Translate this page into:
Hepatitis B and/or C co-infection in HIV infected patients: A study in a tertiary care centre from south India
Reprint requests: Dr Naval Chandra, Associate Professor, Department of General Medicine, Nizam's Institute of Medical Sciences, Punjagutta, Hyderabad 500 082, India e-mail: naval31@yahoo.co.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Co-infection with hepatitis B virus (HBV) and hepatitis C virus (HCV) in human immunodeficiency virus (HIV) infected individuals results in increased hepatic complications. We undertook this study to evaluate the presence of HBV and HCV in HIV infected individuals attending a tertiary care centre in southern India.
Methods:
A total of 120 cases with HIV infection and 120 healthy adult control subjects were included in the study. Samples were tested for hepatitis B surface antigen (HBsAg) and anti-HCV antibodies by enzyme linked immunosorbent assay (ELISA) method. HBsAg and anti-HCV positive serum samples were further tested for the presence of hepatitis B e antigen (HBeAg), anti-HBe antibodies, HBV-DNA and HCV-RNA.
Results:
The most common mode of transmission was sexual promiscuity (79%), followed by spouse positivity (15%) and history of blood transfusion (6%). HBsAg and anti-HCV were positive in 18 (15%) and 10 (8.3%) HIV infected patients; the corresponding figures in healthy controls being 2 (1.6%) 0 (0%) (P<0.0001). Among HIV infected patients, presence of HBeAg and anti-HBe antibodies was seen in 33.3 and 55.5 per cent, respectively; both HBeAg and anti-HBe antibodies were negative in 11.1 per cent. HBV DNA and HCV RNA were positive in 10 of 18 and in all anti-HCV positive samples. Triple infection with HBV, HCV and HIV was seen in three patients. CD4+ T-lymphocyte count less than 200/μl was seen in 22 of 28 co-infected cases.
Interpretation & conclusions:
The findings of our study showed presence of HBV (15%) and HCV (8.3%) co-infections in HIV positive patients which was higher than that seen in HIV negative controls. Co-infection with HBV and HCV is a common problem in HIV infected patients in India. Hence, all HIV patients need to be routinely tested for markers of HBV and HCV infection.
Keywords
Co-infection
hepatitis B virus
hepatitis C virus
human immunodeficiency virus
Human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV) co-infection has emerged as a leading cause of morbidity due to liver disease throughout the world in the last two decades12. Among the HIV infected patients, HBV and HCV co-infections are more prevalent due to overlapping transmission routes3. The introduction of highly-active antiretroviral therapy (HAART) has led to a marked reduction in the morbidity and mortality and has resulted in increased survival in HIV infected patients34. Consequently, the importance of co-morbidities such as chronic liver disease due to HBV and HCV infection is being recognized as significant problems. HIV infection modifies the natural history of chronic parenterally acquired hepatitis C with unusually rapid progression to cirrhosis. Overall survival of HIV positive patients is not affected by the presence of HCV23. However, HCV predisposes to death from liver failure45.
In co-infection, the presence of one virus impacts the natural history of the other virus. HIV accelerates the natural course of HBV and HCV infection and facilitates faster progression of liver disease to cirrhosis and hepatocellular carcinoma. Disease progression to cirrhosis in HIV positive patients is almost three-times faster as compared to HIV negative patients456. Most of the studies678 in HIV-HBV and HIV-HCV co-infected patients have been conducted among western patient populations. Understanding HBV and HCV co-infection with HIV is particularly important in Asian countries due to high background prevalence of HBV and HCV9. The present study was undertaken with the objective to assess the presence of HBV or HCV co-infection in HIV infected patients at a tertiary care centre in southern India.
