Translate this page into:
IgG subclass responses to proinflammatory fraction of Brugia malayi in human filariasis
Reprint requests: Dr. P.K. Murthy, Chief Scientist, Parasitology Division, CSIR-Central Drug Research Institute, Lucknow 226 001, India e-mail: drpkmurthy@yahoo.com k_murthy@cdri.res.in psr_murthy@yahoo.com
-
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Earlier we demonstrated that immunization with F6, a proinflammatory molecular fraction isolated from the human filarial parasite Brugia malayi, protected the host and eliminated the infection in Mastomys coucha by a Th1/Th2 response including IgG2a antibody response. Whether F6 molecules become accessible to human host during natural course of infection and elicit similar response is not known. The present study was undertaken to determine the profile of IgG subclasses specifically reactive to F6 in different categories of bancroftian filariasis cases to infer any relationship between the levels of a particular F6-specific IgG subclass and the infection or disease status.
Methods:
Serum samples of normal individuals from filariasis non-endemic regions of India like Jammu & Kashmir, Uttarakhand, and Chandigarh [(NEN-W; n=10), healthy subjects from USA (NEN-U; n=10) and three categories of bancroftian filariasis cases from endemic areas: endemic normals (EN; n=10) with no symptoms and no microfilariae, asymptomatic microfilaremics (ASM; n=10) and chronic symptomatic amicrofilaremics (CL; n=10) were assayed for F6-specific IgG1, IgG2, IgG3 and IgG4 by ELISA using SDS-PAGE-isolated F6 fraction of B. malayi adult worms.
Results:
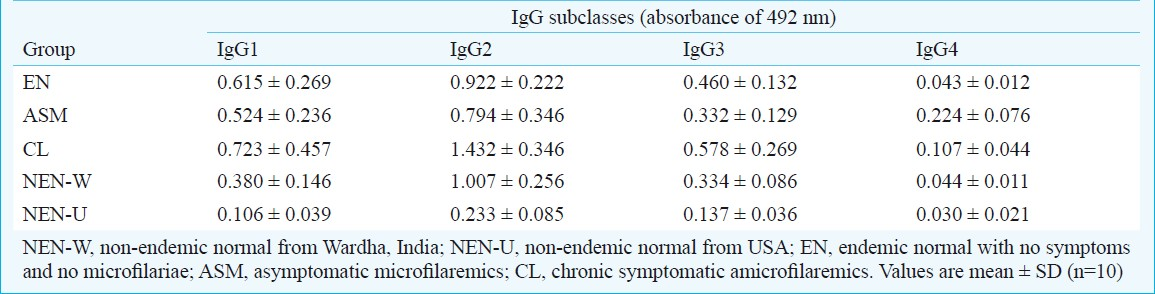
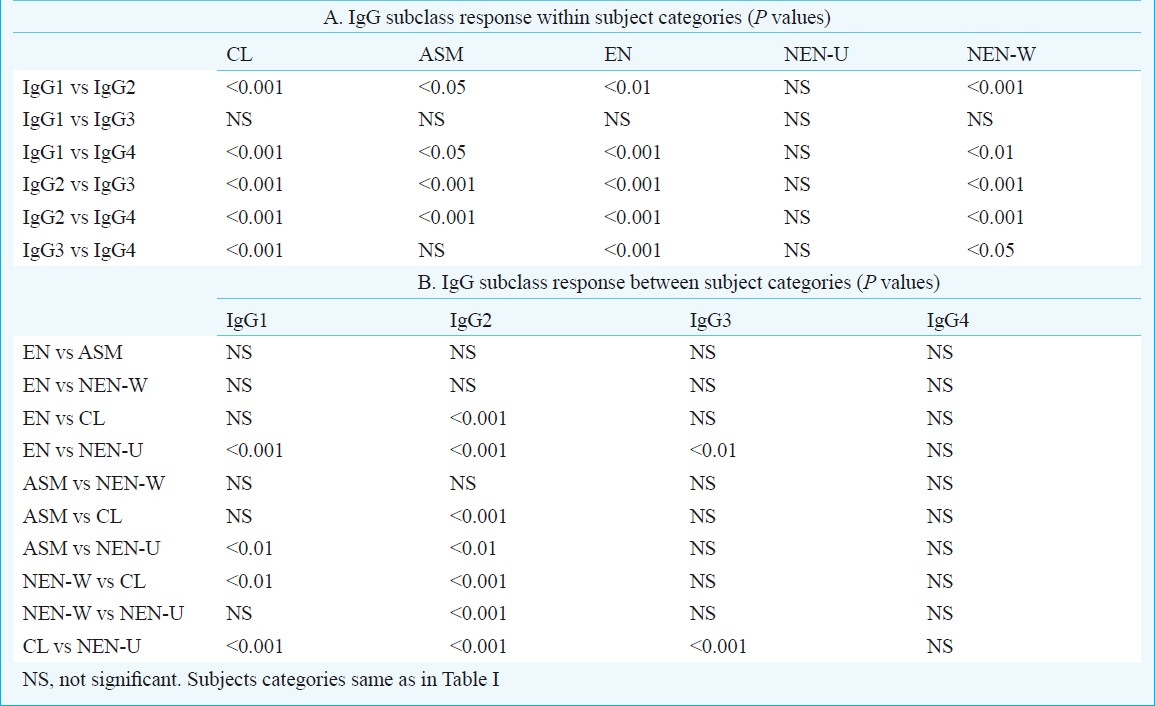
Significantly high levels of F6-specific IgG1, IgG2 and IgG3 were found in CL (P<0.001) and EN (P<0.01-0.001) bancroftian filariasis cases compared to NEN-U. Significant levels of F6-specific IgG1 (P<0.01) and IgG2 (P<0.01) but not IgG3 were found in ASM cases compared to NEN-U. The most abundant was IgG2 which when compared to NEN-U, was significantly high in CL (P<0.001) and EN cases (P<0.001), followed by ASM (P<0.01). F6-specific IgG4 response in EN, ASM and CL subjects was not significantly different from the levels of NEN-U. Among the non-endemic normals, the NEN-W subjects showed significant reactivity with IgG2 (P<0.001) but not with IgG1, IgG3 and IgG4 as compared to NEN-U subjects. IgG subclass levels were different in different categories.
Interpretation & conclusions:
The high levels of F6 reactive IgG1, IgG2 and IgG3 in endemic normals and chronic symptomatic bancroftian patients, and IgG1 and IgG2 in asymptomatic microfilaraemics, suggest that F6 molecules of parasite are accessible in these subjects for IgG subclass-specific immune response and IgG2 may be related to pathogenesis. Studies using individual F6 molecules will be done to identify the molecule(s) involved in infection and protective immunity.
