Translate this page into:
Global emergence of West Nile virus: Threat & preparedness in special perspective to India
For correspondence: Dr Siraj Ahmed Khan, ICMR-Regional Medical Research Centre, Northeast Region, Post Box No. 105, Dibrugarh 786 001, Assam, India e-mail: sirajkhanicmr@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
West Nile virus (WNV) is a mosquito-borne single-stranded RNA neurotropic virus within the family Flaviviridae. The virus was first reported in the West Nile province of Uganda in 1937. Since then, sporadic cases have been reported until the last two decades when it has emerged as a threat to public health. The emergence of WNV with more severity in recent times is intriguing. Considering this phenomenon, the WNV-affected areas of the world were distinguished as old versus new in a depicted world map. The present review showcases the historical and epidemiological perspectives of the virus, genetic diversity of prevailing lineages and clinical spectrum associated with its infection. Emergence of the virus has been discussed in special context to India because of co-circulation of different WNV lineages/strains along with other flaviviruses. Recent laboratory diagnostics, vaccine development and clinical management associated with WNV infection have also been discussed. Further, the research gaps, especially in context to India have been highlighted that may have a pivotal role in combating the spread of WNV.
Keywords
Clinical management
epidemiology
laboratory diagnostic
public health
vaccines
West Nile virus
West Nile virus (WNV) is a neurotropic pathogen maintained through a mosquito–bird–mosquito (enzootic) transmission cycle. It primarily involves mosquitoes belonging to Culex (Cx.) species (spp.) as vectors and birds as natural reservoirs (amplifying) hosts1. Sometimes, due to spill-over from the enzootic cycle; horses and humans get infected. Neither horses nor humans develop sufficient viraemia to transmit the virus, thus acting as ‘dead-end’ hosts2. Originally, WNV was isolated from a febrile patient in the West Nile province of Uganda in 19373. In the last two decades, the virus has emerged as a significant burden to public health worldwide, raising its threat as an emerging global pathogen4.
WNV is a member of the family Flaviviridae and is encoded by an ~11 kb positive-sense single-stranded RNA genome. The genome is first translated into a single polyprotein and then cleaved by viral and host proteases to generate three structural [capsid (C), pre-membrane (prM) and envelope (E)] and seven non-structural (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) proteins5. The structural proteins encompass the viral RNA and form the nucleocapsid region. The NS proteins play an important role in the replication cycle of viral RNA. Virion passes through the host secretory pathway for intracellular trafficking into the cytoplasm as mature virions that are subsequently released from an infected cell by exocytosis6. WNV pathogenesis in humans is poorly defined. Studies on animal models have provided insight on WNV pathogenesis that could be somewhat similar to human infection. WNV pathogenesis can be defined in three distinct phases - initial infection and spread (the early phase); viral amplification phase (visceral-organ dissemination phase) and possibly the neuroinvasion phase involving the central nervous system. However, development and progression of these stages depend on host immune status and viral strains7.
WNV epizootic/epidemic activity in India is of special interest because of co-circulation of different WNV lineages/strains. The presence of the virus in an environment which is endemic to closely related Japanese encephalitis virus (JEV) and other flavivirus including dengue and Kyasanur forest disease viruses further warrants detailed investigation from public health aspect. This review presents the epidemiology, clinical presentations and diagnostic challenges of WNV with special perspective to India. The status of vaccine development, therapeutic intervention and control measures are also highlighted. Knowledge gaps are also discussed that may stimulate areas for WNV research in India and to other regions worldwide where the virus is emerging.
Historical and epidemiological perspective
The first recognized epidemic of WNV was reported from Haifa, Israel, during 1951, where a total of 123 cases were recorded, with symptoms presenting with febrile illness, exanthema, lymphadenopathy and angina8. Concurrently, WNV was isolated from febrile children and Cx. mosquitoes in the Nile delta of Egypt9. Subsequently, in 1953, virus was isolated from some avian species, hooded crows and rock pigeons10. During the 1957 WNV outbreak in Israel, neurological manifestations in 33 per cent of patients and four per cent mortality within a group of elderly nursing home residents became a matter of concern11. Subsequent outbreaks occurred in France and South Africa during 1962 and 1974, respectively, where patients developing encephalitis were recognized1213. During the ensuing two decades, major epidemics of WNV were not reported, until 1996, when a cluster of cases with CNS diseases were diagnosed to be WNV infected near Bucharest, Romania14. The Romanian outbreak was considered to be of significant public health importance because of the first large-scale epidemic of WNV with preponderance of CNS infection in a predominantly urban area15. The epidemic reported 393 hospitalized cases with 17 deaths15. Subsequently, several epidemics with relatively high rates of CNS infection were observed in Morocco (1996), Tunisia (1997), Volga delta region of Russia (1999), America (1999) and Israel (2000)16171819.
