Translate this page into:
Image guided versus palpation guided core needle biopsy of palpable breast masses: a prospective study
Reprint requests: Dr Smriti Hari, Department of Radio-diagnosis, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110 029, India e-mail: smritihari@hotmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Biopsy of palpable breast masses can be performed manually by palpation guidance or under imaging guidance. Based on retrospective studies, image guided biopsy is considered more accurate than palpation guided breast biopsy; however, these techniques have not been compared prospectively. We conducted this prospective study to verify the superiority and determine the size of beneficial effect of image guided biopsy over palpation guided biopsy.
Methods:
Over a period of 18 months, 36 patients each with palpable breast masses were randomized into palpation guided and image guided breast biopsy arms. Ultrasound was used for image guidance in 33 patients and mammographic (stereotactic) guidance in three patients. All biopsies were performed using 14 gauge automated core biopsy needles. Inconclusive, suspicious or imaging-histologic discordant biopsies were repeated.
Results:
Malignancy was found in 30 of 36 women in palpation guided biopsy arm and 27 of 36 women in image guided biopsy arm. Palpation guided biopsy had sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of 46.7, 100, 100, 27.3 per cent, respectively, for diagnosing breast cancer. Nineteen of 36 women (52.8%) required repeat biopsy because of inadequate samples (7 of 19), suspicious findings (2 of 19) or imaging-histologic discordance (10 of 19). On repeat biopsy, malignancy was found in all cases of imaging-histologic discordance. Image guided biopsy had 96.3 per cent sensitivity and 100 per cent specificity. There was no case of inadequate sample or imaging-histologic discordance with image guided biopsy.
Interpretation & conclusions:
Our results showed that in palpable breast masses, image guided biopsy was superior to palpation guided biopsy in terms of sensitivity, false negative rate and repeat biopsy rates.
Keywords
Breast biopsy
breast cancer
image guided
palpation guided
Carcinoma of breast is the most common malignancy diagnosed in women worldwide. An estimated 1.67 million women across the world were diagnosed with breast cancer in 2012, accounting for 25 per cent of all cancers diagnosed in women1. In India, incidence of breast cancer ranged from 31 to 36.6 cases per 100,000 per year in metropolitan cities. In rural India, the incidence was lower and ranged from 7 to 14.4 cases per 100,000 per year2. Unlike in developed countries where screening mammography is practiced, most of the patients in developing countries present with a palpable breast lump. Pathological diagnosis of the breast lump is established using fine needle aspiration cytology (FNAC), core needle biopsy or surgical biopsy.
FNAC is safe, least invasive and inexpensive. However, it is plagued by high false negative rates and insufficiency rates3. Further, FNAC cannot distinguish in situ from invasive cancer. Immunohistochemistry analysis is also difficult with FNAC which is a deciding factor for neo-adjuvant chemotherapy. Open surgical biopsy, although an accurate method for obtaining tissue samples has to be performed in an operation theatre leading to patient anxiety and also results in local scarring and cosmetic deformity. Core needle biopsy provides adequate sample for histological analysis and compared to open surgical biopsy, it has several advantages like better patient tolerance, better cosmetic outcome and low cost4. While non-palpable breast lesions are always biopsied under image guidance, biopsy of palpable masses can be performed either blindly with palpation or under image guidance.
Image guided biopsies are considered to be more accurate than palpation guided biopsies; however, there is insufficient evidence in the literature. This opinion is largely based on personal experiences as well as a few retrospective studies5678. Most of these studies were retrospective analysis of large data in heterogeneous patient population having both palpable and non-palpable breast lesions where various biopsy techniques and guiding modalities were used. Selection bias was another pitfall in these studies, as deep seated or vaguely palpable breast lumps were more likely to be referred for image guided biopsy6. This prospective study was conducted to verify whether image guided core biopsies were more accurate in palpable breast masses and to determine the size of beneficial effect of image guided biopsy over palpation guided biopsy.
