Translate this page into:
Guillain–Barre syndrome: Demographics, clinical profile & seasonal variation in a tertiary care centre of central India
Reprint requests: Dr Manisha Shrivastava, Department of Transfusion Medicine, Bhopal Memorial Hospital & Research Centre, Raisen Bye Pass Road, Near Karond Chowk, Bhopal 462 038, Madhya Pradesh, India e-mail: manishasdr@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Guillain–Barre syndrome (GBS) is an autoimmune disease and a recognized cause of generalized progressive paralysis worldwide. The present study was aimed to document the clinical findings, demographics and seasonal variations amongst the patients with GBS during the hospital stay.
Methods:
A retrospective analysis of 66 referred cases diagnosed as GBS was conducted. Medical records and the data related to age, sex, antecedent illness, duration of symptoms before admission, muscle power graded by the Medical Research Council scale, functional scores, details of Intensive Care Unit complications and need for ventilation were obtained. The patients were divided into four seasonal groups: S1 (spring, February to April), S2 (summer, May to July), S3 (rainy, August to October) and S4 (winter, November to January) and parameters were studied.
Results:
The mean age of the patients was 40.69 yr. Forty one (62.1%) patients had a history of preceding illness. Forty nine (74.2%) patients showed quadriparesis as most common complaint. Thirty three (50%) patients were of acute inflammatory demyelinating polyneuropathy (AIDP) variant. The highest number of GBS cases (60%) was found in S1 and S2. The maximum duration of hospital stay was observed in S3 group (mean 23 days).
Interpretation & conclusions:
GBS seems to affect all age groups with male preponderance. Most common antecedent event and presenting feature were flu-like illness and quadriparesis, respectively. AIDP was the most common variant. Most cases occurred from February to July (S1 and S2 group) (maximum in July) with preceding influenza and diarrhoea and maximum duration of hospital stay was observed in S3 group. Prospective studies with follow up of GBS patients need to be done to confirm findings.
Keywords
Acute motor axonal neuropathy
Guillain–Barre syndrome
seasonal variation
Guillain–Barre syndrome (GBS), also known as Landry's paralysis1, is an immune-mediated disorder of nervous system of acute or subacute onset characterized commonly by generalized progressive weakness of arms and legs, limb paraesthesias and relative or complete areflexia2. GBS patients often develop cranial nerve weakness, usually in the form of facial or pharyngeal weakness. The usual pattern follows the flaccid paralysis typically ascending in nature evolving over a few days to a few weeks. Autonomic dysfunctions are common with usual manifestations as loss of vasomotor control with wide fluctuation in blood pressure, postural hypotension and cardiac arrhythmias. Respiratory failure and oropharyngeal weakness may require ventilatory assistance in about one-third of hospitalized patients making it a disease of vital importance for early management23. It is believed that GBS may not be a single disease, but a variety of acute neuropathies with a number of related immune-mediated pathogenic mechanisms most common being endoneurial inflammation of spinal nerve roots, distal nerve segments and potential nerve entrapment sites2. The reported incidence for GBS is 1-2/100,000 population and increases linearly with age, and men are about 1.5 times more affected than women4. Prior infection such as upper respiratory tract infection is well-established predating event in the development of GBS by 10-14 days3. Many antecedent illnesses associated with GBS have been identified including Campylobacter jejuni gastroenteritis, cytomegalovirus, Mycoplasma pneumoniae, Epstein-Barr virus and influenza virus infections24. Diagnosis of GBS may be confirmed by cerebrospinal fluid (CSF) analysis and electrodiagnostic testing both of which may be normal in the early phase of GBS34. The commonly recognized variants of GBS are considered as syndromes including acute inflammatory demyelinating polyneuropathy (AIDP), acute motor axonal neuropathy (AMAN), acute motor sensory axonal neuropathy (AMSAN) and Miller-Fisher syndrome. AIDP is the most prevalent form and accounts for 70-90 per cent of cases56. GBS can occur in any season although seasonal variability may reflect seasonal peaks of predisposing factors such as infections78. The seasonal occurrence has been described to be peaking in summer season in Asian countries6910. Therapeutic plasma exchange (TPE) and intravenous immunoglobulin (IVIg) are effective immunotherapies for adult and paediatric patients with GBS if given during the first few weeks of the disease1112 along with the diligent supportive care to minimize the risk of mortality and clinical risk and eventually improving the outcomes. Physical therapy is considered as integral part of the supportive management in reducing the incidence of complications such as respiratory complications, deep vein thrombosis (DVT), pain management and delayed mobilization13. The objective of this study was to assess the clinical findings, demographic and seasonal variations amongst the patients with GBS during the hospital stay in a tertiary care institute of central India.