The first outbreak of WNV was reported in 1999 in America. A total of 51,607 WNV cases have been recorded till October, 2019. Almost half (25,161) of cases had CNS involvement. Mortality was recorded in almost five per cent (2369) of cases20. In Europe, during 2018 alone, 2083 WN cases were recorded. This number exceeded the total number of cases recorded during the last seven years21. This emergence of WNV with more number of cases from different areas led to its recognition as an emerging global pathogen. Due to the significance of the Romanian outbreak in recognizing the extent and importance of WNV infection, we propose to distinguish the WNV spread as old versus new world based on the outbreak year of 1996 as a separating point (Figs 1A and B). The virus continued to spread to new areas with cases reported from Germany, Austria, Italy, Greece, China and Australia222324. Among South American countries, the first human case occurred in Colombia during 2005, followed by first recorded human WNV encephalitis case from Argentina during 200625. During 2015-2016 across the Indian sub-continent, WNV neuroinvasive disease cases were reported from the southern province of Sindh in Pakistan26. Though serological evidence of WNV infection was reported from rural Punjab, Pakistan, during the 1970s27, the comparatively late establishment of WNV neuroinvasive cases could be attributed to protective antibodies as virus was endemic and most people were infected during childhood. Another Indian sub-continent country, Sri Lanka reported the first evidence of WNV infection in 2013 during an encephalitis outbreak28.
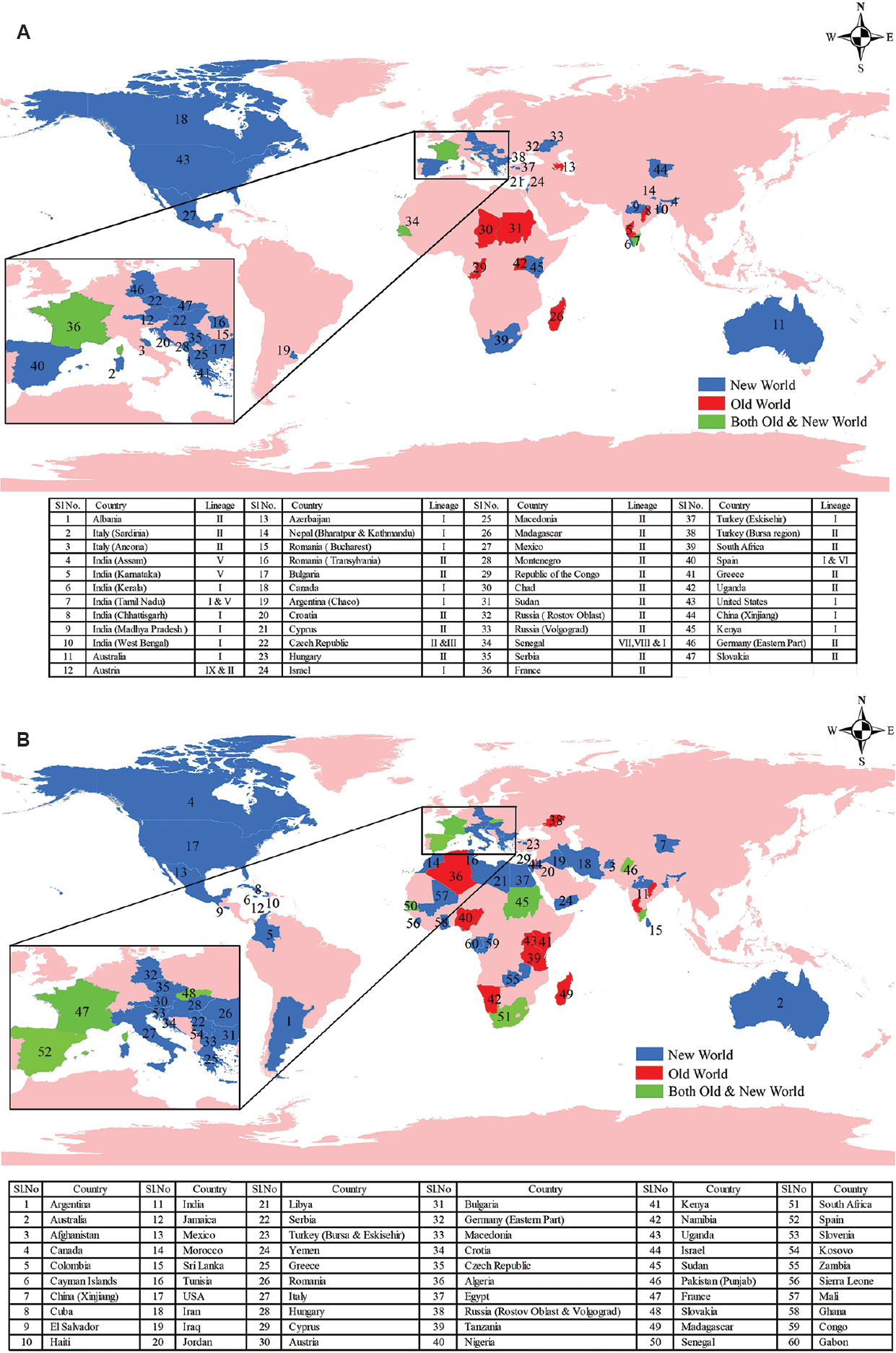
- Schematic world maps: (A) showing distribution of West Nile virus lineages, (B) showing areas with West Nile virus human serological evidence. Data searches were undertaken using online references of the literature site, PubMed (http://www.ncbi.nlm.nih.gov/sites/entrez), supplemented with additional data archives of Centre for Disease Control, (https://www.cdc.gov/westnile/statsmaps/index.html); World Health Organization (http://www.who.int/news-room/fact-sheets/detail/west-nile-virus); European Centre for Disease Prevention and Control (https://ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data/disease-data-ecdc). The maps were prepared using GIS software, ArcGIS 10.2 (Redlands, CA, USA). The source of outline map is a web portal (https://www.diva-gis.org/).
