Translate this page into:
Guiding therapy by fractional exhaled nitric oxide & impulse oscillometry parameters in non-asthmatic individuals with chronic cough
For correspondence: Dr Shuguang Xiong, Department of Respiratory Medicine, The Second Affiliated Hospital of Chengdu Medical College, Chenghua District, Chengdu 610 057, Sichuan, China e-mail: bearguang66@163.com
-
Received: ,
Accepted: ,
Abstract
Background & objectives
Chronic airway inflammation and airway hyperresponsiveness are typical pathophysiological features of cough variant asthma. However, the characteristics of airway inflammation and airflow restriction in individuals with non-asthmatic chronic cough and their guiding value of clinical treatment remain to be determined. This study explored the characteristics and correlations between fractional exhaled nitric oxide (FeNO) and impulse oscillometry (IOS) in non-asthmatic individuals with chronic cough. It also investigated the possibility of chronic cough developing into asthma.
Methods
In total, 65 study participants with negative bronchial provocation test (BPT) were included in this retrospective study. Data were extracted from chronic cough patients’ electronic medical records, including the demographics, FeNO, IOS and spirometric parameters before and after BPT. Study participants were divided into high-FeNO group (FeNO≥25 ppb) and low-FeNO group (FeNO< 25 ppb) based on FeNO levels. The correlation between the markers was investigated using the Spearman rank correlation test.
Results
We observed that individuals with non-asthmatic chronic cough exhibited significant increases in Z5, Fres, R5 and R5-R20 after BPT compared to before BPT. In addition, the IOS values of Z5, Fres, R5, and Rc were higher in the low-FeNO group than in the high-FeNO group, but a decrease in FEV1. Correlation analysis: IOS parameters showed a negative correlation with FeNO. However, there were positive correlations of FeNO with FEV1 and PEF.
Interpretation & conclusions
Our findings showed that individuals with non-asthmatic chronic cough may have varying levels of small airway resistance and inflammation severity. A combined use of FeNO and IOS measurements is conducive to the early clinical treatment of individuals with non-asthmatic chronic cough.
Keywords
Asthma
chronic cough
fractional exhaled nitric oxide
impulse oscillometry
therapy
Cough is one of the most common respiratory symptoms with multiple or unclear causes, including cough variant asthma (CVA), upper airway cough syndrome (UACS), eosinophilic bronchitis (EB), gastroesophageal reflux cough (GERC), allergic cough (AC), etc1. Rare causes include cerebellar ataxia with neuropathy and vestibular reflex syndrome (CANVAS)2, Chiari malformation type 1, and especially refractory cough3. The prevalence of chronic cough in China has been estimated to be 7.11 per cent, indicating that over 90 million people suffer from the disease in the country4. Chronic cough is the body’s reflex defense action to clear secretions and foreign bodies from the respiratory tract. Still, severe coughing can lead to hemoptysis, torn diaphragm, arrhythmia, hernia, and spontaneous pneumothorax5. Chronic cough has a negative impact on work, daily life, psychological health and other aspects6-8. A study of children with chronic cough followed up for more than 10 yr showed that 45 per cent of children with chronic cough developed asthma, and an important risk factor for asthma development is hyperresponsive airways in chronic cough patients9. Prospective clinical research showed that the risk of chronic obstructive pulmonary disease (COPD) was nearly threefold higher for subjects with chronic cough than for those who were asymptomatic. The progression of airflow obstruction is a continuous and progressive process, and chronic cough may be a dangerous stage in the airway obstructive diseases such as COPD and asthma, characterised by chronic airway inflammation and airflow restriction10. Airway eosinophilic inflammation and airway hyperresponsiveness are important pathophysiological features of cough variant asthma. However, the characteristics of airway inflammation and airflow restriction in patients with non-asthmatic chronic cough and their guiding value of clinical treatment remain to be determined. We hypothesised that airway inflammation and changes in airway resistance may have occurred during the chronic cough stage, early intervention may prevent or delay the further development of chronic cough into more burdensome and impactful chronic respiratory diseases.