Material & Methods
This study was conducted at Bhopal Memorial Hospital and Research Center (BMHRC), Bhopal, India, after obtaining the ethical approval by the Institutional Ethics Committee. In this retrospective analysis, medical records of 66 referred cases with the diagnosis of GBS admitted to BMHRC from 2002 to 2013 were reviewed and analyzed during April to June, 2014. The data related to age, sex, date of admission, antecedent illness, duration of symptoms before admission, muscle power graded by the Medical Research Council (MRC) scale14, Hughes' functional scores (F-Scores)15, details of Intensive Care Unit (ICU) complications if any, need for ventilation, details of investigations including CSF and electrodiagnostic analysis, complete blood profile, lipid profile, serum electrolytes, coagulation profile, blood grouping and information about TPE as therapy instituted were obtained. As per the hospital policy, all patients received TPE as the treatment of choice. Critical and supportive care comprising respiratory care including mechanical ventilation as and when required, cardiac monitoring, DVT prophylaxis, management of infections, nutritional care and physiotherapy were integral part of the treatment. Patients were classified according to MRC Manual Muscle Testing grading system (0-5) and functional grading scales: grade 0 - healthy, grade 1 - minor symptoms and signs of neuropathy, grade 2 - able to walk five min without assistance, grade 3 - able to walk five min with assistance, grade 4 - confined to bed or chair bound, and grade 5 - requiring assisted ventilation. All patients were divided into four groups based on the four seasons of the year depending on the time of their admission in the hospital. The groups were named as S1 spring season (February to April), S2 summer season (May to July), S3 rainy season (August to October) and S4 winter season (November to January) considering the geographical situation of central India1016.
Statistical analysis: The seasonal preponderance was compared by goodness of fit Chi-square test assuming a null hypothesis of no seasonal variation. The duration of hospital stay was compared by one-way ANOVA and other parameters were compared using Chi-square test.
Results
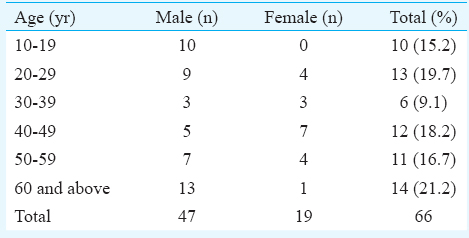
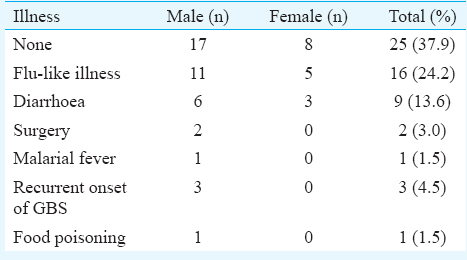
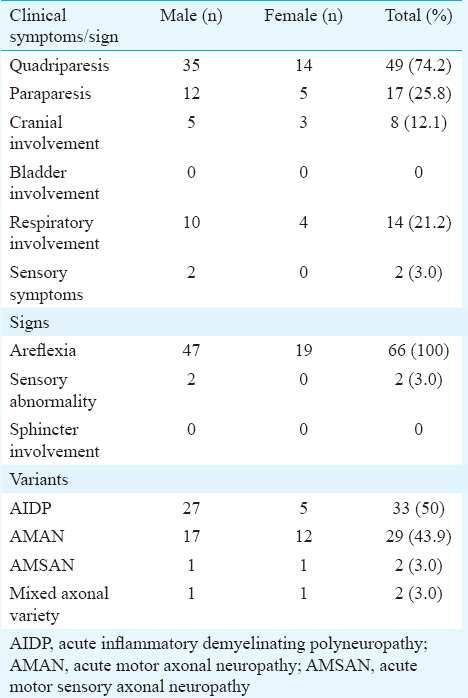