Material & Methods
This study was conducted in the departments of Radio-diagnosis and Surgery at All India Institute of Medical Sciences (AIIMS), New Delhi, India, over the period of 18 months from October 2008 to April 2010. Patients with palpable breast masses which could be seen on ultrasound and/or mammography were invited to participate in the study. Masses which were reported as definitely benign on imaging (e.g. simple cysts) were excluded. During this period, 72 consecutive women, who met the inclusion criteria, agreed to participate in the study and gave informed consent. The study protocol was approved by the ethics committee of the institute. The median age was 45 yr (range 16-70 yr). All the patients were subjected to ultrasound examination. Mammography was available with 61 patients in whom Breast Imaging-Reporting and Data System (BI-RADS) categories were assigned as per American College of Radiology (ACR) guidelines9. Mammography was not performed in 11 patients as they were younger than 30 yr of age. Ultrasound features alone were used to assign BI-RADS categories to these 11 patients using breast ultrasound BI-RADS. Patients whose lesions were definitely benign (BI-RADS 2) on imaging were excluded from the study. Sixty patients had BI-RADS 4 or 5 lesions. Twelve patients had BI-RADS 3 lesions which were subjected to biopsy on surgeons’ or patients’ request. Patients were randomized into two arms of 36 each to palpation guided and image guided biopsy, using block randomization with allocation ratio of 1:1 (Fig. 1).
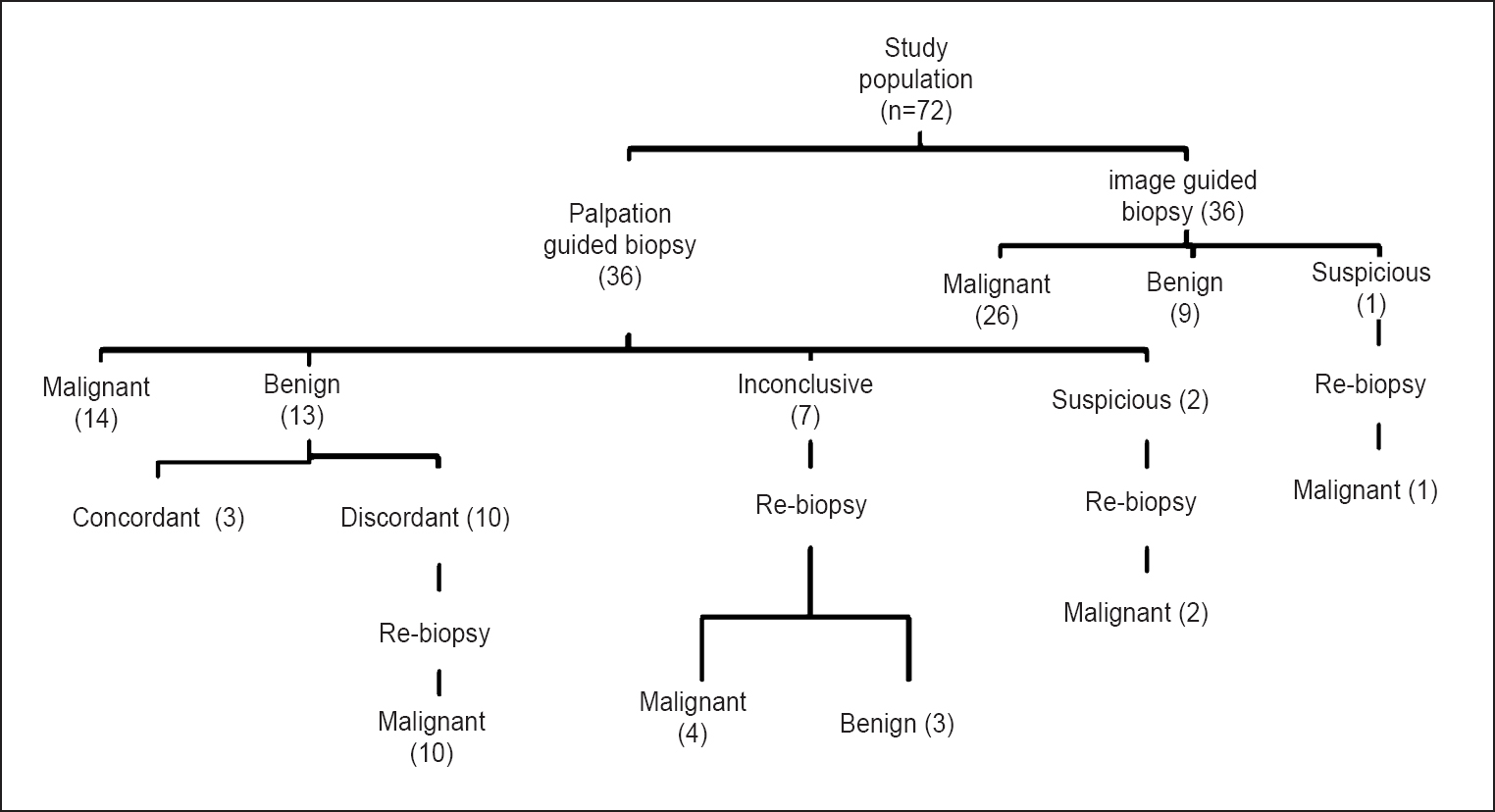
- Flow chart showing inclusion of patients in the study.
