Translate this page into:
High-risk human papillomavirus in Turkish patients with clinically suspicious cervical lesions analyzed by multiplex-PCR
For correspondence: Prof Hamiyet Donmez-Altuntas, Department of Medical Biology, Faculty of Medical, Erciyes University, Kayseri 38030, Turkey e-mail: donmezh@erciyes.edu.tr
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Human papillomavirus (HPV) infection is known to be the main cause of cervical cancer. This study aimed to determine the prevalence of high-risk HPV genotypes in smear specimens taken from women who had normal or abnormal cytology using a multiplex PCR method.
Methods:
The study included 270 women aged between 19 and 69 yr with or without suspicious cervical abnormalities. A Pap smear sample from each patient was cytologically examined, and HPV typing was performed using a multiplex fluorescent PCR method. Those who were high-risk HPV positive and had a normal or abnormal cytology were further evaluated by colposcopy and biopsy.
Results:
The total HPV positivity was 43 per cent (116/270). HPV positivity in the patients with an abnormal cytology was 77 per cent (33/43), whereas it was only 37 per cent (83/227) in women with normal cytology, which showed a significant difference (P<0.05). HPV positivity was also related to the age group when all the subjects were considered (P<0.05), and the highest prevalence of HPV infection was in the 30-39 yr age group. High-risk HPV types 16, 18, 31, 35, 51 and 56 were more common in the normal cytology patients, whereas high-risk HPV types 16, 31, 35, 45, 58 and 68 were commonly found in the abnormal cytology patients.
Interpretation & conclusions:
The determination of high-risk HPV genotypes in women with clinically suspicious cervical lesions should be conducted during an annual follow-up, irrespective of a normal or abnormal cytology by the age of 30 years or above.
Keywords
Abnormal cytology
cervical cancer
high-risk human papillomavirus
multiplex fluorescent PCR
ThinPrep Pap test
Turkish women
The International Agency for Research on Cancer (IARC) Global Burden of Cancer (IARC) (GLOBOCAN) 2020 database, estimated that from about 604,127 cases of cervical cancer that were diagnosed, around 341,831 died from this disease globally1. Cervical cancer is the fourth most diagnosed cancer in women, though low- and middle-income countries have a higher incidence than developed countries1-3. In Turkey, cervical cancer is the 19th ranked cancer, with 2532 new cases and 1245 deaths per year4. In Turkey, the Ministry of Health began a Pap smear screening programme, and thus, cervical cancer has been screened using human papillomavirus (HPV)-DNA tests since 20145. Based on women who utilized the Cancer Early Diagnosis, Screening and Training Centers (KETEM) for screening in Turkey, HPV-DNA positivity was found to be 2.7 per cent in 7992 women using the Hybrid Capture II method6 and 3.29 per cent in 33,649 women using the PCR method7.
HPVs belonging to the Papillomaviridae family cause various benign and malignant lesions, most commonly in the skin and mucous membranes. HPV can convert a non-permissive cell to an oncogenic one, facilitating lytic viral infections in permissive cell types8,9. Currently, data indicate that HPV has more than 200 genotypes, and the whole genome sequence is known for about 100 genotypes. Approximately 40 HPV types, known as anogenital types, are sexually transmitted and are closely linked with anogenital tract cancers. HPV genotypes are divided into low-risk, high-risk and possible high-risk types according to their oncogenic potential10,11. The fifteen high-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73 and 82) are known to be responsible for 99 per cent of cervical cancer cases9. Worldwide, HPV type 16 is found in 50-60 per cent of cervical cancers, and HPV type 18 in 10-12 per cent9,12-14. Furthermore, in addition to cervical cancer, HPV types 16 and 18 are also associated with other cancer types, including vulvar, anal vaginal, oral and oropharyngeal cancers9.