Of the 66 patients, 47 were male. The mean age of the patients was 40.69±18.8 yr. The mean ages of male and female patients were 40.82±21.19 yr (10-74 yr) and 40.36±11.34 yr (20-60 yr), respectively. The male-to-female ratio was 2.4:1. Maximum number of patients (21.2%, n=14) were in the age group of 60 yr and above. The next common age group was 20-29 yr in which 19.7 per cent (n=13) patients were seen (Table I). Nearly 62.1 per cent patients (n=41) had history of preceding illness (Table II). Flu-like illness as evidenced by fever and cough was found to be the most common antecedent event preceding GBS in 24.2 per cent patients (n=16) followed by gastroenteritis in 13.6 per cent patients (n=9). One patient each presented with the uncommon antecedent events as food poisoning and malarial fever. All patients developed neurological illness within two weeks of the onset of the symptoms (Table III). Majority of the patients were admitted to the hospital with progressive weakness in all four limbs (quadriparesis) in 74.2 per cent patients (n=49) as a common clinical feature followed by paraparesis in 25.8 per cent patients (n=17). Dysphagia and respiratory distress were noted in eight patients (12.1%) each. None of the patients were found to have bladder and bowel involvement. All patients had areflexia and two patients (3%) developed sensory involvement (Table III). All patients underwent nerve conduction velocity testing as the diagnostic testing. The majority (n=33, 50%) were found to be of AIDP followed by AMAN variants (n=29, 43.9%) and 3.0 per cent patients (n=2) were diagnosed as AMSAN (Table III).
The disease occurred sporadically throughout the year; month-wise occurrence was found to be highest in S1 and S2 groups followed by S3 group. The least number of cases were seen in S4 group. The number of GBS cases was highest in summer and spring seasons (60%) spanning from February to July with equal number of cases (n=40, S1 and S2) occurring in the two groups. However, the variants seen in the two seasons differed, S1 having preponderance of AIDP in 21.2 per cent (n=14) patients and S2 having mainly AMAN in 16.7 per cent (n=11) patients. The analysis of various parameters studied did not show any significant association with various seasons of occurrence of GBS (Table IV). Gender, motor impairment, respiratory involvement and duration of hospital stay were not significantly different between all four seasons. Almost equal number of patients of GBS got admitted to the hospital in all months except in the months of October and November. The maximum number of admissions was in July with preceding influenza and diarrhoea (Figs 1 and 2). The mean duration of hospital stay was 14 days (range 2-109). The maximum duration of hospital stay was found to be in S3 group followed by S2 group.

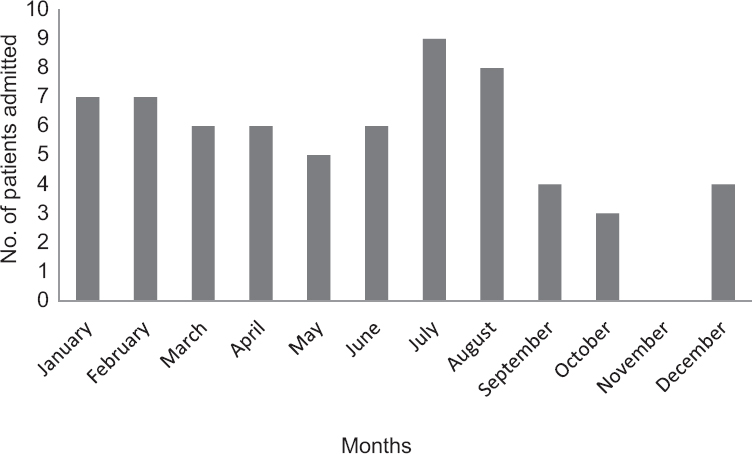
- Month-wise distribution of patients of Guillain–Barre syndrome.
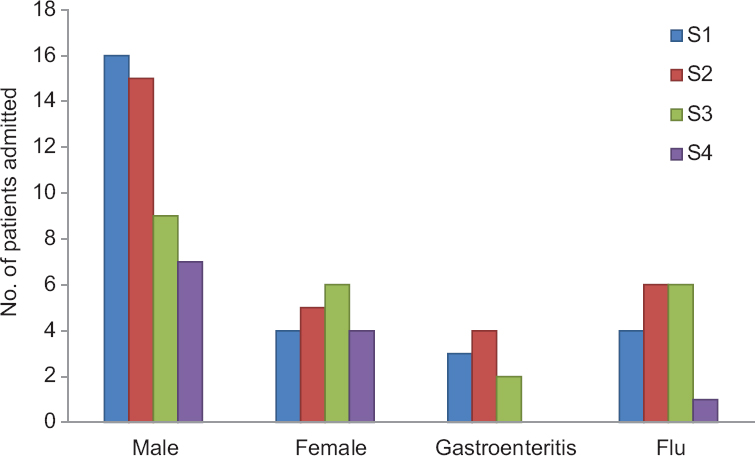
- Seasonal variation in gender and antecedent events. S1, S2, S3, S4 are four seasonal groups.