Keywords
Brugia malayi
IgG subclasses
proinflammatory antigen
Lymphatic filariasis, a blood-borne disease caused by Wuchereria bancrofti, Brugia malayi and B. timori and transmitted by mosquitoes is recognized as one of the world's most incapacitating diseases in tropical areas. Worldwide around 120 million people are affected by the infection of whom 40 million show the chronic disease manifestations: elephantiasis and hydrocele1 and a further one billion (18% of the world's population) are at risk of infection2. The adult worms inhabit the lymphatics, where they survive for prolonged periods, and produce millions of first-stage larvae (microfilariae; mf), which circulate in the peripheral blood. Following ingestion of blood by mosquitoes, mf develop to the third larval stage (L3) in the mosquito. The cycle of infection is re-initiated by the mosquito during next blood meal.
A major enigma is the identity of parasite products that modulate host's immune response leading to the two extremes: (i) largely peaceful survival of the parasite in the host without causing disease (asymptomatic microfilaremics), or (ii) development of the chronic disease manifestations such as elephantoid deformities and hydrocele through repeated episodes of adenolymphangitis and lymphoedema. Inflammatory cytokines and immunological hyperactivity of the host may, on one hand, promote establishment of the infection3 and on the other, lead to disease manifestations4. Such diverse responses are thought to be due to the ability of live and dead parasite products to stimulate release of either predominantly pro- or anti-inflammatory cytokines under different conditions. Our earlier studies revealed that live stages of the parasites are capable of stimulating release of both pro- and anti-inflammatory cytokines5. Maizels and Lawrence6 also showed that an acute exposure to mf induced an inflammatory type 1 response whereas L3 and adults induced primarily type 2 responses in a mouse model. We recently isolated BmAFII, a Sephadex G-200 eluted fraction of B. malayi adult worm extract, and found it to be predominantly proinflammatory, and it protected the rodent host Mastomys coucha from B. malayi7 and hamsters from Leishmania donovani infections8. Further studies revealed that the strong proinflammatory proteins are localized to a 54-68kDa fraction F6 and immunization with F6 protected jird and M. coucha from B. malayi infection via Th1/Th2 type responses including IgG2a antibody response9. MALDI-TOF analysis of the fraction revealed five proteins, of which three were immunostimulatory (viz. elongation factor 2, heat-shock protein 60 and intermediate filament)9. Whether F6 molecules of the adult parasite become accessible to human host during natural course of infection and elicit similar response is not known.