Early screening programmes using the Pap test along with HPV testing and the implementation of the HPV vaccine programme may lead to the possible elimination of cervical cancer worldwide by the end of this century and are necessary to prevent cervical cancer. This is possible because pre-cancerous lesions can be detected in the early stages by HPV screening programmes, and there is a long latency period between HPV infection and the development of cervical cancer. Thus, this study aimed to determine the prevalence of the high-risk HPV genotypes in cervical smear specimens taken from female patients with clinically suspicious cervical lesions, irrespective of a normal or abnormal cytology, by a multiplex PCR method.
Material & Methods
This study was conducted in the department of Obstetrics and Gynaecology, Erciyes University, Kayseri, Turkey, between February 2017 and November 2018, after approval from the Intitutes Ethics Committee. The smear samples were taken from the patients after obtaining their written consent.
Study participants: This study included 270 female patients with clinically suspicious cervical lesions who were admitted to the Obstetrics and Gynaecology outpatient clinic at University Hospital in the Central Anatolia region of Turkey between February 2017 and November 2018. The mean age of the patients was 38.63±10.76 yr (range: 19-69 yr). Women with an intact uterus, cervical erosion, chronic cervicitis, hypertrophied cervix, bleeding on touch or suspicious growth/polyp/ulcer on cervix were included in this study. Women below the age of 19 yr and those with a current pregnancy, a history of a gynaecologic malignancy, a history of cervical conization and uterine prolapsus were excluded from the study. Women between 19-69 yr age who came to the clinic with distinct gynaecologic complaints were included in this study even though the international guidelines, do not recommend screening for cervical cancer below the age of 21 years and conducting HPV testing below the age of 30 years15-17. Although in women below 30 yr, HPV infections may be transient, women <30 with an abnormal cervical lesion that required a clinical assessment and biopsy to exclude neoplasia were included in this study for HPV screening and typing.
Sample collection: Two cervical smear samples were obtained from each patient at the same time using two separate broom-like devices (Cervex-Brush, Rovers Medical Devices, the Netherlands), and these were rinsed into two vials of PreservCyt solution (ThinPrep Pap Test Solution; Cytyc Corporation, Boxborough, MA, USA) because the residual cells after the ThinPrep Pap test are not typically sufficient for HPV testing. The vials were sent to the Pathology department. The first vial was used to prepare the ThinPrep Pap test slides using the ThinPrep 2000 System (TP2000; Cytyc Corporation, Boxborough, MA, USA), and the second vial was used for HPV typing.
Cytology: Two pathologists evaluated the slides, and the slides from HPV positive women with a normal cytology were re-evaluated. The cytological evaluations of the slides were made according to the 2014 Bethesda system18. The cytological classifications included normal (negative), low-grade squamous intraepithelial lesion (LGSIL or LSIL), atypical squamous cells of undetermined significance (ASC-US), high-grade squamous intraepithelial lesion (HGSIL or HSIL), atypical glandular cells (AGC), ASC-cannot exclude HSIL (ASC-H), squamous cell carcinoma (SCC), adenocarcinoma (ADC) and adenocarcinoma in situ (AIS). The patients who were low-risk HPV positive and had a normal cytology were followed up every six months during the first years. The patients who were high-risk HPV positive and had a normal cytology or were HPV positive and had an abnormal cytology were followed up by conducting a colposcopic examination, and cervical biopsy or biopsies were taken if suspicious areas were present. The cervical biopsies were classified and studied according to the World Health Organization classification19. Among the 46 (41%) patients whose cervical biopsy samples were taken, 16 (35%) had a normal histology, and there were 16 (35%) HGSIL, 10 (22%) LGSIL, 3 (6%) ASC-US and 1 (2%) SCC. Based on the histological evaluations, the patients with HGSIL/cervical intraepithelial neoplasia (CIN2 and CIN3) underwent the loop electrosurgical excision procedure (LEEP).