As a biomarker of type 2 airway inflammation, fractional exhaled nitric oxide (FeNO) is widely used to detect and quantify airway inflammation in clinical practice11. When type 2 airway inflammation induced by external stimuli, inducible NO synthase is activated through an NF-kB dependent mechanism, leading to a significant increase in NO12,13. The cutoff values of FeNO (25 ppb) are useful for etiological detection of cough with high sensitivity14. Glucocorticoids can alleviate airway eosinophilic inflammation and reduce FeNO levels by inhibiting the effect of the enzyme inducible nitric oxide synthase. A meta-analysis evaluating the relationship between FeNO and response to inhaled corticosteroids (ICS) in patients with chronic cough suggested that a high FeNO baseline value may help identify patients with chronic cough who respond to ICS therapy, and FeNO ≥ 25 ppb is thought to be relevant for this clinical application15.
Impulse oscillometry (IOS) is a technique recommended by the American Thoracic Society (ATS) and European Respiratory Society (ERS) guidelines for measuring airflow obstruction, which is more sensitive than spirometry in detecting small airway dysfunction (SAD) in objects with chronic respiratory symptoms but preserved pulmonary function16. The oscillation measurement method can detect peripheral airway obstruction before symptoms, which helps implement early treatment to prevent disease progression17. One study found that patients with cough had increased resonant frequency (Fres) values at baseline, suggesting some degree of baseline peripheral airway abnormality. Additionally, objects with cough had an increase in baseline R5–R20, which was comparable to classic asthma and cough variant asthma. To determine whether cough is a unique illness state or a ‘pre-pathological’ phenotype that could eventually deteriorate into severe classic asthma or cough variant asthma, more research is required18,19. Therefore, this study intended to retrospectively analyse the characteristics of airway inflammation and airway resistance in individuals with chronic cough, and further explore the relationship between FeNO and IOS, so as to clarify the possibility of chronic cough developing into asthma and provide feasible clinical strategies for preventing and delaying the development of chronic cough into airway obstructive disease.
Materials & Methods
This retrospective observational study was conducted at department of Respiratory, The Second Affiliated Hospital of Chengdu Medical College, Chengdu, China after obtaining the ethical approval from the Institutional Ethics Committee for the study. Since this study was a retrospective observational study without any intervention measures for the study participants, and the information was anonymised, we applied for the exemption of patients’ informed consent.
Study participants
The data of individuals with chronic cough was collected retrospectively who visited the department of Respiratory, The Second Affiliated Hospital of Chengdu Medical College, Chengdu, China, from November 2021 to October 2022, and 65 study participants were finally included after screening (Figure).
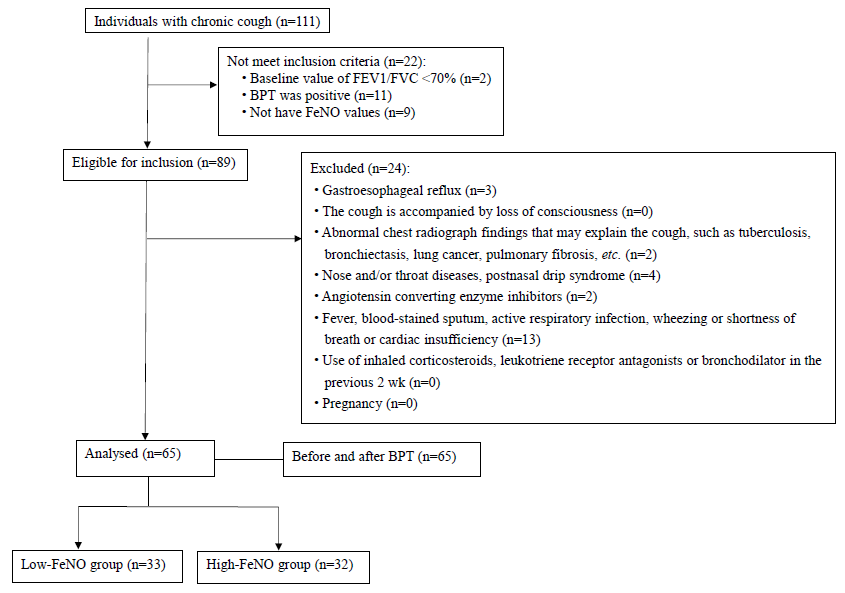
- Enrollment flow chart of the study. BPT, bronchial provocation test; FeNO, fractional exhaled nitric oxide.