Biopsies were performed under local anaesthesia using 14G automated core needle biopsy guns (Bard, Arizona, USA or US biopsy gun, Franklin, IN, USA). Ultrasound was the preferred modality for image guidance and was used in 33 patients. Broad based multi-frequency probe was used and all procedures were performed on same ultrasound unit (Envisor HD, Philips Medical Systems, Bothel, WA, USA). Three patients were subjected to stereotactic biopsy as they had pleomorphic microcalcifications on mammogram in the region of palpable lump. An add-on stereotactic biopsy device with digital spot imaging (Opdima, Philips Medical systems, The Netherlands) was used for this purpose.
On ultrasound guided biopsy, visualization of needle tip in the lesion in orthogonal planes was mandatory before taking the samples (Fig. 2a-d). The adequacy of cores was assessed visually based on size and consistency of the samples. Complete unfragmented cores that sank in the formalin jar were considered optimal. In addition, specimen radiography was performed after stereotactic biopsy to confirm calcification retrieval. We attempted to obtain minimum of five good cores in each case as per the recommendation in the literature5610111213.
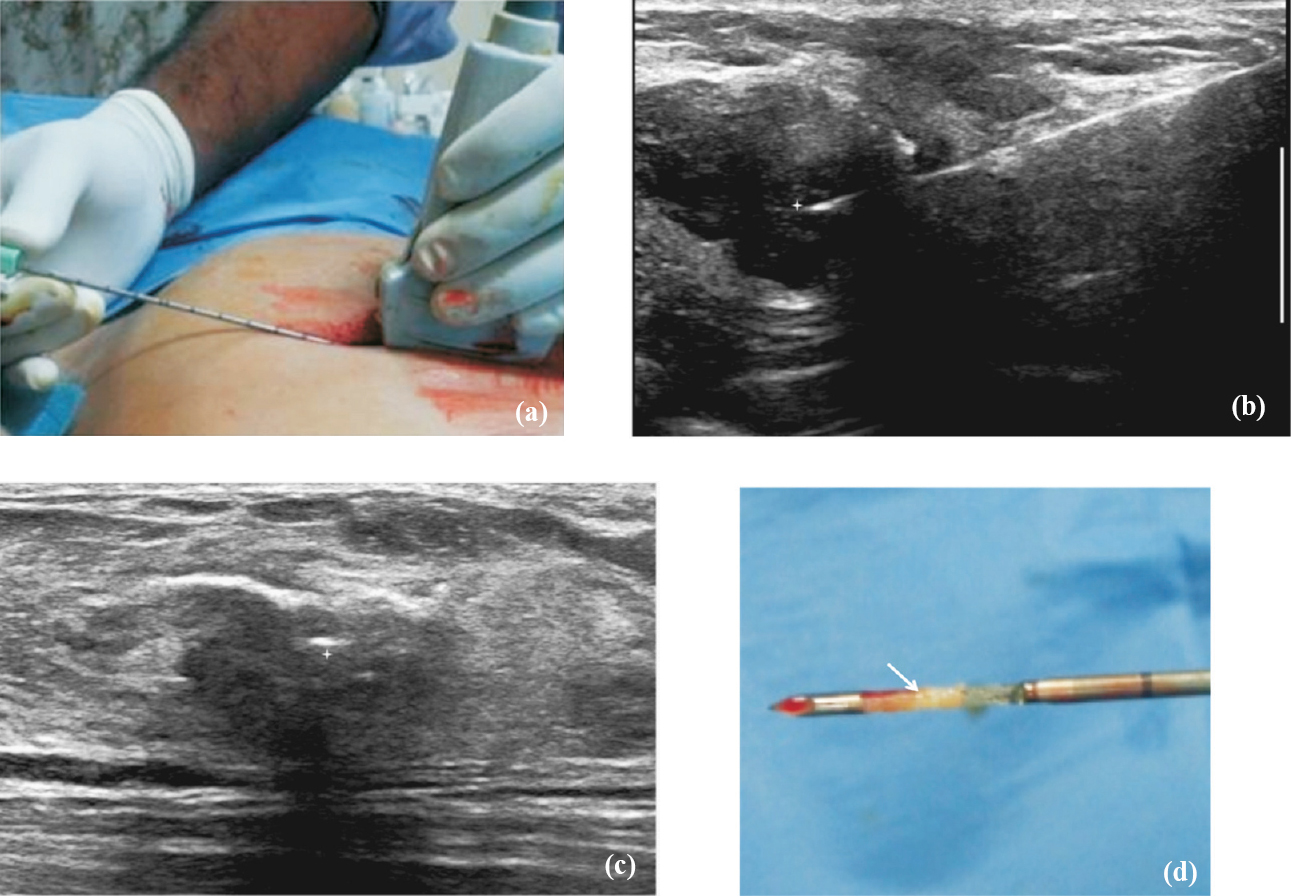
- Technique of ultrasound guided biopsy. (a) After preliminary scanning to determine appropriate approach to the lesion, local anaesthesia is administered at the skin entry site and biopsy needle is advanced into the lesion under real time ultrasound guidance. (b) The position of the needle tip (star) is confirmed to be within the lesion in two orthogonal planes. (c) The position of the needle tip (star) is confirmed to be within the lesion in two orthogonal planes. (d) Biopsy is obtained, the core (arrow) is harvested from the sample notch and put into a formalin bottle.
Results of biopsy were related with imaging findings to establish imaging-histologic concordance. Those with concordant malignant diagnosis were offered definitive treatment. Repeat biopsy was performed if the biopsy results were inconclusive, suspicious or showed imaging-histologic discordance. Six month imaging and clinical follow up was advised for concordant benign results. Final diagnosis was based on surgical histopathology in operated patients and on six month clinical and imaging follow up in others.
