Translate this page into:
High degree of fluoroquinolone resistance among pulmonary tuberculosis patients in New Delhi, India
For correspondence: Dr Surendra Kumar Sharma, Adjunct Professor, Department of Molecular Medicine, Jamia Hamdard Institute of Molecular Medicine, Hamdard University, Hamdard Nagar, New Delhi 110 062, India e-mail: sksharma.aiims2@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The fluoroquinolones (FQs) group of antibiotics is the backbone drugs for the management of drug-resistant tuberculosis (TB). In routine clinical practice, drug susceptibility testing (DST) for FQs is not performed, and the patients are empirically treated. A limited information exists regarding FQs resistance among pulmonary TB cases. The present study was conducted to determine the FQs resistance among drug sensitive and drug-resistant pulmonary TB patients in a tertiary care centre in north India.
Methods:
A total of 1619 sputum/smear-positive specimens of pulmonary TB patients were subjected to DST for first-line drugs (FLDs) and second-line drugs. In addition, FQs DST was also performed using automated Mycobacterial Growth Indicator Tube-960 liquid culture technique. The immuno-chromatographic assay was performed to distinguish Mycobacterium tuberculosis complex (MTBC) from non-MTBC.
Results:
Mycobacterium tuberculosis (Mtb) was isolated in 1499 sputum specimens; 1099 culture specimens were sensitive to FLDs, 249 grew as multidrug-resistant (MDR) Mtb and the remaining 151 isolates revealed any drug resistance to FLDs. While FQs monoresistance among the FLD sensitive isolates was 3.1 per cent (35/1099), 27.3 per cent (68/249) among MDR Mtb isolates had additional FQs resistance.
Interpretation & conclusions:
FQs resistance among drug sensitive and MDR Mtb isolates was high in Delhi, India. Based on these findings, it is recommended that the DST for FQs should be routinely performed to avoid further amplification of drug resistance.
Keywords
Drug susceptibility testing
extensively drug-resistant tuberculosis
multidrug-resistant tuberculosis
pre-extensively drugresistant tuberculosis
Fluoroquinolones (FQs) are a group of bactericidal antibiotics acting on DNA gyrase and have a high activity against Mycobacterium tuberculosis (Mtb). These are classified as group A drugs by the World Health Organization (WHO) and form the backbone of multidrug/extensively drug-resistant TB (M/XDR-TB) treatment due to their good oral bioavailability, affordable cost and excellent safety profile1. Due to widespread prescription of FQs for trivial infections and their easy availability over the counter (OCT) in India, resistance to these drugs has remarkably increased, and this poses a major challenge to the clinicians, as it takes away a very potent weapon from the armamentarium against M/XDR-TB treatment2. The emergence of resistance to FQs also accelerates the progression of disease among multidrug-resistant (MDR) patients and is a risk factor for the development of XDR-TB3. Even treatment-naïve and drug-sensitive TB patients are at risk of FQs resistance4 making it imperative to assess FQs resistance at the outset before initiating therapy, especially in TB high-endemic areas5.
According to the WHO TB Report (2017), the prevalence of any FQs resistance [drug susceptibility testing (DST) done for ofloxacin (OFX), levofloxacin (LFX) and moxifloxacin (MFX)] among MDR-TB patients was 21 per cent globally5. Testing for FQs resistance before starting therapy is rarely done in sputum specimens in treatment-naïve as well as in treatment-experienced TB patients, and this leads to lack of quality data regarding the same. The present observational study was carried out in a tertiary care centre in north India to find FQs resistance among smear-positive pulmonary TB patients.
Material & Methods
A total of 1619 consecutive smear-positive pulmonary TB (PTB) patients were enrolled for diagnosis of MDR-TB at the Intermediate Reference Laboratory (IRL) of the All India Institute of Medical Sciences, New Delhi, between August 2013 and January 2016 after obtaining written informed consent and detailed drug treatment history. Institutional Ethics Committee approved the study protocol. Two slides for acid-fast bacilli were made directly from each sample collected from spot and morning sputum specimens and stained by Ziehl-Neelsen method as per the Revised National Tuberculosis Control Programme (RNTCP) guidelines6. All samples were handled in the biosafety level III (BSL-III) laboratory in class-II biosafety cabinet following standard decontamination protocol, using NALC-NaOH (N-acetyl-L-cysteine-sodium hydroxide) method7. After centrifugation, the supernatant was discarded, and the pellet was dissolved in 1-1.5 ml of phosphate buffer saline. Only 0.5 ml of this concentrated sample was inoculated on BACTEC Mycobacterial Growth Indicator Tube (MGIT-960) liquid culture medium (Becton Dickinson, Sparks, MD, USA) containing 7 ml of 7H9 supplemented with 0.8 ml of oleic acid-albumin-dextrose-catalase along with polymyxin B-amphotericin B-nalidixic acid-trimethoprim-azlocillin. The positive tubes by MGIT-960 underwent sterility check on brain heart infusion agar to rule out contamination. The immunochromatographic assay (ICA) was performed to distinguish Mtb complex (MTBC)from non-MTBC. The DST for first-line [streptomycin (S) 1.0 μg/ml, isoniazid (H) 0.1 μg/ml, rifampicin (R) 1.0 μg/ml and ethambutol (E) 5.0 μg/ml], and the second-line [kanamycin (KM) 2.5 μg/ml, OFX 2.0 μg/ml, LFX 2.0 μg/ml and MFX 2.0 μg/ml] anti-TB drugs was performed on pure Mtb isolates using BACTEC MGIT-960 system as per the standard operating procedure according to the manufacturer's instruction7.