Measurements
All study participants underwent measurement of FeNO, IOS, spirometry and the bronchial provocation test (BPT). Specific indicators included FeNO value, forced vital capacity (FVC), forced expiratory volume in the first second (FEV1), FEV1/FVC ratio, peak expiratory flow as a percentage of predicted value (PEF% pred), forced expiratory flow instantaneous 50 per cent (FEF50%), forced expiratory flow instantaneous 75 per cent (FEF75%), maximum mid-expiratory flow rate (MMEF), total respiratory impedance (Z5), airway resistance at 5 Hz, namely total airway resistance (R5), airway resistance at 20 Hz, namely central airway resistance (R20), airway resistance difference at 5 Hz and 20 Hz, namely surrounding airway resistance (R5-R20), and oscillation frequency at 5 Hz Time reactance (X5), resonance frequency (Fres), central airway resistance (Rc), and surrounding airway resistance (Rp). According to the guidelines from Europe20 and the United States21, the diagnostic criteria for chronic cough are defined as a cough lasting more than eight wk. A drop in FEV1 from baseline of less than 20 per cent is considered a negative bronchial provocation test result22. In this research, the subjects were divided into two groups: high-FeNO (FeNO≥25 ppb) and low-FeNO groups (FeNO<25 ppb).
Inclusion/exclusion criteria
The conditions for inclusion were as follows: (i) age ≥18 yr old; (ii) the cough lasts more than eight wk; (iii) the results of the BPT showed that fall in FEV1, from a baseline of <20 per cent; and (iv) baseline value of FEV1/FVC ≥70 per cent. The exclusion criteria were as follows: (i) history of gastroesophageal reflux disease; (ii ) the cough accompanied by loss of consciousness; (iii) abnormal chest radiograph findings that may explain the cough, such as tuberculosis, bronchiectasis, lung cancer, pulmonary fibrosis, etc.; (iv) nose and/or throat diseases, postnasal drip syndrome; (v) long-term use of angiotensin-converting enzyme inhibitors; (vi) blood-stained sputum, fever, active respiratory infection, wheezing or shortness of breath, cardiac insufficiency; (vii) bronchodilators, leukotriene receptor antagonists, or inhaled corticosteroids had been used in the previous two wk; and (viii) pregnancy.
Statistical analysis
Analyses were performed with the SPSS statistical package, Version 25 (IBM Corp., Armonk, NY, USA). The distribution of continuous data was assessed by the Shapiro-Wilk test. The quantitative data with a normal distribution are described as the mean (SD) that the student’s t-test was used for comparison between groups. The non-normally distributed variables are presented as the median (IQR) and the Mann-Whitney U test was used to compare between groups. Data before and after BPT were compared using paired t-test or Wilcoxon signed rank sum test. A 95% CI (confidence interval) was estimated. Qualitative data are expressed as n (%) and were compared with chi-squared test. The relationship between variables was evaluated using Spearman’s rank correlation coefficient; P<0.05 indicated significance.
Results
Study participants’ characteristics
Data from 65 study participants were statistically analysed. The average age was 40 yr, the range being 20 to 64 yr, and males accounted for 43.1 percent. The demographics of the study participants are shown in table I.
| Demographic parameter | All study participants (N=65) |
|---|---|
| Age (yr) | |
| Median (IQR), range | 40 (24), 20-64 |
| 18-40, n (%) | 31 (47.7) |
| 40-60, n (%) | 28 (43.1) |
| >60, n (%) | 6 (9.2) |
| Gender | |
| Male, n (%) | 28 (43.1) |
| Female, n (%) | 37 (56.9) |
| Body mass index, kg/m2 | |
| Mean (SD), range | 23.92 (4.14), 14.69-36.68 |
| <18.5, n (%) | 5 (7.7) |
| 18.5-24.9, n (%) | 38 (58.5) |
| 25-29.9, n (%) | 18 (27.7) |
| >30, n (%) | 4 (6.2) |
N refers to the total population; n refers to the subgroup population. SD, standard deviation; IQR, inter-quartile range
Parameters before and after BPT
The values of Z5, Fres, R5 and R5-R20 showed significant differences before and after the BPT (P<0.05); the remaining parameters did not show a significant difference (P>0.05) (Table II).