Cancers diagnosed on the basis of core needle biopsy and confirmed on surgical excision were considered true-positive (TP). Cancers for which the core needle biopsy did not show cancer but that resulted in repeat biopsy/surgical excision because of questionable pathologic findings or imaging-histologic discordance were considered false-negative (FN). Benign diagnoses on core needle biopsy were considered true-negative (TN) if they were confirmed by surgical excision or remained stable at six month follow up imaging. A cancer diagnosed on the basis of core needle biopsy, not confirmed on surgical excision was considered false-positive (FP).
Statistical analysis: Data analysis was performed using commercially available software (SPSS for windows version 17, Inc., Chicago, USA). Technical success, sensitivity, specificity, false positive rate and false negative rate of core biopsy were determined for each arm. Diagnostic accuracy for correct diagnosis (benign as well as malignant) was calculated in both the palpation and image guided groups as a proportion of correctly classified lesions (TP+TN) among all subjects (TP+TN+FP+FN).
Results
Mean diameter of lumps undergoing image guided biopsy was 4 cm (range 1-13 cm) and of those undergoing palpation guided biopsy was 4.4 cm (range 2-13 cm). Good quality, unfragmented cores were obtained in 30 of 36 palpation guided biopsies and 31 of 36 image guided biopsies. There was no significant procedure related complication.
Forty five of 72 patients were operated (42 malignant and three benign lesions) and the diagnoses was confirmed on postoperative histopathology. Three benign lesions were operated because of patients’ preferences. Fifteen malignant lesions were subjected to neoadjuvant chemotherapy and hence, biopsy diagnosis was considered as final diagnosis in them. Nine lesions with a benign result on biopsy were stable on at least six month follow up imaging; this was taken as confirmation of their benign nature.
Palpation guided biopsy: Twenty seven of the 36 patients had definitive histopathological diagnosis; malignancy in 14 patients and benign results in 13 patients. Biopsy results were inconclusive in nine patients; seven biopsy samples were considered inadequate and other two were reported as suspicious. Ten of 13 benign results were nonspecific benign diagnoses which were discordant with their highly suspicious imaging features (BI-RADS 5) (Fig. 1).
All 19 patients with inconclusive or discordant palpation guided biopsy results were subjected to ultrasound guided re-biopsy. Malignancy was diagnosed in all 10 patients with imaging-histologic discordance (Fig. 3a and b). Among seven inconclusive results, four proved to be malignant and three were benign on re-biopsy (Fig. 4). Two suspicious results were also confirmed as malignant. All the 14 malignant masses were invasive ductal carcinoma (IDC).