All patients were given TPE as a choice of the standard treatment protocol. On presentation, there was a diffuse weakness of all four extremities. The lower limbs were more severely involved than the upper limbs and distal extremities were affected more than the proximal extremities. Most of the patients were reported to be active on their F-scores before the onset of GBS. The F-score on admission and discharge were 4 and 3, respectively. About 75.75 per cent patients (n=50) improved at the time of discharge on their F-scores with mild disability. One patient required ventilator support and none of the patients died during the hospital stay.
Discussion
In our study, there was a male preponderance (more than twice that of females) which conformed to the findings of a systematic review which reported that the incidence increased with increase in age, 50 or more years and the distribution of age existed with two peaks17. In our study, progressive muscular weakness of all four limbs was the common presenting clinical feature and flu-like illness followed by gastroenteritis the most common antecedent illness, similar to that reported earlier7. The most common variant of GBS was AIDP followed by AMAN. Other studies from different parts of the world have reported 80-90 per cent frequency from Europe and the USA1819 and Indian studies reporting 48.8 to 85.2 per cent202122. A large study from northern India comprising 328 patients reported AIDP in 73.8 per cent patients and better outcome compared to AMAN6. Contrary to our findings, AMAN has been reported at a frequency of 67 per cent in a study from Bangladesh23.
In this retrospective analysis, two peaks were found with equal number of GBS patients, one in S1 group (February to April) and the other in S2 group (May to July). Sharma et al10 have reported maximum cases in summer (May to July), with majority of patients presenting in the month of May. The seasonal variation may be attributed to the sudden temperature differences in the seasonal conditions making certain months more prone to infections of gastrointestinal and respiratory tract, important antecedent factors of GBS. A study from Southern Iran reported significant seasonal and monthly variation with 50 per cent patients being admitted from February to June and maximum occurrence in spring and winter24. Sriganesh et al16 reported a higher incidence between March and August, similar to our study. Zaheer et al9 reported a bimodal incidence of GBS during April-May (24%) and July-August (32%) as compared to the other months of the year. Kalita et al6 have reported poor prognosis in AMAN variant mostly occurring in summers and complete recovery in AIDP variant which was frequent in rainy season. Our study also demonstrated that in GBS and its variants, respiratory complications (maximum in S4) and duration of hospital stay (maximum in S3) showed a seasonal variation. However, the sample size was small to establish this association clearly and retrospectively limited parameters were studied. About 12.12 per cent patients reported respiratory complications and one patient required mechanical ventilation and scored poor grade on F-score and muscle power grading of 1/5 on MCR scores. Many factors seem to be predictors for respiratory complications and thereby mechanical ventilation including progressively rapid muscular weakness, ineffective cough, bulbar involvement, rapid decrease in vital capacity16. Critical care unit is required for the management of GBS patients with respiratory involvement25. Hughes et al13 reported rehabilitation as important as the immunotherapy and considered it as an integral part of the treatment of the patients with GBS. In our study, proper turning and positioning of patients to prevent bed sores and exercise therapy for maintaining muscle tone and further improvements of power were an important part of multidisciplinary care. None of the patients died during the time of the treatment and only one patient required ventilator support. All patients were advised physiotherapy treatment during the time of discharge. Early detection of the symptoms and early interventions are important factors for better prognosis of GBS26.
The efficacies of various modalities of treatment in GBS have been a matter of discussion and debate. Hughes et al11 reported both TPE and IVIg as equally effective treatment modalities of GBS. Another study reported similar effectiveness of TPE in the treatment of various neurological diseases including GBS27. In this retrospective analysis, TPE was used as the standard treatment of choice in all seasons. All patients were stable and recovered well and improved in their functional grade and MRC scores at the time of discharge. No mortality related to pulmonary embolism was noted.