Material & Methods
The study was carried out at the departments of General Medicine and Medical Gastroenterology, Nizam's Institute of Medical Sciences, Hyderabad, Andhra Pradesh, India, during November 2009 to May 2011. The study included 120 newly diagnosed HIV patients attending the outpatient of General Medicine and were confirmed by Western Blot (HIV W. Blot, J Mitra & Co. Pvt Ltd, New Delhi, India) (Group 1). The sample size was calculated using online calculator using www.openEpi.com. The study was prospective observational study. Age and sex matched 120 healthy controls (Group 2) were also included in the study. These healthy controls were taken simultaneously from a study on HBV vaccination which was simultaneously done in Department of Gastroenterology. Five ml blood was drawn for various investigations. These individuals had normal renal functions, liver functions and normal haemogram. The patients were recruited randomly. Written informed written consent was taken from all the subjects. The study protocol was approved by the Institutional Ethics Committee, Nizam's Institute of Medical Sciences, Hyderabad.
A detailed history of sexual activities, blood transfusion, intravenous drug abuse and other opportunistic infections was obtained. All patients with HIV infection underwent complete haemogram, liver function tests (LFT), chest X-ray, Mantoux test, and CD4+ T-lymphocyte count. HIV infected patients and healthy controls were also tested for HBsAg (Hepanostica HBsAg, Biomerieux, Netherland) and anti-HCV antibodies (General Biologicals Corp., Taiwan) by enzyme linked immunosorbent assay (ELISA) as per the manufacturer's instructions. All HBsAg positive patients were further tested for hepatitis B e antigen and antibodies (HBeAg and anti-HBe) (General Biologicals Corp., Taiwan) by ELISA method. Qualitative HBV-DNA and HCV-RNA were tested using polymerase chain reaction (PCR) technique10111213 in all HBsAg and anti-HCV positive patients, respectively. HBV-DNA was isolated from serum by modified protocol and detected by PCR amplification1011. The appearance of a 566-bp fragment corresponding to the core gene, which is conserved in all strains, was taken to be indicative of a positive reaction. Presence of HCV-RNA was detected by the guanidium thiocyanate-phenol method12. Amplification by reverse transcriptase-PCR (RT-PCR) was done by the method of Das et al13. RT-PCR was carried out in a single tube using a programmable thermocycler (Gene AmpR PCR System 2400, Applied Biosystems, CA, USA). PCR products were analyzed on 2 per cent agarose gels followed by staining with ethidium bromide and visualized under a UV illuminator. A negative control, a positive control and water blank were used for quality control and to exclude false positive results in the PCR due to cross-contamination.
Ultrasonography of the abdomen was done in all HBV, HCV positive patients.
Statistical analysis: The presence of HBsAg and anti-HCV was compared in the controls and patient groups using Chi-square test. The distribution of variables like CD4+ count, ALT and serum albumin were used as continuous variables. The mean, median and distribution of these variables in the four groups, i.e., HIV alone; HIV plus HBV; HIV plus HCV; and HIV plus HBV plus HCV co-infection were studied using one-way analysis of variance (ANOVA) and P trend was obtained to explore whether this variable was altering in co-infection. Further, using different cut-offs for different variables Fisher exact test was conducted for categories above and below the cut-off and corresponding odds ratios, 95% confidence intervals (95% CI) were obtained.
Results
The mean age of group 1 patients was with 35.6 ± 8.7 yr; there were 100 males. The mean age of group 2 individuals was 34.2 ± 7.56 yr; there were 104 males. The common presenting features in group 1 were fever, loss of appetite, loss of weight and cough. The predominant mode of transmission of HIV was sexual activity (79%, n=95; heterosexual) followed by spouse positivity in 15 per cent (n=18) and history of blood transfusion in 6 per cent (n=7). None of the HIV infected patients or controls gave history of intravenous drug abuse. Among the seven cases who received blood transfusion, five had HCV co-infection.