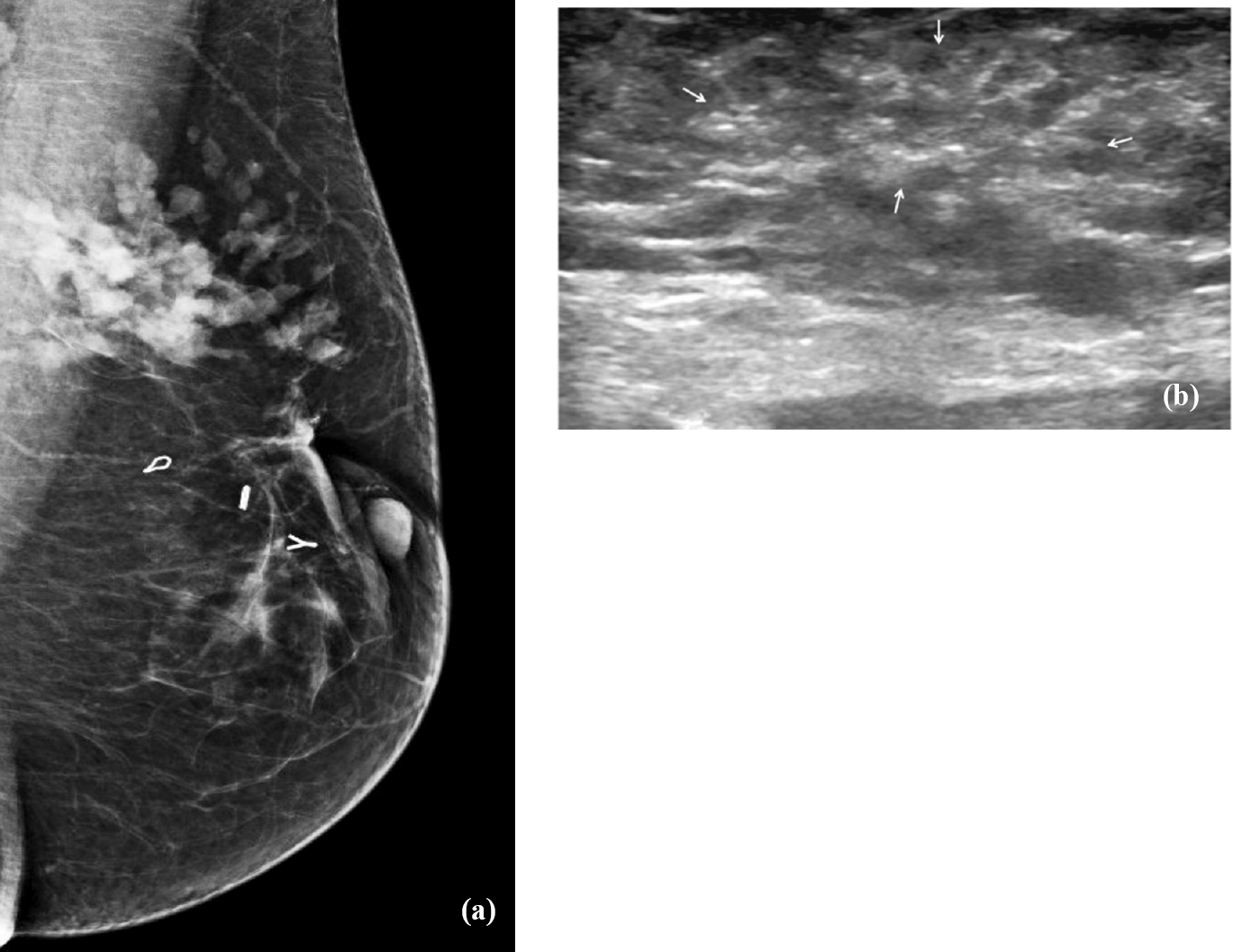
- Mucinous carcinoma diagnosed on image guided biopsy. (a) Mammogram (Rt MLO view) of a 34 yr old lady presenting with a recurrent palpable lump shows lobular high density mass in the upper outer quaderant. (Surgical clips are also seen). (b) Ultrasound shows a poorly defined, isoechoic mass lesion (marked by arrows). Palpation guided biopsy was reported as chronic inflammation, assessed as discordant with imaging findings. Repeat ultrasound guided biopsy correctly diagnosed it to be mucinous carcinoma.