HPV genotyping: Total DNA was isolated from the second set of vials without storage using the QIAamp DNA FFPE Tissue Kit (QIAGEN Inc., Hilden, Germany) and following the manufacturer’s protocols. HPV typing was performed using a multiplex fluorescent PCR kit (f-HPV typing Kit, Molgentix, Barcelona, Spain) following the protocols described in the user’s manual. The kit is designed for simultaneously detecting 13 high-risk HPV types (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68), one probable high-risk HPV type (type 66) and the 2 low-risk HPV types (types 6 and 11). The fluorescently labelled PCR products were analyzed using capillary electrophoresis in an Applied Biosystems’ 3130 Genetic Analyzer (Thermo Fisher Scientific, UK).
Statistical analysis: The statistical analyses were done using SPPS version 15 (SPSS Inc., Chicago, IL, USA) software. The variables were either nominal or ordinal and were analyzed using a Fisher’s exact test. P<0.05 was considered as significant.
Results & Discussion
Human papillomavirus (HPV) positivity in patients with normal and abnormal cytology: According to the cytological evaluations of the 270 women included in this study, 227 (84%) had a normal cytology and 43 (16%) had abnormal cytological findings (Figure). HPV positivity was observed in 116 (43%) of the smear samples taken from all the patients. HPV positivity was detected in 83 (37%) of the 227 patients with a normal cytology, whereas HPV positivity was found in 33 (77%) out of 43 patients with an abnormal cytology (Figure). High-risk HPV positivity significantly correlated with the cytology results for all the patients (P<0.001).
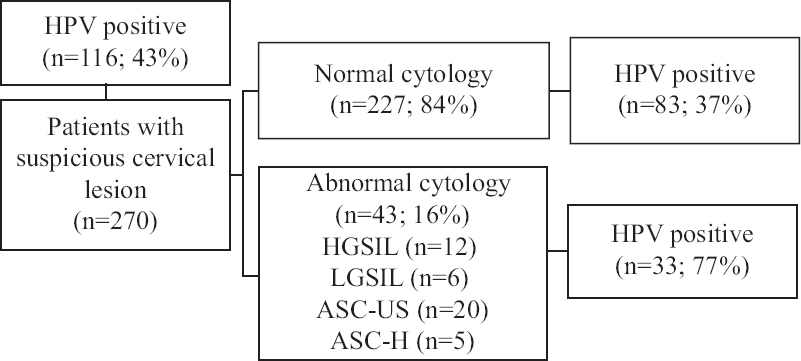
- Flow diagram showing prevalence of HPV in smear samples taken from patients with suspicious cervical lesion who had normal or abnormal cytology using a multiplex-PCR method. HPV, human papillomavirus; HGSIL, high-grade squamous intraepithelial lesion; LGSIL, low-grade squamous intraepithelial lesion; ASC-US, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells-cannot exclude HSIL
The HPV positivity rates in our results were similar to those reported in previous studies conducted in Turkey20-22. For instance, Çalişkan et al22 found an HPV positivity of 42 per cent in consecutive cervical specimens by real-time PCR. In two other studies, HPV types were determined in patients who presented to the clinic with various gynaecological complaints (menstrual irregularity, dyspareunia, vaginal discharge, lower abdominal pain and post-coital bleeding)20,21. The first study included 61 female patients and the HPV positivity rate was 44 per cent (36% and 49% of the patients with normal and abnormal cytologies, respectively) by PCR and reverse hybridization methods20. In the second study, which included 201 female patients, the HPV positivity rate was 45 per cent (49% and 43% of the patients with normal and abnormal cytologies, respectively) by the multiplex PCR method21. However, in our study, the HPV positivity was significantly higher in the patients with an abnormal cytology (P<0.05) (Table I).
