google Scholar]
- Intracellular monocyte cytokine production and CD 14 expression are up-regulated in severe vs mild chronic heart failure. J Heart Lung Transplant. 2005;24:854-9.
- [Google Scholar]
- Expression of interferon-gamma and interleukin-4 production in CD4+ T cells in patients with chronic heart failure. Heart Vessels. 2007;22:178-83.
- [Google Scholar]
- Cardiotrophin-1 is expressed in adipose tissue and upregulated in the metabolic syndrome. Am J Physiol Endocrinol Metab. 2008;294:E52-60.
- [Google Scholar]
- Augmented expression of cardiotrophin-1 in failing human hearts is accompanied by diminished glycoprotein 130 receptor protein abundance. Circulation. 2002;106:1442-6.
- [Google Scholar]