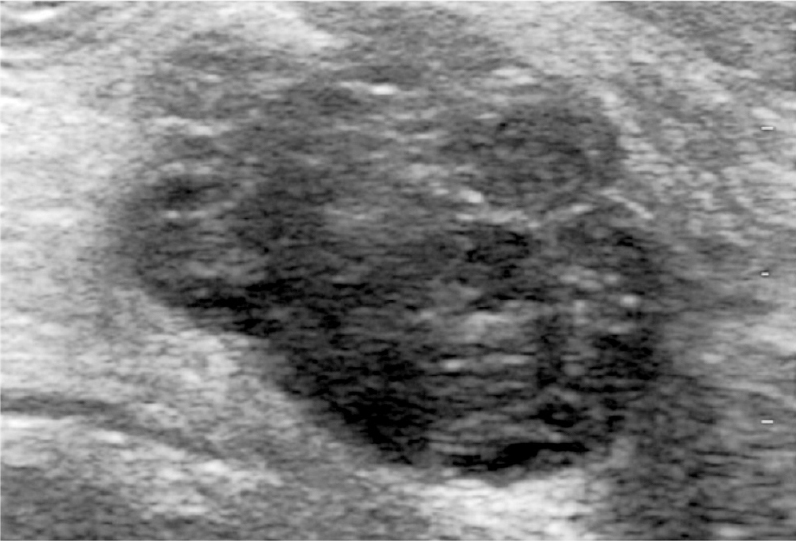
- Ultrasound image of a 38 yr old lady presenting with a palpable lump shows an irregular, heterogenous, predominantly hypoechoic mass. Palpation guided biopsy was inconclusive and ultrasound guided biopsy was reported as fibroadenoma.
Image guided biopsy: Definitive histopathological diagnosis was made in all but one of the 36 patients; 26 malignant and 9 benign (Table I). There was no case of imaging-histologic discordance in image guided biopsy group; however, there was one suspicious result. This patient subsequently underwent 11G vacuum assisted biopsy which confirmed the malignancy. Among 26 malignancies, 18 were IDC. Two lesions each were diagnosed as ductal carcinoma in situ (DCIS) and lymphoma, respectively. One lesion each was diagnosed as mucinous, tubular, metaplastic and papillary carcinoma. Specific histopathological diagnosis of benign diseases was obtained in three of 13 benign results in palpation guided biopsy and eight of nine benign results in image guide biopsy group. Specific diagnosis of fibroadenoma was made in masses. While three were diagnosed as fibrocystic disease, one each was diagnosed as tubular adenoma and phylloides tumour.
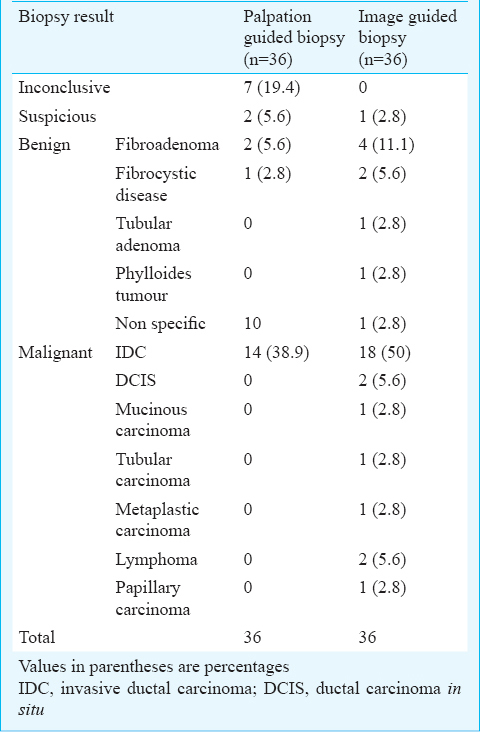
Comparison of diagnostic performance of biopsy methods: Diagnostic performance of image guided biopsy was superior than the palpation guided biopsy (Table II). The sensitivity of image guided biopsy for diagnosing a malignant lesion was 96.3 per cent [26 of 27; 95 per cent confidence interval (CI), 81-99.4%]. On the other hand, palpation guided biopsy group yielded sensitivity of 46.7 per cent (14 of 30; 95% CI, 28.4-65.7%). Specificity as well as positive predictive value in both the groups was 100 per cent for diagnosis of malignancy. There was a significant (P<0.001) difference in the negative predictive value between both the groups for diagnosis of malignancy; 90 per cent (9 of 10; CI, 55.4-98.3%) in the image guided biopsy group as against 27.3 per cent (6 of 22; CI, 10.8-50.2%) in the palpation guided biopsy group.