In India, antibodies against WNV in humans were first detected from Bombay (now Mumbai) during 195229. Subsequently, serologically confirmed WNV cases were reported from Vellore and Kolar districts of Karnataka during encephalitis epidemics in 1977, 1979 and 198130. In western countries, WNV infections were found to be higher among elderly patients31. However, in India, children succumbing to WNV infection were frequently observed. WNV was isolated from brain tissue of three children who died of encephalitis in the southern region of India. One isolate was obtained from encephalitis presenting case in Mysuru district during 1980 and another during encephalitis epidemic in Kolar district in 198130. Details for the third case are not available. WNV-infected fatal paediatric cases were reported in 2006 from the State of Assam situated in North-east India32. During the late 2009 and the early 2010, WNV cases were reported from patients presenting with ocular complications in Tamil Nadu33. Acute flaccid paralysis (AFP) due to WNV infection has also been reported from Kerala during 201434. This was an unusual phenomenon because poliovirus has been the common cause of AFP in India. Characterization of WNV PCR-positive samples revealed circulation of lineage I WNV during 2011 Kerala outbreak35. In the eastern State of West Bengal, WNV was reported in 201736. In the central State of Madhya Pradesh, during 2015, infection of lineage I WNV was found in paediatric population presented with acute encephalitis syndrome (AES)37. These scenarios of WNV infection delineate an emerging threat to public health in India (Fig. 2). This advocates for inclusion of WNV screening as routine diagnosis for AES cases in the country. The differences observed in the clinico-epidemiological scenario of WNV may be due to strain variations, which necessitate the identification of the circulating strains in different endemic settings for understanding WNV pathobiology.