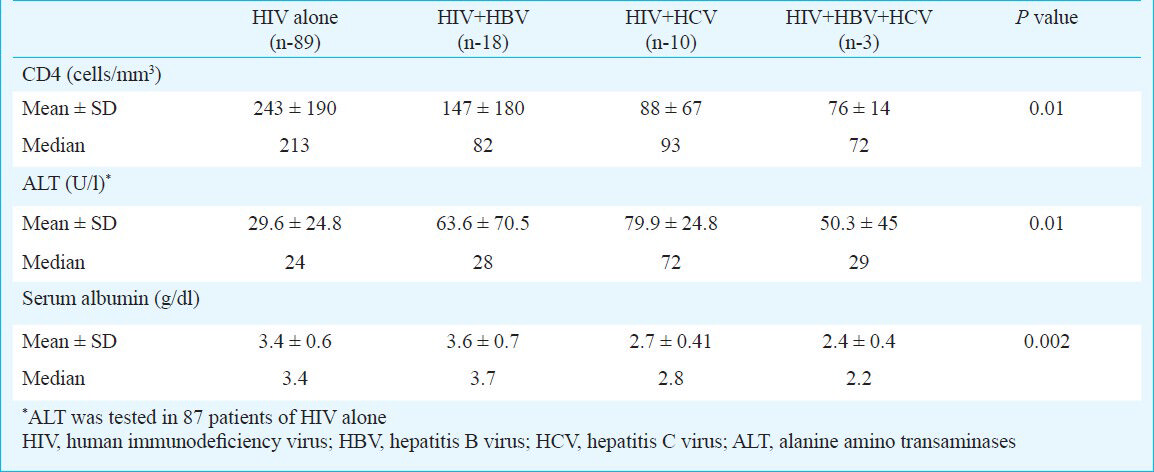
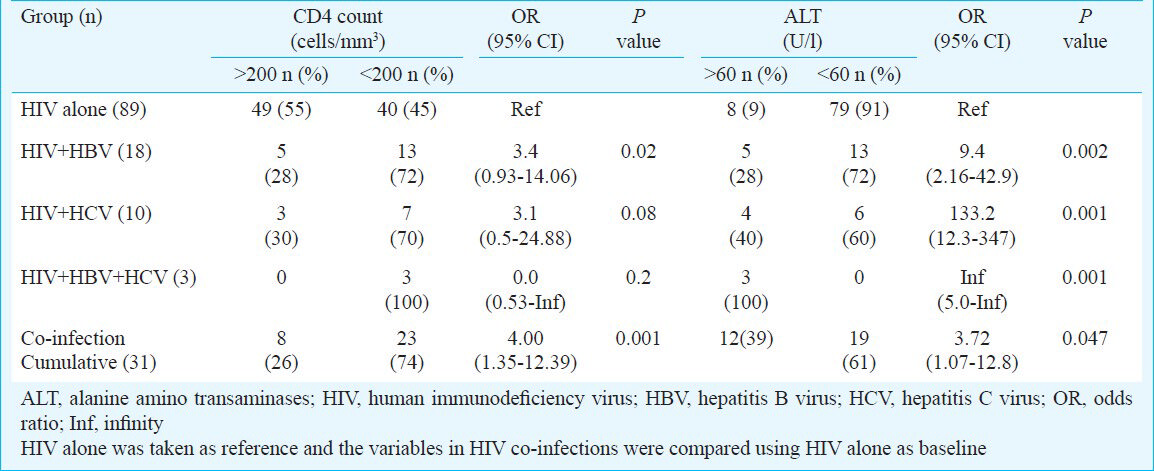
The presence of HBsAg was found in 15 per cent (n=18) HIV patients and 1.6 per cent (n=2) controls (P<0.001). Anti-HCV antibodies were positive in 10 (8.3%) patients in HIV group while none of the controls was positive (P<0.001). Among the 28 HBsAg and HCV positive patients, three had triple infection with HBV, HCV and HIV. Hepatitis B envelope antigen and antibody (HBeAg and anti HBe) were positive in 33.3 per cent (6/18), and 55.5 per cent (10/18), respectively. HBV-DNA was positive in 55.6 per cent (10/18) (6 were HBeAg positive, 4 were anti-HBe positive). HCV-RNA was positive in all anti-HCV positive patients. The mean and median CD4 count, alanine aminotransferase (ALT) and serum albumin levels in the various groups are shown in Table I. The mean CD4 count was significantly low in HIV patients with HBV and/or HCV co-infection (Table I). The CD4+ count less than 200 per μl was seen in 40/89 (45%) of patients with HIV infection alone while it was seen in 23/31 (74%) in HIV co-infection (Table II). The CD4 count <200 cells/mm3 was seen in 13/18 (72%) in HBV co-infection, 7/10 (70%) in HCV co-infection and 3/3 (100%) in triple infection Table II (P<0.002). There was a four-fold increase in patients having CD4 count less than 200 cells/μl in HIV-HBV and/or HCV co-infection than HIV alone (Table II) odds ratio=4.0. To compare HIV vs HIV + co-infections P trend test using ANOVA was conducted to explore whether this variable was altered in co-infection. The mean and median ALT in co-infections was high in comparison to HIV infection alone (Table I, P trend 0.01). ALT more than 1.5 times the upper normal limits (40 U/l) was seen in 27.7 per cent (15/18) of HBV co-infected patients, 40 per cent (4/10) of HCV co-infected patients as against 9.1 per cent (8/87) in patients of HIV alone and 3/3 (100%) in triple infection respectively (Table II). Taking 60 U/l as cut-off value, the HIV patients with HBV/HCV co-infection had a 3.7 fold increase in liver damage (as indicated by raised ALT) compared to HIV alone (odds ratio 3.7, Table II). Hypoalbuminaemia was seen in 10 per cent (n=9/89) of HIV patients and in 22 per