IgG is the major immunoglobulin detectable in all categories of filariasis patients and the clinical severity of the infection is reflected by distinct IgG subclass profiles in the subjects10. The objective of the present study was to determine the profile of IgG subclasses specifically reactive to F6 in different categories of bancroftian filariasis cases in order to infer any relationship between the levels of a particular F6-specific IgG subclass and the infection or disease status.
Material & Methods
Serum samples of different categories of bancroftian filariasis cases and normal individuals were obtained from Serum Bank, Mahatma Gandhi Institute of Medical Sciences, Sevagram, India. The serum bank complies with the Institutional Ethics Committee regulations. The categories of filarial subjects which were from Sevagram and surrounding areas were endemic normal individuals with no symptoms and no microfilaraemia (EN; n=10), asymptomatic microfilaremics (ASM; n=10) and chronic symptomatic amicrofilaremics (CL; n=10). Serum samples of normal individuals who had come from filariasis non-endemic areas of India like Jammu & Kashmir, Uttarakhand, and Chandigarh (NEN-W; n=10) to get admission in 1st year of MSBS in Sevagram, Wardha, India, were collected following the Institutional Ethics Committee permission. The NEN-W individuals were microfilaria-negative but recent exposure to filarial L3-carrying mosquitoes could not be ruled out. Serum of healthy subjects (NEN-U; n=10) were obtained from USA (kind courtesy of Division of Bacteriology and Parasitology, Tulane National Primate Research Center, Tulane University Health Sciences Center, Lousiania).
Isolation of F6 fraction: Adult worms of B. malayi were collected from peritoneal cavity of infected jirds11 having 120-150 days old infection. Soluble somatic extract of the worms was prepared and resolved by 10 per cent sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) as described by Laemmli12. Resolved fraction F6 (54-68kDa) identified with the help of pre-stained molecular weight markers run simultaneously was cut out using sharp and clean scalpel9.
Proteins from gel strips were electro-eluted (Electroeluter, Millipore, India), concentrated (Centricon®; 10kDa cut-off; Millipore, India), and estimated13. The molecular weight of the purified proteins was confirmed in SDS-PAGE (Fig.) and stored in aliquots at -20°C till use.
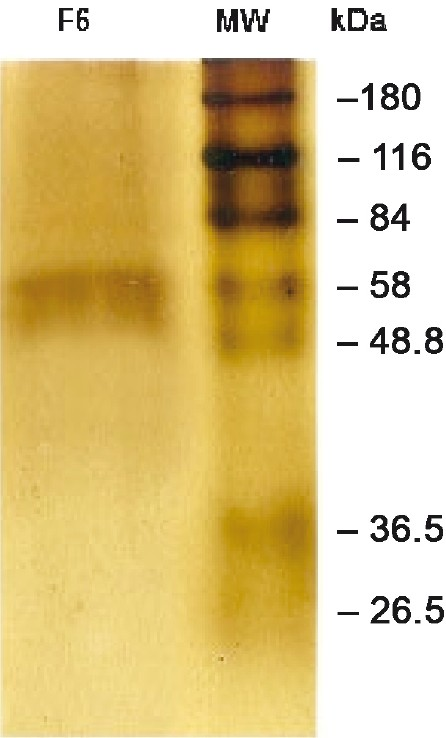
- SDS-PAGE showing F6 of B. malayi. B. malayi adult worm somatic extract (BmAS) was run on SDS-PAGE and the F6 band was cut out, eluted with gel eluter (Electroeluter, Millipore, India) and run again on SDS-PAGE to confirm the molecular location.