Results & Discussion
Of the 1619 smear-positive PTB patients, 1104 (68%) were male and 515 (32%) were female patients with mean age of 34±14.5 yr. A total of 1520 (93.9%) samples from the patients were culture positive by MGIT-960; among these 1499 (98.6%) were identified as MTBC and 21 (1.4%) were negative for MTBC by ICA. All the MDR-TB isolates among treatment naïve and treatment experienced patients were subjected to second-line DST (Figure). In the remaining 1499 Mtb culture specimens, 385 patients were newly diagnosed and 1114 patients were TB treatment-experienced patients. A total of 249 MDR-TB patients were detected in this study, of whom 19 (4.9%; 19/385) were new cases and 230 (20.6%; 230/1114) were previously treated TB patients. The second-line drug (SLDs) DST patterns were available for 249 MDR Mtb isolates; 67.5 per cent (168/249) isolates were found to be susceptible to all the SLDs. Resistance to OFX was found in 27.3 per cent (68/249) isolates, and resistance to KM was observed in 0.8 per cent (2/249) MDR Mtb isolates. The FQ resistance (OFX) among drug-sensitive Mtb isolates was 3.2 per cent (35/1099). The prevalence of XDR-TB among MDR Mtb isolates was 3.21 per cent (8/249) (Table). The cross-resistance between LFX and OFX was observed in two isolates while between LFX, OFX and MFX, was seen in one isolate. All cases of KM resistance and XDR-TB occurred in treatment-experienced TB patients only.
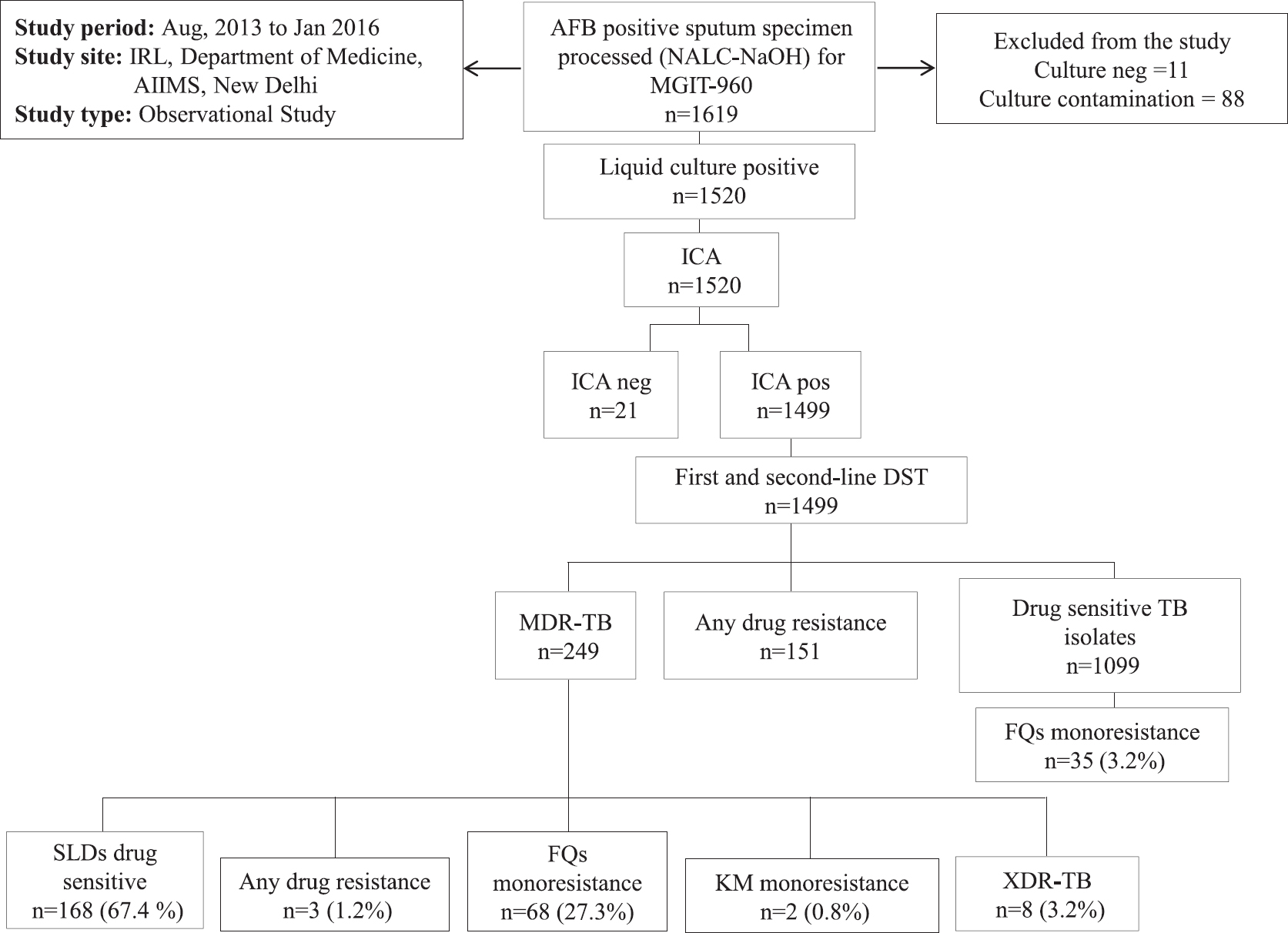
- Schematic flow of study design. IRL, intermediate reference laboratory Accredited laboratory by Ministry of Health & Family Welfare, Government of India and Revised National Tuberculosis Control Programme, India; AIIMS, All India Institute of Medical Sciences, New Delhi; AFB, acid-fast bacilli; MGIT-960, mycobacterial growth indicator tube; ICA, immunochromatographic assay; FQs, fluoroquinolones; KM, kanamycin; DST, drug susceptibility testing; SLDs, second-line drugs; Mtb, Mycobacterium tuberculosis; TB, Tuberculosis; MDR-TB, multidrug-resistant tuberculosis; XDR-TB, extensively drug-resistant TB; Pre-XDR-TB Mtb isolates resistant to first-line drugs (FLDs) and resistant to second-line injectables or any one of the fluoroquinolones.