cent (n=4/18) HIV with HBV co-infection and 50 per cent (n=5/10) in HCV co-infection. These parameters (ALT, serum albumin and CD4) were compared only between HIV infection subgroups (HIV alone and/or HIV with co-infection) to see the impact of co-infection, hence controls were not included for this comparison. The other opportunistic infections noted were tuberculosis in 12 patients (8 pulmonary and 4 extrapulmonary), Pneumocystis carinii pneumonia (PCP) in 8 patients, candidiasis in 4 patients, chronic diarrhoea in 2 patients and herpes zoster in one patient.
Discussion
Co-infection with HBV and/ or HCV complicates the clinical course of HIV in infected patients, and may also adversely affect treatment of HIV infection. The prevalence of HBV and HCV co-infection in HIV has been variably reported in different studies14151617. The prevalence of HBV varies markedly among different HIV infected population and geographical location is one of the major determinants of prevalence. The prevalence of HBV co-infection varies from 5-7 per cent in low endemicity areas715. In intermediate and high endemicity, it varies from 6-20 per cent16. The prevalence of HCV co-infection varies from 9-16 per cent817. TREAT Asia HIV Observational Database study from Taiwan has reported HBV-HCV co-infection to be approximately 10 per cent each18.
There are only a few reports19202122 from our country on prevalence of HBV/HCV in HIV patients and the observations have been highly variable. Co-infection observed in these studies was 30.4 per cent from Nagpur19, 2.25 per cent from Lucknow20, 7.7 per cent from Chennai21 and 3.5 per cent from Mumbai22. We found HBV co-infection in 15 per cent HIV positive individuals which was higher than previous reports from other parts of India202122.
The HCV co-infection in the present study was 8.3 per cent (10/120). None of the healthy controls were anti-HCV antibody positive. The HCV co-infection was higher in our study compared to 1.6 per cent in Lucknow20, 7.2 per cent in Nagpur19 and 2.3 per cent in Chennai21. The HCV co-infection from Mumbai was reported as 8 per cent similar to the present study22.
The route of transmission of HIV in most of our patients was sexual contact.