| Parameters | Before BPT | After BPT | t/z | Difference & 95% CI | P |
|---|---|---|---|---|---|
| FEV1 (% predicted)† | 95.40 (17.20) | 94.60 (18.25) | -0.399 | 0.25 (-0.90,1.40) | 0.693 |
| PEF (% predicted)# | 97.36 (15.74) | 96.78 (15.81) | 0.530 | 0.57 (-1.59,2.74) | 0.598 |
| Z5 [cmH2O/(1/s)]† | 4.45 (1.70) | 4.69 (2.17) | -3.297 | 0.37 (0.14,0.61) | 0.001* |
| Fres [1/s]# | 14.69 (3.01) | 15.76 (3.18) | -4.247 | -1.06 (1.56, 0.56) | <0.001* |
| R5 [cmH2O/(1/s)]† | 4.35 (1.20) | 4.74 (1.56) | -3.419 | -0.39 (-0.62,0.16) | 0.001* |
| R20 [cmH2O/(1/s)]† | 3.13 (1.02) | 2.86 (0.95) | -1.877 | 0.31 (-0.01,0.63) | 0.061 |
| R5-R20 [cmH2O/(1/s)]† | 1.07 (0.78) | 1.17 (1.11) | -4.120 | 0.23 (0.12,0.37) | <0.001* |
| X5 [cmH2O/(1/s)]† | -1.46 (0.41) | -1.26 (0.51) | -1.828 | -0.21 (-0.46,0.02) | 0.072 |
| Rc [cmH2O/(1/s)]† | 2.56 (0.63) | 2.65 (0.70) | -0.762 | 0.04 (-0.08,0.17) | 0.449 |
| Rp [cmH2O/(1/s)]† | 3.06 (1.53) | 3.06 (1.79) | -1.337 | 0.00 (0.00,0.26) | 0.184 |
P*<0.05. †Median (IQR). #Mean (SD); t, t test value. z, the Mann–Whitney U test value. IOS, impulse oscillometry; CI, confidence interval; BPT, bronchial provocation test; FEV1, forced expiratory volume in 1s; PEF, peak expiratory flow; Z5, impedance at 5 Hz; Fres, resonant frequency; R5, resistance at 5 Hz; R20, resistance at 20 Hz; R5-R20, the difference between R5 and R20; X5, reactance at 5 Hz; Rc, central resistance, Rp, peripheral resistance
Comparison of the discrepancies in IOS and spirometric parameters between the two groups
The study participants were divided into two groups: 33 (50.8%) and 32 (49.2%) were identified with FeNO< 25 ppb and FeNO ≥ 25 ppb, respectively. Demographics about the two groups of study participants regarding gender distribution, age and BMI (body mass index) were not statistically significant (P > 0.05) and thus were comparable. The values of FEV1 (P=0.024), Z5 (P=0.037), Fres (P=0.010), R5 (P=0.043) and Rc (P=0.035) showed significant differences between FeNO< 25 ppb group and FeNO ≥ 25 ppb group, but there was no difference in other parameters. The results are shown in table III.