Determination of IgG subclasses: All the antibodies and conjugates were procured from Sigma Chemical Co., USA. Circulating F6-specific IgG subclasses were assayed by ELISA914. Briefly, polystyrene 96-well ELISA plates (Nunc-Immunoplate Maxisorp, Denmark) were coated with F6 protein (0.25 μg/ml) in carbon-ate buffer (0.06 M; pH 9.6) overnight at 4 °C. Unsaturated sites of the surface were blocked with 1 per cent BSA in phosphate buffered saline (BSA-PBS) followed by incubation with optimally diluted human (1:25) serum in BSA-PBS containing 0.01 per cent Tween-20 (B-PBS-T). In order to eliminate plate-to-plate differences in absorbance values, 10 samples of a given group were dispensed in 10 wells of a row (e.g. group NEN-W in row A, NEN-U in row B, EN in row C and so on). In each row well no. 11 and 12 carried reagent blanks. Each such plate (and similarly made replicate plates) was used for assaying a given IgG subclass. The plates were washed with PBS-T and incubated with optimally diluted mouse monoclonal anti-human IgG1 (1:7,500), IgG2 (1:5000), IgG3 (1:5000) and IgG4 (1:7500). The plates were again washed and incubated with 1:1000 dilution of goat anti-mouse IgG-horse raddish peroxidase conjugate. After antigen coating step all the incubations were carried out for 90 min at 37°C. The plates were washed and incu-bated with substrate (0.08% each of O-phenylenediamine and H2O2 in pH 5.0 citrate buffer). The reaction was stopped with 2.5N H2SO4 and optical density read at 492 nm in an ELISA reader (PowerWaveX, BioTek, USA).
Statistical analysis: The values of IgG subclasses in different groups were compared by two-way ANOVA followed by Newman- Keuls test for individual comparisons. Differences were considered significant if P<0.05.
Results
Table I shows values of F6-specific IgG subclasses (IgG1, IgG2, IgG3 and IgG4) in serum samples of bancroftian filariasis cases (ASM, CL, EN) and non-endemic normals (NEN-W and NEN-U). It was found that if the level of one IgG subclass was higher in a category, the level of another IgG subclass was lower in the same clinical category or vice versa. Significant difference (P<0.001) was found between different categories of bancroftian filariasis with respect to F6-specific IgG subclasses. Significance levels in IgG subclass responses within and between clinical categories of bancroftian filariasis cases are given in Table IIA and B. Significantly high levels of F6-specific IgG1, IgG2 and IgG3 were found in CL (P<0.001) and EN categories (P<0.01-0.001) of bancroftian filariasis cases compared to NEN-U. Significant levels (P<0.01) of F6-specific IgG1 and IgG2 but not IgG3 were found in ASM cases compared to NEN-U. The most abundant was IgG2 which when compared to NEN-U, was significantly high in CL (P<0.001), and EN (P<0.001), followed by ASM (P<0.01). F6-specific IgG4 response in EN, ASM and CL subjects was not significantly different from the levels of NEN-U. Among the non-endemic normals, the NEN-W subjects showed significantly higher levels of anti F6-IgG2 antibodies (P<0.001) compared to NEN-U subjects. However, the levels of other subclasses of IgG were quite low in NEN-W as compared to F6-specific IgG2 antibodies and there was no difference in their levels in the NEN subjects of the two regions (Tables I and II).
Discussion
The major immunoglobulins involved in the antifilarial antibody responses in human host are IgG, IgM, and IgE15–17, of which IgG is detectable in all categories of filarial subjects. Further, different categories of filarial subjects have different IgG subclass profiles which can be related to clinical severity of the infection10. In the present study, there was a difference in the levels of IgG subclasses among the different categories of bancroftian filariasis subjects. Filarial parasite specific IgG1 IgG2 and IgG3 are shown to be predominant in chronic lym-phatic filariasis18. In the present study, F6-reactive IgG1, IgG2 and IgG3 were high in CL, but the most prominent increase was found in IgG2. This suggests that much of the increase in these three IgG subclasses, especially IgG2, may be attributed to F6 molecules that were accessible to host largely in the chronic stage of infection during which the dying or dead parasites also produce lymphatic pathology. We also found that exposure of M. coucha to F6 alone induced epithelioid granulomas in the draining lymph nodes (unpublished observation) indicating a possible role of F6 in filarial pathology. High IgG2 levels in ASM subjects appear to be related to pathology as ASM subjects are now known to show hidden early lymphatic pathology19. However, Noordin et al18 found that IgG2 levels were higher in CL subjects than in ASM subjects similar to our study and they suggested that IgG2 may be used as diag-nostic tool for B. malayi-induced chronic elephantiasis. It, therefore, appears that the IgG2 levels, whether broadly filarial parasite-specific or F6-specific, correlate with the pathology.