| Cytology | HPV positive, n (%) | HPV negative, n (%) | Total (n) | P |
|---|---|---|---|---|
| Total | 33 | 10 | 43 | |
| HGSIL | 12 (36.4) | 0 (0) | 12 | 0.025 |
| LGSIL | 4 (12.1) | 2 (20.0) | 6 | |
| ASC-US | 12 (36.4) | 8 (80.0) | 20 | |
| ASC-H | 5 (15.1) | 0 (0) | 5 |
ASC-US, atypical squamous cells of undetermined significance; HGSIL, high-grade squamous intraepithelial lesion; LGSIL, low-grade squamous intraepithelial lesion; ASC-H, atypical squamous cells-cannot exclude HSIL
HPV type distribution in HPV-positive patients with a normal or abnormal cytology: Table II shows the distribution of the HPV types in the HPV-positive patients with a normal or abnormal cytology. In our study, the six most common high-risk HPV types were HPV 16, 51, 31, 18, 35 and 56, in descending order, and HPV type 16 had the highest rate (19% and 24% in the HPV-positive patients with normal or abnormal cytologies, respectively). Similarly, in many studies from Turkey, Eastern Asia and Europe, the six most common HPV types are HPV 16, 18, 31, 51, 52 and 58, whereas the most common high-risk HPV type is HPV type 16 in patients with both normal and abnormal cervical cytologies by the Hybrid Capture II and PCR methods6,7,14,20,21,23-30. It should be noted that, except for HPV 16, the most common HPV types may differ from country to country worldwide and even from region to region within a country.
| Cytology | HPV types | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16a | 18a | 31a | 33a | 35a | 39a | 45a | 51a | 52a | 56a | 58a | 59a | 66b | 68a | 6c | 11c | Multiple* | Total | |
| Normal cytology, n (%) | 16 (19) | 5 (6) | 6 (7) | 3 (4) | 4 (5) | 2 (2) | 2 (2) | 8 (10) | 2 (2) | 4 (5) | 3 (4) | 3 (4) | 3 (4) | 3 (4) | 2 (2) | 1 (1) | 16 (19) | 83 (100) |
| Abnormal cytology | ||||||||||||||||||
| HGSIL | 4 | - | 1 | - | - | - | 1 | - | - | - | 1 | - | - | - | - | - | 5 | 12 |
| LGSIL | 1 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - | - | 2 | 4 |
| ASC-US | 2 | 1 | - | - | 1 | - | - | - | 1 | - | 1 | - | - | 1 | 2 | 1 | 2 | 12 |
| ASC-H | 1 | - | 1 | - | 1 | - | 1 | - | - | - | - | - | - | - | - | - | 1 | 5 |
| n (%) | 8 (24) | 1 (3) | 2 (6) | - | 2 (6) | - | 2 (6) | - | 1 (3) | - | 2 (6) | - | - | 2 (6) | 2 (6) | 1 (3) | 10 (31) | 33 (100) |
*Multiple HPV types are positive in the same patient with normal cytology: 6/16; 11/33; 16/33; 16/51 (in 2 patients); 16/56; 16/58; 16/66; 31/45; 33/56; 39/51; 39/52; 52/66; 16/51/58; 51/52/66; 16/35/51/59. Multiple HPV types are positive in the same patient with abnormal cytology: 6/33 (in 2 patients); 6/45; 11/18; 6/51; 33/35; 6/39/66; 6/18/59; 16/31/45/52; 11/51/66/68, aHigh-risk HPV types; bPossible high-risk HPV type; cLow-risk HPV types. ASC-US, atypical squamous cells of undetermined significance; HGSIL, high-grade squamous intraepithelial lesion; LGSIL, low-grade squamous intraepithelial lesion; ASC-H, atypical squamous cells-cannot exclude HSIL
Furthermore, in our study, 26 (22%) of the 116 HPV-positive patients had multiple HPV genotypes, including 10 (31%) of the 33 HPV-positive patients with an abnormal cytology and 16 (19%) of the 83 HPV-positive patients with a normal cytology (Table II). Of these, high-risk HPV types 16, 51 and 33 and low-risk HPV type 6 were the most common genotypes particularly in women with multiple HPV infections, irrespective of the cytology (normal or abnormal). Consistent with our results, several studies reported multiple HPV infections, namely 21 per cent in all HPV-positive patients24 and 23 per cent in HPV-positive women with a normal pathology26. However, one study from Turkey30 reported that 36 per cent of their cases had multiple HPV infections, which is higher than the value reported in the present study (22%).