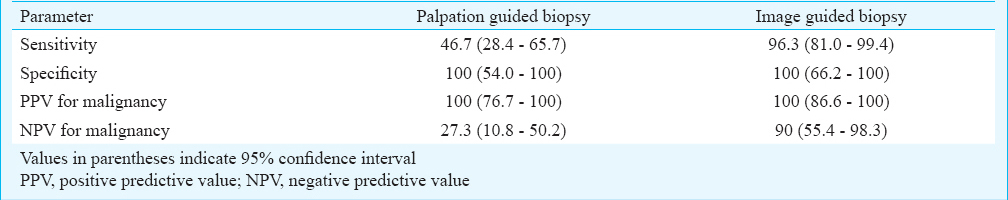
Palpation guided biopsy group had a high repeat biopsy rate (19 of 36, 52.8%) due to inconclusive (7 of 19), suspicious (2 of 19) pathological findings or imaging-histologic discordance (10 of 19) as well as a high false negative rate (16 of 36, 44.4%). On the other hand, there was only one repeat biopsy in the image guided biopsy group, which was due to suspicious pathological findings. There were no inconclusive biopsy samples or reports showing imaging-histologic discordance. This group also had a very low false negative rate (1 of 36, 0.03%). Image guided biopsy gave correct definitive diagnosis in 35 of 36 lesions yielding a higher diagnostic accuracy of 97.2 per cent than that of palpation guided biopsy (55.6%) which was accurate in diagnosing 20 of 36 lesions.
Discussion
Both palpation guided and image guided biopsy are routinely used for histopathological sampling of palpable breast masses. In this prospective study comparing these two biopsy techniques, average number of cores obtained was five in each arm. It was comparable to earlier studies3510111213. Quality of cores was also same in both biopsy techniques and there was no significant complication. Hence, technical success of both biopsy techniques was similar.
The results of our study showed that the outcomes of the image guided biopsy were better than those of palpation guided biopsy in terms of repeat biopsy rates, false negative rates, sensitivity and negative predictive value for diagnosing breast cancer. The sensitivity of 96.3 per cent for image guided biopsy was similar to the reported sensitivity of image guided biopsy that ranged from 94.5-100 per cent1415. The sensitivity of 46.7 per cent for palpation guided biopsy was lower than that reported (65-87%) previously1617. This may be due to the larger clinically palpable size of malignant masses in our study with a larger component of peritumoral induration leading to sampling error.
Biopsy specimens were considered as inadequate for histopathological analysis in seven patients in palpation guided biopsy arm while there was no case of inadequate specimen with image guided biopsy. Similar experiences have been reported in previous studies81114. Four (57.1%) of these seven lesions had malignancy on re-biopsy. Ten patients in palpation guided biopsy arm had biopsy results which were discordant to imaging findings. All these lesions proved to be malignant in subsequent image guided biopsy and surgery. Malignancy rate in lesion with imaging-histologic discordance is generally less than 30 per cent613 however, similar to our results, Schueller et al18 also found malignancy in all of their patients with discordant biopsy results. Histologic underestimation is another limitation of percutaneous biopsy19, however; we did not encounter this in our study.
The higher rate of inconclusive results and misdiagnosis in palpation guided biopsy is likely due to sampling errors. Large malignant masses are more likely to be associated with peritumoral infiltration, desmoplasia, inflammatory reaction or lymphatic oedema. It may sometimes be difficult to reliably differentiate these changes from true tumour mass on palpation. Fixity to chest wall or skin may also hamper satisfactory assessment on palpation. All these factors increase the chances of erroneous sampling on palpation guided biopsy; however, these are not major limitations for image guided biopsy. Small deep seated mobile palpable lumps are also likely to slip off the needle tip, which is difficult to appreciate in palpation guided biopsy. Needle position can be easily verified in image guided biopsies.
Even though image guided biopsies are known to be superior, a high false negative rate of palpation guided biopsy in our study may be considered unexpected. This can partly be attributed to our patients’ profile. Most of our patients presented late and were symptomatic with large tumours. Many of them also had gross oedema, induration and fixity to skin or chest wall. Image guidance is more useful in these situations. Median diameter was 4.4 and 4 cm in palpation and image guided biopsy arms, respectively and it was larger than the same in some of the previous studies5611. However, selection bias was possible in these retrospective studies as bigger lesions were likely to be subjected to palpation guided biopsy while deep seated or vaguely palpable lesions were more likely to be referred for image guided biopsy6. Size of the lesions was comparable in both arms and it supported the superiority of image guided biopsy over palpation guided biopsy in palpable breast lesions.