| Parameters | FeNO< 25 (ppb) | FeNO ≥ 25 (ppb) | t/z/χ2 | Difference & 95% CI | P |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 12 (36.4%) | 16 (50%) | 1.232 | ____ | 0.321 |
| Female | 21 (63.6%) | 16 (50%) | |||
| Total | 33 (100%) | 32 (100%) | |||
| Age(yr)# | 41.61 (12.31) | 39.63 (13.19) | 0.626 | 1.98 (-4.34,8.30) | 0.533 |
| BMI (kg/m2)† | 23.42 (4.07) | 24.43 (4.22) | -0.989 | -1.02 (-3.07,1.04) | 0.327 |
| FEV1 (L)† | 2.52 (0.83) | 2.80 (1.18) | -2.244 | -0.39 (-0.76, -0.05) | 0.024* |
| FEV1 (% predicted)† | 91.70 (19.25) | 98.75 (16.33) | -1.489 | -5.00 (-11.10,1.00) | 0.138 |
| FEV1/FVC# | 83.88 (7.43) | 83.48 (5.94) | 0.236 | 0.40 (-2.95,3.74) | 0.814 |
| PEF (L/s)# | 6.69 (2.07) | 7.56 (1.74) | -1.840 | -0.87 (-1.82,0.08) | 0.070 |
| PEF (% predicted)# | 94.36 (16.87) | 100.45 (14.09) | -1.578 | -6.09 (-13.81,1.62) | 0.120 |
| FEF50 (% predicted)† | 75.60 (32.65) | 77.80 (26.75) | -0.925 | -5.40 (-15.60, 6.60) | 0.359 |
| FEF75 (% predicted)† | 54.30 (31.55) | 69.20 (25.90) | -1.483 | -8.45 (-19.10, 2.70) | 0.140 |
| MMEF (% predicted)† | 65.30 (32.40) | 73.5 (18.35) | -1.378 | -7.15 (-15.90,3,50) | 0.170 |
| Z5 [cmH2O/(1/s)]† | 4.71 (1.98) | 3.83 (1.367) | 2.132 | 0.14 (0.01,0.27) | 0.037* |
| Fres (1/s)# | 15.63 (2.69) | 13.73 (3.05) | 2.666 | 1.90 (0.48,3.33) | 0.010* |
| R5 [cmH2O/(1/s)]† | 4.48 (1.96) | 3.63 (1.33) | 2.069 | 0.13 (0.01,0.26) | 0.043* |
| R20 [cmH2O/(1/s)]† | 3.13 (1.02) | 2.82 (0.95) | 1.808 | 0.10 (-0.01,0.22) | 0.075 |
| R5-R20 [cmH2O/(1/s)† | 1.21 (0.56) | 0.88 (0.88) | -1.831 | 0.25 (-0.02,0.51) | 0.067 |
| X5 [cmH2O/(1/s)]# | -1.46 (0.41) | -1.26 (0.51) | -1.828 | -0.21 (-0.44,0.02) | 0.072 |
| Rc [cmH2O/(1/s)]† | 2.72 (0.82) | 2.52 (0.46) | -2.106 | 0.22 (0.01,0.58) | 0.035* |
| Rp [cmH2O/(1/s)]† | 3.57 (1.53) | 3.06 (1.40) | 0.787 | 0.06 (-0.09,0.21) | 0.434 |
P*<0.05. †Median (IQR); #Mean (SD); t, t test value; z, the Mann–Whitney U test value; χ2, Chi-square value. BMI, body mass index; FVC, forced vital capacity; FEF50, forced expiratory flow at 50% of the FVC; FEF75, forced expiratory flow at 75% of the FVC; MMEF, forced expiratory flow between 25 and 75%
Correlation analysis between spirometric data, IOS parameters and FeNO
By Spearman rank correlation analysis, FeNO was actively correlated with FEV1 (P<0.05). FeNO values were negatively correlated with Z5, Fres, R5, R20, Rc and R5-R20 (P<0.05), and FeNO levels were weakly positively correlated with X5 (P<0.05). FeNO had no correlation with FEV1%pred, FEV1/FVC, PEF%pred, PEF50%, PEF75%, MMEF% and Rp (P>0.05) (Table IV).