HPV positivity for the different age groups in patients with normal or abnormal cytology: The patients were divided into five age groups, and the HPV positivity and cytology results were evaluated according to the age groups. HPV infection is more common in young women. In some studies, the highest proportion of HPV positivity is reported in women aged 31-40 or 35-45 and in those younger than 20 years24,25,31,32. However, in studies performed in Turkey, it is reported that women between the 30 and 39 yr have the highest HPV positivity6,29,30. In the present study, we also found that the highest HPV positivity in the 30-39 yr age group for patients with both normal (40%) and abnormal (40%) cytologies (Table III, P<0.05). The high HPV positivity in this age range may be explained by the fact that women in this sexually active period have a higher chance of being infected with HPV.
| Age groups | HPV positivity in 270 patients*, n (%) | HPV positivity in 43 patients with an abnormal cytology**, n (%) | HPV positivity in 227 patients with a normal cytology***, n (%) |
|---|---|---|---|
| Total | 116 | 33 | 83 |
| 19-29 | 21 (18) | 8 (24) | 13 (16) |
| 30-39 | 46 (40) | 13 (40) | 33 (40) |
| 40-49 | 22 (19) | 7 (21) | 15 (18) |
| 50-59 | 24 (21) | 4 (12) | 20 (24) |
| 60-69 | 3 (2) | 1 (3) | 2 (2) |
*HPV positivity for different age groups is statistically significant in all patients (P=0.014); **HPV positivity for different age groups is not statistically significant in patients with an abnormal cytology (P=0.959); ***HPV positivity for different age groups is statistically significant in patients with a normal cytology (P=0.007).
The limitations of this study are that the study population was relatively small and was limited to a single-centre investigation. Thus, our findings cannot be extrapolated much. Our results demonstrated that regular gynaecological check-ups, as well as HPV detection methods, are important for identifying high-risk types of HPV at an early stage as this is a known cause of cervical cancer. However, factors such as cigarette smoking, a weak immune system and human immunodeficiency virus (HIV) infection could increase the persistence of HPV infection. The other limitation of our study is that the percentage of abnormal cytology patients was low. This could be due to the use of a Cervex-Brush instead of a combination of an extended tip spatula and an endocervical cytobrush during the sampling.
Patients with normal cytology had a higher prevalence (6%) of high-risk HPV18 in this study, whereas it was low (3%) in women with abnormal cytology as shown in Table II. This may be because of low sample size.
Taken together, this study revealed that high-risk HPV types could be determined in smear samples taken from patients who had normal or abnormal cytologies using a multiplex PCR method. Therefore, our results indicated that identifying high-risk HPV genotypes in women with clinically suspicious cervical lesions is crucial and this should be conducted during an annual follow up, irrespective of a normal or abnormal cytology by the age of 30 yr or above.
Financial support & sponsorship: This work was financially supported by the Research Fund of the Erciyes University (Project Number: TYL-2017-7316).
Conflicts of Interest: None.
References
- 2020. International Agency for Research on Cancer. World Health Organization. Cervix Uteri: GLOBOCAN; Available from: https://gco.iarc.fr/today/data/factsheets/cancers/23-Cervix-uteri-fact-sheet.pdf
- Global cancer statistics. 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- [Google Scholar]
- Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-53.
- [Google Scholar]
- 2020. International Agency for Research on Cancer. World Health Organization. Population fact sheets, Turkey: GLOBOCAN; Available from: https://gco.iarc.fr/today/data/factsheets/populations/792-turkey-fact-sheets.pdf
- General Directorate of Public Health, T. C. Ministry of Health. Cervical cancer screening program national standards. Available from: https://hsgm.saglik.gov.tr/tr/kanser-tarama-standartlari/ listesi/serviks-kanseri-tarama-program%C4%B1-ulusal-standartlar%C4%B1.html
- Prevalence and distribution of high-risk human papillomavirus in Amasya region, Turkey. Biomed Res. 2016;27:769-72.