Small sample size was a major limitation of this study. Although palpation guided breast biopsies are still widely performed; there is increased availability and tendency for image guided biopsy as the latter technique is considered better. The positivity rate of biopsy for malignancy was high, which could be explained by small sample size and high pretest probability of lesions being cancers. All patients were symptomatic and had presented with large breast lumps which were clinically suspicious for malignancy.
Image guided biopsy of breast was found to be more accurate than palpation guided biopsy in women presenting with palpable breast lumps. It has been noted that palpation guided biopsy can miss or delay the diagnosis in significant number of patients. Hence, if available, image guided biopsy should be preferred for sampling breast masses even if they are palpable. Regardless of the technique, breast biopsy results should always be correlated with the imaging features to detect discordant results. This helps to decrease the cancer miss rates and improves the accuracy of percutaneous breast biopsy.
References
- GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11. 2013. Lyon, France: International Agency for Research on Cancer; Available from: http://globocan.iarc.fr
- [Google Scholar]
- Three year report of population based cancer registries 2009-2011. Bangalore, India: National Centre for Disease Informatics and Research, Indian Council of Medical Research; 2013. p. :55.
- Fine-needle aspiration biopsy of nonpalpable breast lesions in a multicenter clinical trial: Results from the Radiologic Diagnostic Oncology Group V1. Radiology. 2001;219:785-92.
- [Google Scholar]
- US-guided core breast biopsy: use and cost-effectiveness. Radiology. 1998;208:717-23.
- [Google Scholar]
- False-negative core needle biopsies of the breast: an analysis of clinical, radiologic, and pathologic findings in 27 concecutive cases of missed breast cancer. Cancer. 2003;97:1824-31.
- [Google Scholar]
- Palpable breast masses: is there a role for percutaneous imaging-guided core biopsy? AJR Am J Roentgenol. 2000;175:774-87.
- [Google Scholar]
- Freehand versus ultrasound-guided core biopsies of the breast: reducing the burden of repeat biopsies in patients presenting to the breast clinic. Breast. 2010;19:105-8.
- [Google Scholar]
- The diagnostic accuracy of core biopsy in palpable and non-palpable breast lesions. Eur J Radiol. 1997;24:120-3.
- [Google Scholar]
- American College of Radiology. BI-RADS: mammography. In: Breast imaging reporting and data system: BI-RADS atlas (4th ed). Reston, Va, USA: American College of Radiology; 2003.
- [Google Scholar]
- Percutaneous core biopsy of the breast: effect of operator experience and number of samples on diagnostic accuracy. AJR Am J Roentgenol. 1996;166:341-6.
- [Google Scholar]
- Ultrasound-guided core needle biopsy as an initial diagnostic test for palpable breast masses. J Vasc Interv Radiol. 2001;12:1313-7.
- [Google Scholar]
- Lessons from mammographic-histopathologic correlation of large-core needle breast biopsy. Radiographics. 1996;16:1111-30.
- [Google Scholar]
- Sonographically guided 14-gauge core needle biopsy of breast masses: a review of 2,420 cases with long-term follow-up. AJR Am J Roentgenol. 2008;190:202-7.
- [Google Scholar]
- Percutaneous core needle biopsy of palpable breast tumours. Do we need ultrasound guidance? Rofo. 2002;174:1142-6.
- [Google Scholar]
- Systematic review: comaparative effectiveness of core needle and open surgical biopsy to diagnose breast lesions. Ann Intern Med. 2010;154:238-46.
- [Google Scholar]
- The role of tru-cut needle biopsy in the diagnosis of carcinoma of the breast. Surg Gynecol Obstet. 1990;170:407-10.
- [Google Scholar]
- Automatic needle biopsy in the diagnosis of early breast cancer. Eur J Surg Oncol. 1991;17:237-9.
- [Google Scholar]
- US-guided 14-gauge core-needle breast biopsy: results of a validation study in 1352 cases. Radiology. 2008;248:406-13.
- [Google Scholar]
- Present state of and problems with core needle biopsy for non-palpable breast lesions. Breast Cancer. ;206(13):32-7.
- [Google Scholar]