F6-specific IgG3 was significantly high in CL and EN subjects but not in ASM, NEN-W over NEN-U. Mak20 reported increase in IgG3 in ASM subjects but in the present study, increase in IgG3 in EN cases who had no active infection is not clear. EN subjects, who are constantly exposed to L3, show antibodies against the parasite and are refractive to infection, this increase may be related to protective immunity to filarial parasites mounted naturally2122. However, different B. malayi antigens are known to elicit different IgG subclass profile in different clinical categories of subjects2324. L3 antigen BmNIP3 showed elevated levels of IgG1 and IgG2 antibodies in EN subjects and largely IgG3 in chronically infected patients and strong reactivity with IgG1 in microfilaraemic individuals25. The IgG3 reactivity of F6 in EN subjects thus appears to be common between L3 and adult derived (F6) antigens. However, it is clear that the profile of all IgG subclasses reactive to adult derived F6 need not be identical to that shown by L3 antigens. The implications of the F6 reactive profile need further study.
Elevated IgG4 levels were reported in mf carriers (ASM) and tropical pulmonary eosinophilia (TPE) cases26–30. In the present study, F6-specific IgG4 levels in ASM cases were not significantly high as compared to NEN-U. This difference may be due to the fact that F6 which does not elicit IgG4 response is only one fraction of the complex parasite antigens available in the mf carriers and TPE cases and the antigens other than F6 might be responsible for the IgG4 levels in these cases. IgG4 has been used as a biomarker in the evaluation of antifilarial efficacy in microfilaraemic human subjects143132. Another possible reason for absence of F6-specific IgG4 is that although F6 is isolated from a mixture of both male and female worms, it is likely that F6 contains relatively very few antigens of mf (of uterine mf).
An interesting observation in the present study was the presence of high IgG2 levels also in NEN-W subjects. Presently, the reason for this is not clear, but this could be due to recent exposure of the subjects to filarial L3-carrying mosquitoes before their blood sampling.
We had earlier analyzed the F6 by MALDI-TOF and found five proteins9. Three of these proteins were elongation factor 2 (EF2), heat-shock protein 60 (hsp60) and intermediate filament, and all of these were immunostimulatory. Using B. malayi-Wolbachia hsp60, Suba et al33 reported that clinical groups of individuals mounted responses of all 4 subclasses (IgG1-IgG4) and IgG1 were especially higher in the serum of patients with chronic pathology compared to microfilaraemics and endemic normals. As IgG2 was high in our CL, NEN-W, EN and relatively less in ASM subjects, perhaps hsp60 in F6 fraction was different from that of B. malayi-Wolbachia and/or the other two immunostimulatory proteins in our F6 were probably responsible for the heightened IgG2 response. Further studies to elucidate the role of immunostimulatory proteins of F6 are planned.
In conclusion, high levels of F6 reactive IgG1, IgG2 and IgG3 in endemic normals and chronic symptomatic bancroftian patients, and IgG1 and IgG2 in asymptomatic microfilaraemics, suggest that F6 molecules of parasite are accessible in these subjects for IgG subclass-specific immune response and IgG2 may be related to pathogenesis. Studies using individual F6 molecules are planned to identify the molecule(s) involved in infection/pathology and protective immunity.
Acknowledgment
The authors thank the Director, CDRI, Lucknow, for his encouragement during the study. Thanks are due to Dr V.A. Dennis, Tulane University Health Sciences Center, Lousiania, USA, for providing healthy human serum. Technical assistance rendered by Shri V.K. Bose is acknowledged. MKS and SKJ was supported by Senior Research Fellowship from CSIR, SKV from CSIR-UGC, New Delhi.
CDRI communication number: 8043.
References
- WHO. Global programme to eliminate lymphatic filariasis: Annual report on lymphatic filariasis 2005. Wkly Epidemiol Rec. 2006;22:221-32.
- [Google Scholar]
- Are inflammation and immunological hyperactivity needed for filarial parasite development? Trends Parasitol. 2001;17:70-3.
- [Google Scholar]
- Potential role of infections in chronic inflammatory diseases. ASM News. 2005;71:529-35.
- [Google Scholar]
- Inflammatory antigens of Brugia malayi and their effect on rodent host Mastomys coucha. Parasite Immunol. 2004;26:397-407.
- [Google Scholar]
- Immunological tolerance: The key feature in human filariasis? Parasitol Today. 1991;7:271-6.
- [Google Scholar]
- Protection against L3 induced Brugia malayi infection in Mastomys coucha pre-immunized with BmAFII fraction of the filarial adult worm. Vaccine. 2006;24:5824-31.