- [Google Scholar]
- Evaluation of the frequency of human papillomavirus (HPV) in women admitted to cancer early diagnosis and screening training centers (KETEM) and analysis of HPV genotypes. Turk Hij Den Biyol Derg. 2019;76:163-8.
- [Google Scholar]
- Human papillomavirus: Biology and pathogenesis. Human papillomavirus and related diseases – From bench to bedside – A clinical perspective 2012:3-40.
- [Google Scholar]
- Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401:70-9.
- [Google Scholar]
- Human papillomavirus and control of cervical cancer in India. Expert Rev Obstet Gynecol. 2010;5:329-46.
- [Google Scholar]
- Human papillomavirus epidemiology in populations with normal or abnormal cervical cytology or cervical cancer in the Middle East and North Africa: A systematic review and meta-analysis. J Infect Public Health. 2020;13:1304-13.
- [Google Scholar]
- Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131:2349-59.
- [Google Scholar]
- American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis. 2012;16:175-204.
- [Google Scholar]
- Use of primary high-risk human papillomavirus testing for cervical cancer screening: Interim clinical guidance. J Low Genit Tract Dis. 2015;19:91-6.
- [Google Scholar]
- 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102-31.
- [Google Scholar]
- The bethesda system for reporting cervical cytology, definitions, criteria, and explanatory notes. 2015
- [Google Scholar]
- PathologyOutlines.com. Cervix general: WHO classification. Available from: https://www.pathologyoutlines.com/topic/cervixWHO.html
- Serviksin prekanseröz lezyonlarında human papilloma virus (HPV) tiplerinin belirlenmesi. Van Med J. 2013;20:70-5.
- [Google Scholar]
- The prevelance of human papillomavirus (HPV) genotypes detected by PCR in women with normal and abnormal cervico-vaginal cytology. Ginekol Pol. 2018;89:62-7.
- [Google Scholar]
- Analysis of HPV genotypes and liquid-based cervical cytology: Results from a tertiary academic center in northwestern turkey. Jpn J Infect Dis. 2021;74:69-72.
- [Google Scholar]
- Prevalence of human papillomavirus genotypes and relative risk of cervical cancer in China: A systematic review and meta-analysis. Oncotarget. 2018;9:15386-97.
- [Google Scholar]
- Human papillomavirus genotype distribution among HPV-positive women in Sichuan province, Southwest China. Arch Virol. 2018;163:65-72.
- [Google Scholar]
- Prevalence of specific types of human papiloma virus in cervical intraepithelial lesions and cervical cancer in macedonian women. Med Arch. 2018;72:26-30.
- [Google Scholar]
- High-risk human papillomavirus genotype distribution and attribution to cervical cancer and precancerous lesions in a rural Chinese population. J Gynecol Oncol. 2017;28:e30.
- [Google Scholar]
- Do clinical data and human papilloma virus genotype influence spontaneous regression in grade I cervical intraepithelial neoplasia? J Turk Ger Gynecol Assoc. 2017;18:1-8.
- [Google Scholar]
- Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789-99.
- [Google Scholar]
- Human papillomavirus (HPV) subtypes and their relationships with cervical smear results in cervical cancer screening: A community-based study from the central Anatolia region of Turkey. Int J Clin Exp Pathol. 2019;12:1391-8.
- [Google Scholar]
- HPV genotype distribution among women with normal and abnormal cervical cytology in Turkey. Rev Esp Quimioter. 2019;32:516-24.
- [Google Scholar]
- Comparison of the diagnostic value of cervical cytology and HPV HR DNA testing for the diagnosis of low-grade and high-grade squamous intraepithelial lesions across different age groups. Ginekol Pol. 2017;88:141-6.
- [Google Scholar]
- Human papillomavirus genotype distribution and E6/E7 oncogene expression in Turkish women with cervical cytological findings. Asian Pac J Cancer Prev. 2014;15:3997-4003.
- [Google Scholar]