| Drug resistance pattern | Number of isolates (%) |
|---|---|
| One-drug resistance | |
| OFX | 68 (27.3) |
| KM | 2 (0.80) |
| LFX | – |
| MFX | – |
| Two-drug resistance | |
| KM + OFX | 6 (2.40) |
| LFX + OFX | 2 (0.80) |
| Three-drug resistance | |
| LFX + OFX + MFX | 1 (0.40) |
| LFX + OFX + KM | 1 (0.40) |
| Four-drug resistance | |
| KM + OFX + LFX + MFX | 1 (0.40) |
| All sensitive isolates | |
| KM + OFX + LFX + MFX | 168 (67.4) |
Data in parentheses are percentages. MDR-TB, multidrug-resistant tuberculosis; KM, kanamycin (2.5 µg/ml); OFX, ofloxacin (2 µg/ml); LFX, levofloxacin (2 µg/ml); MFX, moxifloxacin (2 µg/ml)
Indiscriminate use of FQs for a variety of infections such as respiratory tract, genitourinary tract, gastrointestinal tract, skin and ocular infections and initiating anti-TB therapy with FQs in drug sensitive TB patients in a high-endemic country like India has contributed to the rapid emergence of resistance to this class of anti-TB drugs891011. Studies also report that as small as 13 days of exposure to FQs can lead to the development of FQs resistance among treatment naïve and treatment-experienced TB patients8. Jain et al4 from Lucknow, north India, and Selvakumar et al9 from Tamil Nadu reported FQs resistance among MDR-TB patients as 26 and 29 per cent, respectively, which was similar to the present study. A drug resistance surveillance (DRS) study conducted by Ramachandran et al12 in Gujarat, 2009, reported FQs monoresistance to be 24 per cent with no significant difference between treatment-naïve and treatment-experienced TB patients. A recent study from a national reference laboratory in India showed 32 per cent of FQ resistance among MDR-TB isolates before treatment with second-line anti-TB drugs; the study had a higher rate of FQs resistance as their facility received mostly defaulter cases13. India's first National DRS (NDRS) for MDR-TB was completed by the National Tuberculosis Institute, Bengaluru, Karnataka and the report of NDRS will have important public health implications in the prevention and control of TB disease as it will be truly representative sample of various States of India14.