| Index | r | P |
|---|---|---|
| FEV1 (L) | 0.295 | 0.017 |
| PEF (L/s) | 0.297 | 0.016 |
| Z5 [cmH2O/(1/s)] | -0.318 | 0.010 |
| Fres (1/s) | -0.325 | 0.008 |
| R5 [cmH2O/(1/s)] | -0.310 | 0.012 |
| R20 [cmH2O/(1/s)] | -0.262 | 0.035 |
| R5-R20 [cmH2O/(1/s)] | -0.268 | 0.031 |
| Rc [cmH2O/(1/s)] | -0.318 | 0.010 |
| X5 [cmH2O/(1/s)] | 0.265 | 0.033 |
r, correlation coefficient
Discussion
This study compared spirometric and IOS parameters before and after BPT in individuals with chronic cough. The finding was that the Z5, R5, R5-R20 and Fres for chronic cough individuals with negative BPT were significantly higher after BPT than before BPT. During the acute phase of asthma, airway resistance increases, especially in small airways, showing an increase in R5, R20, R5-R20, Fres, and a decline of X523. This study provides further evidence that there are significant small airway obstructions in chronic cough, reflecting early changes in obstructive airway function and the tendency of chronic cough to develop into cough variant asthma.
In the study, half of the study participants had low levels of FeNO. Compared to the high-FeNO group, the low-FeNO group had lower FEV1 and higher Z5, Fres, R5 and Rc, with statistical differences. A previous study proposed that FeNO, in addition to being a biomarker of type 2 airway inflammation, may also serve as a marker of change in the diameter of the peripheral airways by establishing a new model of NO production and transport in airway epithelium to truly simulate changes in airway calibre, which theoretically supports that the specific changes in FeNO are related to changes in airway calibre at specific locations in the lung in the absence of inflammation, and the deeper the airway contraction, the greater the reduction in FeNO13. In the past, we generally believed that a high FeNO level is meaningful to guide the direction of clinical treatment, but perhaps a low FeNO level is also clinically significant.
Elevated FeNO is associated with eosinophil infiltration and upregulation of expression in type 2 inflammatory cytokines such as IL-4, IL-5, and IL-1324. FeNO and cough symptoms of CVA patients were significantly improved after glucocorticoid treatment. A prospective study by Zhang et al25 reported, in cough variant asthma (CVA) or eosinophilic bronchitis (EB). In patients treated with inhaled corticosteroids, baseline FeNO values were noticeably higher in patients with cough relief than in those without cough relief. Hahn et al26 reported FeNO cutoff values greater than or equal to 38ppb were found to identify patients with chronic cough who responded to ICS. FeNO≧33.9 ppb is considered suitable for guiding ICS treatment in patients with chronic cough as reported by Hsu et al27. Lamon et al28 found that the proportion of patients who responded to ICS was significantly greater in the high FeNO group compared to the normal FeNO level group (FeNO<25ppb). Although the optimal thresholds for FeNO to guide the application of ICS in patients with chronic cough vary across countries, regions, and centres, the conclusion is that FeNO is a good tool for predicting the efficacy of ICS in patients with chronic cough. The research demonstrates that FeNO is negatively correlated with IOS. We speculate that individuals with non-type 2 inflammatory chronic cough with low FeNO have higher airway resistance than indiviudals with type 2 inflammatory chronic cough; long-acting inhaled β-agonists and anticholinergics may be used to improve the symptoms of these individuals. It is even beneficial to long-term prognosis, prevent disease progression, and reduce the occurrence of asthma. Our research has some shortcomings. As this study was a retrospective one, we were unable to get follow up data, and we could not present the progression of chronic cough developing into asthma. More research using a prospective design is needed to evaluate changes of airway inflammation and airway resistance in chronic cough.
Overall, chronic cough has some degree of airway reactivity, and FeNO is negatively correlated with IOS parameters. We propose a hypothesis for the treatment of chronic cough that individuals with low FeNO are unlikely to benefit from corticosteroid therapy, but the use of long-acting inhaled β-agonists and anticholinergics may be effective. Chronic cough with FeNO greater than 25 ppb may be treated with inhaled corticosteroids, but this conclusion requires support further prospective studies.
Financial support & sponsorship
None
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:1S-23S.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Chronic cough as a genetic neurological disorder? Insights from cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS) Lung. 2023;201:511-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Management of chronic refractory cough in adults. Eur J Intern Med. 2020;81:15-21.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence of chronic cough in China: A systematic review and meta-analysis. BMC Pulm Med. 2022;22:62.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Complications of chronic cough. Zhonghua Jie He He Hu Xi Za Zhi. 2022;45:10-12.