The CD4+ count less than 200 cells/μl was seen in significantly more number of patients in HBV, HCV co-infection than in HIV alone. This reflects that immune status in HIV co-infection was more compromised than in HIV alone. The mean ALT levels were high in HIV co-infections than in HIV alone. The study from Mumbai22 reported that 9.5 per cent of HBV/HIV and 50 per cent of HCV/HIV co-infected patients had raised aspartate aminotransferase (AST) and ALT. ALT levels more than 1.5 times normal are an indicator of liver damage, and serum albumin levels less than 3.0 g/dl should raise the possibility of chronic liver disease14. Hypoalbuminaemia is more common in chronic liver diseases and usually reflects severe liver damage and decreased albumin synthesis. However, hypoalbuminemia is not specific for liver diseases and may occur in other conditions like protein malnutrition and protein losing enteropathies14. In our study the hypoalbuminaemia seen in HIV co-infection may be due to chronic liver disease.
In conclusion, it is evident from the present study the HIV-infected patients in this region have a high risk of acquiring HBV/HCV co-infections through the shared routes of transmission. Hence, HIV patients should routinely be tested for HBV and HCV markers. Screening the high-risk population for these infections would aid in prompt diagnosis and treatment with improved outcomes in these patients which in turn may decrease the further spread of these chronic viral infections.
Acknowledgment
Authors gratefully acknowledge Professor V.R. Srinivasan, Prof & Head, Department of General Medicine, Nizam's Institute of Medical Sciences (NIMS), Hyderabad for his guidance. We also thank Shri Shaik Mohamaad Naushad, Senior Research Fellow, Department of CP & T, NIMS, for the statistical analysis of the data.
References
- Epidemiology of viral hepatitis and co-infection. J Hepatol. 2006;44(Suppl 1):S6-9.
- [Google Scholar]
- Decline in AIDS and death rates in EuroSIDA study; an observational study. Lancet. 2003;362:22-9.
- [Google Scholar]
- Human immunodeficiency virus infection modifies the natural history of chronic parentally acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1-5.
- [Google Scholar]
- Natural history and predictors of severity of chronic hepatitis C virus (HCV) and human immunodeficiency virus (HIV) co-infection. J Hepatol. 2006;44:528-34.
- [Google Scholar]
- Hepatitis B and HIV: Prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19:593-601.
- [Google Scholar]
- Clinical implications of HIV and hepatitis B coinfection in Asia and Africa. Lancet Infect Dis. 2007;6:402-9.
- [Google Scholar]
- Comparison of methods for the detection of HBV DNA. J Clin Microbiol. 1994;32:2088-91.
- [Google Scholar]
- Modified protocol for HBV DNA isolation and detection. Anal Biochem. 2003;314:142-3.
- [Google Scholar]
- Single step method of RNA isolation by guanidium thiocynatephenol-choloroform extraction. Anal Biochem. 1987;162:152-9.
- [Google Scholar]
- Evaluation of liver function. In: Longo Dl, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, eds. Harrison's principles of internal medicine. New York: Mc Graw Hill; 2012. p. :2527-30.
- [Google Scholar]
- HIV 1, hepatitis B virus and risk of liver related mortality in the multicenter cohort study (MACS) Lancet. 2002;306:1921-6.
- [Google Scholar]
- Hepatitis B and human immunodeficiency virus coinfection. Hepatology. 2009;49(Suppl):S138-45.
- [Google Scholar]
- Prevalence and characteristics of hepatitis C vius coinfection in a human immunodeficiency virus clinical trials groups: The Terry Beirn Community Programs for Clinical Research on AIDS. Clin Infect Dis. 2003;36:1313-7.
- [Google Scholar]
- Hepatitis B & C virus co-infection in the TREAT. J Gastroenterol Hepatol. 2007;22:1510-8.
- [Google Scholar]
- Seroprevalence of anti HCV and hepatitis B surface antigen in HIV infected patients. Indian J Med Microbiol. 2003;21:268-70.
- [Google Scholar]
- Low prevalence of hepatitis B virus and hepatitis C virus co infection in patients with human immunodeficiency virus in Northern India. J Assoc Physicians India. 2007;55:429-31.
- [Google Scholar]
- Co-infetion of hepatitis B & hepatitis C in HIV infected patients in South India. World J Gastroenterol. 2007;13:5015-20.
- [Google Scholar]