- [Google Scholar]
- Influence of Brugia malayi life stages and BmAFII fraction on experimental Leishmania donovani infection in hamsters. Acta Trop. 2008;106:81-9.
- [Google Scholar]
- Immunization with inflammatory proteome of Brugia malayi adult worm induces a Th1/Th2-immune response and confers protection against the filarial infection. Vaccine. 2009;27:4263-71.
- [Google Scholar]
- The profile of IgG and IgG subclasses of onchocerciasis patients. Clin Exp Immunol. 1992;88:258-63.
- [Google Scholar]
- Fate of infective larvae of Brugia malayi in the peritoneal cavity of Mastomys natalensis and Meriones unguiculatus. Folia Parasitol (Praha). 1997;44:302-4.
- [Google Scholar]
- Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-5.
- [Google Scholar]
- A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-54.
- [Google Scholar]
- Response of IgG subclasses to diethylcarbamazine therapy in bancroftian filariasis. Curr Sci. 1997;72:265-7.
- [Google Scholar]
- Filarial antibodies and eosinophilia in human subjects in an endemic area. Trans R Soc Trop Med Hyg. 1969;63:796-800.
- [Google Scholar]
- Serological diagnosis of bancroftian and malayan filariasis. Am J Trop Med Hyg. 1978;27:508-13.
- [Google Scholar]
- Detection, quantitation, and specificity of antiparasite IgE antibodies in human Schistosomiasis mansoni. Am J Trop Med Hyg. 1981;30:1228-37.
- [Google Scholar]
- Potential use of IgG2-ELISA in the diagnosis of chronic elephantiasis and IgG4-ELISA in the follow-up of microfilaraemic patients infected with Brugia malayi. Biochem Biophys Res Commun. 1994;205:202-7.
- [Google Scholar]
- The clinical manifestations of lymphatic filariasis. In: Nutman TB, ed. Lymphatic filariasis. London: Imperial College Press; 2000. p. :103-25.
- [Google Scholar]
- Recent advances in the laboratory diagnosis of filariasis. Malaysian J Pathol. 1989;11:1-5.
- [Google Scholar]
- Protective immunity in bancroftian filariasis.Selective recognition of a 43-kD larval stage antigen by infection-free individuals in an endemic area. J Clin Invest. 1989;83:14-22.
- [Google Scholar]
- Cloning and characterization of a potentially protective chitinase-like recombinant antigen from Wuchereria bancrofti. Infect Immun. 1994;62:1901-8.
- [Google Scholar]
- Antibody responses to filarial infective larvae are not dominated by the IgG4 isotype. Parasite Immunol. 1998;20:9-17.
- [Google Scholar]
- Specificity of predominant IgG4 antibodies to adult and microfilarial stages of Brugia malayi. Parasite Immunol. 1998;20:155-62.
- [Google Scholar]
- Cloning and characterization of a novel immunogenic protein 3 (NIP3) from Brugia malayi by immuno screening of a phage-display cDNA expression library. Parasitol Res. 2005;97:49-58.
- [Google Scholar]
- Prominence of IgG4 in the IgG antibody response to human filariasis. J Immunol. 1985;134:2707-12.
- [Google Scholar]
- Enhanced diagnostic specificity in human filariasis by IgG4 antibody assessment. J Infect Dis. 1988;158:1034-7.
- [Google Scholar]
- IgG antibody subclasses in human filariasis.Differential subclass recognition of parasite antigens correlates with different clinical manifestations of infection. J Immunol. 1987;139:2794-8.
- [Google Scholar]
- Filarial-specific IgG4 response correlates with active Wuchereria bancrofti infection. J Immunol. 1990;145:4298-305.
- [Google Scholar]
- Multicentre evaluations of two new rapid IgG4 tests (WB rapid and panLF rapid) for detection of lymphatic filariasis. Filaria J. 2007;6:9-12.
- [Google Scholar]
- Confirmation of elimination of lymphatic filariasis by an IgG4 enzyme-linked immunosorbent assay with urine samples in Yongjia, Zhejiang Province and Gaoan, Jiangxi Province, People's Republic of China. Am J Trop Med Hyg. 2007;77:330-3.
- [Google Scholar]
- Brugia malayi Wolbachia hsp60 IgG antibody and isotype reactivity in different clinical groups infected or exposed to human bancroftian lymphatic filariasis. Exp Parasitol. 2007;116:291-5.
- [Google Scholar]