In this study, the outcome analysis was limited to the period of hospital stay of the patients. A further study can be designed to assess the outcome analysis of the patients on regular follow up after discharge. Further, climatic conditions might vary from region to region within the country and in other parts of the world making the observations differ. Finally, being a tertiary care centre, patients do not come directly and are referred early for TPE mainly before developing serious complications. Thus, only one of our patients was shifted to ventilator and hence the study analyzed the non-ventilated patients in majority. However, it can serve as an adjunct to other studies done on the ventilated patients. A well-designed prospective analysis needs to be planned to study the effect of seasonal variations in the patients with GBS across the various geographic locations along with markers to establish the associations in terms of clinical features, demographics and type of immunotherapy given.
Conflicts of Interest: None.
References
- Adams and victor's principles of neurology (9th ed). New York: McGraw-Hill Medical Publishing Division; 2005. p. :1117-27.
- Guillain-Barré syndrome (acute inflammatory demyelinating polyneuropathy) and related disorders. In: Katirji B, Kaminski HJ, Preston DC, eds. Neuromuscular disorders in clinical practice. Boston Mass: Butterworth-Heinemann; 2002. p. :544-66.
- [Google Scholar]
- Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. 2008;7:939-50.
- [Google Scholar]
- Campylobacter jejuni infection and Guillain-Barré syndrome. N Engl J Med. 1995;333:1374-9.
- [Google Scholar]
- Guillain-Barré syndrome: Subtypes and predictors of outcome from India. J Peripher Nerv Syst. 2014;19:36-43.
- [Google Scholar]
- Guillain-Barré syndrome and influenza virus infection. Clin Infect Dis. 2009;48:48-56.
- [Google Scholar]
- Epidemiology of the Guillain-Barré syndrome in the county of Hordaland, Western Norway. Acta Neurol Scand. 1985;71:43-7.
- [Google Scholar]
- Seasonal variation and sex distribution in patients with Guillain-Barré syndrome. Pak J Neurol Sci. 2008;3:6-8.
- [Google Scholar]
- Seasonal, age & gender variation of Guillain Barre syndrome in a tertiary referral center in India. Neurosci Med. 2013;4:23.
- [Google Scholar]
- Immunotherapy for Guillain-Barré syndrome: A systematic review. Brain. 2007;130(Pt 9):2245-57.
- [Google Scholar]
- Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2012;7:CD001798.
- [Google Scholar]
- Supportive care for patients with Guillain-Barré syndrome. Arch Neurol. 2005;62:1194-8.
- [Google Scholar]
- Medical Research Council. Aids to the investigation of the peripheral nervous system. London: HMSO; 1943.
- Seasonal variation in the clinical recovery of patients with Guillain Barré syndrome requiring mechanical ventilation. Neurol India. 2013;61:349-54.
- [Google Scholar]
- The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009;32:150-63.
- [Google Scholar]
- Gastroenteritis-associated Guillain-Barré syndrome on the Caribbean Island Curaçao. Neurology. 2001;56:1467-72.
- [Google Scholar]
- Patterns of Guillain-Barre syndrome in children: Results from a Mexican population. Neurology. 2007;69:1665-71.
- [Google Scholar]
- Electrodiagnostic and clinical aspects of Guillain-Barré syndrome: an analysis of 142 cases. J Clin Neuromuscul Dis. 2008;10:42-51.
- [Google Scholar]
- Campylobacter jejuni infection in Guillain-Barré syndrome: A prospective case control study in a tertiary care hospital. Neurol India. 2011;59:717-21.
- [Google Scholar]
- Clinical, electrophysiological subtypes and antiganglioside antibodies in childhood Guillain-Barré syndrome. Neurol India. 2011;59:727-32.
- [Google Scholar]
- Axonal variant of Guillain-Barre syndrome associated with Campylobacter infection in Bangladesh. Neurology. 2010;74:581-7.
- [Google Scholar]
- Seasonal variation of Guillain-Barré syndrome admission in a large tertiary referral center in Southern Iran: A 10 year analysis. Acta Neurol Taiwan. 2012;21:60-3.
- [Google Scholar]
- Therapeutic plasma exchange in patients with neurological diseases: Multicenter retrospective analysis. Transfus Apher Sci. 2013;48:349-52.
- [Google Scholar]