- [CrossRef] [PubMed] [Google Scholar]
- Disease burden and quality of life of patients with chronic cough in Japan: A population-based cross-sectional survey. BMJ Open Respir Res. 2021;8:e000764.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prevalence, progression and impact of chronic cough on employment in Northern Europe. Eur Respir J. 2021;57:2003344.
- [CrossRef] [PubMed] [Google Scholar]
- Chronic cough from the patient’s perspective. Mayo Clin Proc. 2007;82:56-60.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between bronchial hyperresponsiveness and development of asthma in children with chronic cough. Pediatr Pulmonol. 2001;31:412-8.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med. 2007;175:32-9.
- [CrossRef] [PubMed] [Google Scholar]
- Summary for Clinicians: Clinical Practice Guideline for the Use of Fractional Exhaled Nitric Oxide to Guide the Treatment of Asthma. Ann Am Thorac Soc. 2022;19:1627-30.
- [CrossRef] [PubMed] [Google Scholar]
- Contribution of exhaled nitric oxide measurement in airway inflammation assessment in asthma. A position paper from the French speaking respiratory society. Rev Mal Respir. 2015;32:193-215.
- [CrossRef] [PubMed] [Google Scholar]
- A new role for the exhaled nitric oxide as a functional marker of peripheral airway caliber changes: A theoretical study. J Appl Physiol (1985). 2018;124:1025-33.
- [CrossRef] [PubMed] [Google Scholar]
- Association between fractional exhaled nitric oxide (FeNO) cutoff values (25 ppb) and risk factors of cough. Clin Respir J. 2018;12:193-9.
- [CrossRef] [PubMed] [Google Scholar]
- Performance of fractional exhaled nitric oxide in predicting response to inhaled corticosteroids in chronic cough: A meta-analysis. Ann Med. 2021;53:1659-72.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Impulse oscillometry for detection of small airway dysfunction in subjects with chronic respiratory symptoms and preserved pulmonary function. Respir Res. 2021;22:68.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Impulse oscillometry as a predictor of asthma exacerbations in young children. Respiration. 2016;91:107-14.
- [CrossRef] [PubMed] [Google Scholar]
- Small-airway obstruction, dynamic hyperinflation, and gas trapping despite normal airway sensitivity to methacholine in adults with chronic cough. J Appl Physiol (1985). 2019;126:294-304.
- [CrossRef] [PubMed] [Google Scholar]
- Methacholine-induced cough in the absence of asthma: insights from impulse oscillometry. Front Physiol. 2020;11:554-679.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J. 2020;55:1901136.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Classification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest. 2018;153:196-209.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309-29.
- [CrossRef] [PubMed] [Google Scholar]
- Impulse oscillometry in the diagnosis of cough variant asthma in children. BMC Pediatr. 2024;24:296.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Understanding the cellular sources of the fractional exhaled nitric oxide (FeNO) and its role as a biomarker of Type 2 inflammation in asthma. Biomed Res Int. 2022;2022:5753524.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The values of fractional exhaled nitric oxide in the diagnosis and treatment of chronic cough. Zhonghua Jie He He Hu Xi Za Zhi. 2011;34:504-8.
- [PubMed] [Google Scholar]
- Use of exhaled nitric oxide in predicting response to inhaled corticosteroids for chronic cough. Mayo Clin Proc. 2007;82:1350-5.
- [CrossRef] [PubMed] [Google Scholar]
- Optimal value of fractional exhaled nitric oxide in inhaled corticosteroid treatment for patients with chronic cough of unknown cause. J Chin Med Assoc. 2013;76:15-9.
- [CrossRef] [PubMed] [Google Scholar]
- Exhaled nitric oxide in chronic cough: A good tool in a multi-step approach. Respir Med Res. 2019;76:4-9.
- [CrossRef] [PubMed] [Google Scholar]