In the present study 3.2 per cent FQs monoresistance was observed among drug sensitive Mtb isolates, which further highlighted the need to use these drugs judiciously and regulate their availability stringently. The presence of FQs resistance among drug sensitive Mtb isolates in the present study may be due to indiscriminate use of some commonly available quinolones for the treatment of various infections151617.
The strength of the present study was that isolation, identification and DST of all positive Mtb isolates were carried out in the RNTCP-certified IRL laboratory having quality assured BSL-III laboratory facility and the limitations included the use of liquid culture with high contamination rates. Acquisition of FQ resistance among Mtb isolates is a matter of concern and is a contribution to the development of XDR-TB14.
In conclusion, increasing FQ resistance among treatment-naive and treatment-experienced TB patients is a matter of concern. It is suggested to test sputum and other specimens of pulmonary TB patients for FQ and other second-line anti-TB drugs resistance. Since we have limited options for the treatment of XDR-TB, all efforts should be directed to create awareness among practicing doctors to use these drugs with discretion.
Acknowledgment
The authors thank the All India Institute of Medical Sciences (AIIMS) administration for providing appropriate infrastructure; Central TB Division, Ministry of Health and Family Welfare, Government of India; State TB cell, Foundation of Innovative New Diagnostics, India for logistic support. Authors also acknowledge the help of the staff at Intermediate Reference Laboratory (IRL), AIIMS, New Delhi, for sample collection and processing.
Financial support & sponsorship: The study was supported by J.C. Bose Fellowship awarded to the second author (SKS) (SB/S2/JCB-04/2013), from the Ministry of Science and Technology, Government of India.
Conflicts of Interest: None.
References
- Guidelines for the programmatic management of drug-resistant tuberculosis: Emergency Update. 2008. Available from: http://apps.who.int/iris/bitstream/handle/10665/43965/9789241547581_eng.pdf
- [Google Scholar]
- Empirical treatment with a fluoroquinolone delays the treatment for tuberculosis and is associated with a poor prognosis in endemic areas. Thorax. 2006;61:903-8.
- [Google Scholar]
- Increasing incidence of fluoroquinolone-resistant Mycobacterium tuberculosis in Mumbai, India. Int J Tuberc Lung Dis. 2009;13:79-83.
- [Google Scholar]
- Pre-XDR & XDR in MDR and ofloxacin and kanamycin resistance in non-MDR Mycobacterium tuberculosis isolates. Tuberculosis (Edinb). 2012;92:404-6.
- [Google Scholar]
- Central TB Division. Revised National Tuberculosis Control Programme: Guidelines on programmatic management of drug resistant TB (PMDT) in India. New Delhi: Directorate General of Health Services, Ministry of Health and Family Welfare; 2012.
- [Google Scholar]
- MGIT™ procedure manual: For BACTEC™ MGIT 960™ TB system. Franklin Lakes: Becton Dickinson; 2006.
- [Google Scholar]
- The rapid development of fluoroquinolone resistance in M. tuberculosis. N Engl J Med. 2003;349:1977-8.
- [Google Scholar]
- High rates of ofloxacin resistance in Mycobacterium tuberculosis among both new and previously treated patients in Tamil Nadu, South India. PLoS One. 2015;10:e0117421.
- [Google Scholar]
- Fosfomycin: Use beyond urinary tract and gastrointestinal infections. Clin Infect Dis. 2008;46:1069-77.
- [Google Scholar]
- Detection and prevention of ocular phototoxicity of ciprofloxacin and other fluoroquinolone antibiotics. Photochem Photobiol. 2010;86:798-805.
- [Google Scholar]
- Surveillance of drug-resistant tuberculosis in the state of Gujarat, India. Int J Tuberc Lung Dis. 2009;13:1154-60.
- [Google Scholar]
- Baseline resistance and cross-resistance among fluoroquinolones in multidrug-resistant Mycobacterium tuberculosis isolates at a national reference laboratory in India. J Glob Antimicrob Resist. 2018;12:5-10.
- [Google Scholar]
- Central TB Division. TB India 2017. Revised National TB Control Programme. In: Annual Status Report. New Delhi: Central TB Division, Ministry of Health and Family Welfare, Government of India; 2017.
- [Google Scholar]
- National patterns in the treatment of urinary tract infections in women by ambulatory care physicians. Arch Intern Med. 2002;162:41-7.
- [Google Scholar]
- Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis. 2000;31:347-82.
- [Google Scholar]
- A study on pre-XDR & XDR tuberculosis & their prevalent genotypes in clinical isolates of Mycobacterium tuberculosis in North India. Indian J Med Res. 2016;143:341-7.
- [Google Scholar]