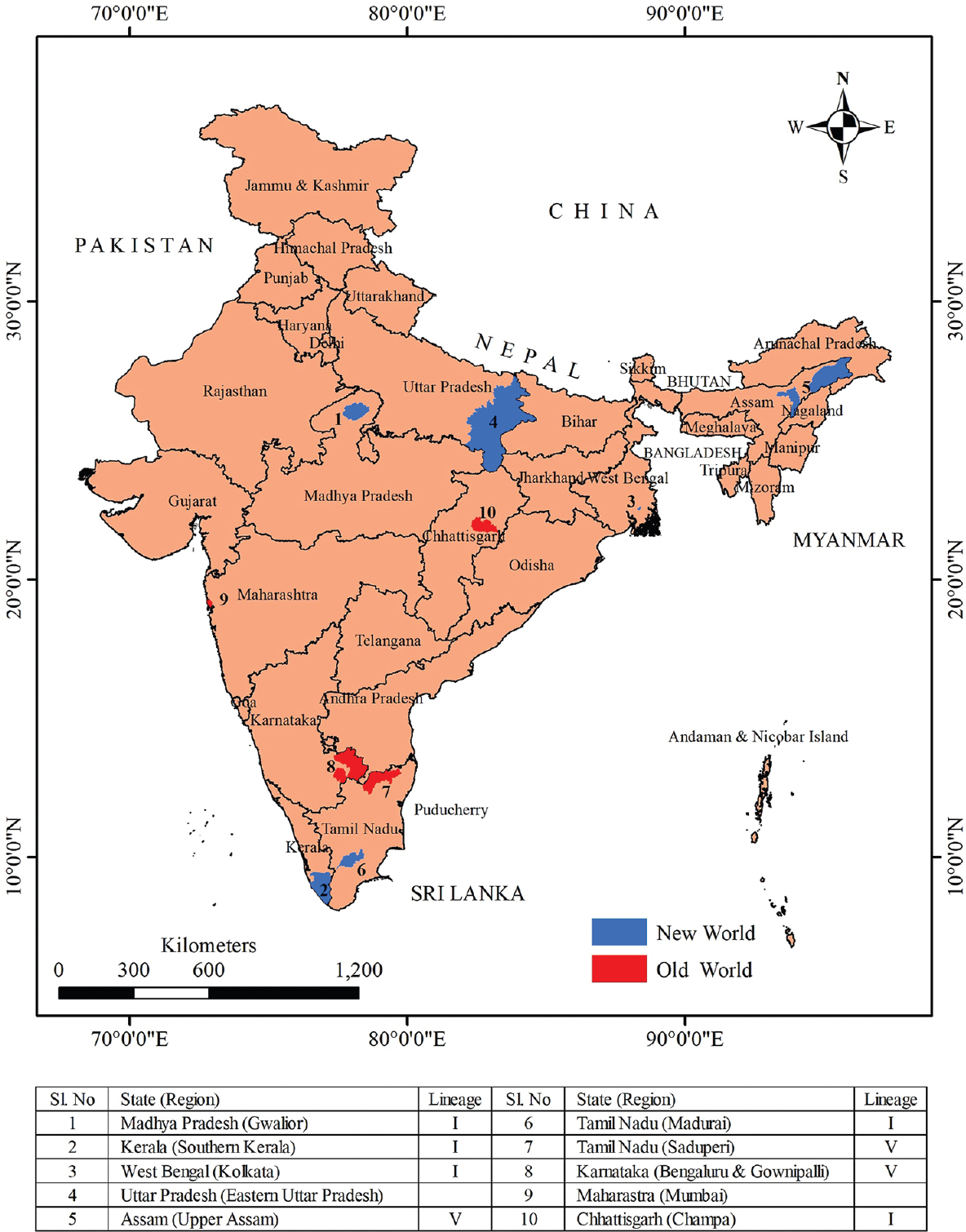
- Schematic map of India showing distribution of West Nile virus lineages and West Nile virus human serological evidence. Data searches were undertaken using online references of the literature site, PubMed (http://www.ncbi.nlm.nih.gov/sites/entrez). The source of outline map is a web portal (https://www.diva-gis.org/).
Ecology, transmission cycle and host range
In nature, WNV is maintained or circulates through sylvatic transmission cycle, also called as enzootic cycle. The virus is maintained between birds and ornithophilic mosquitoes. The intensity of infection in humans or other mammalian species depends on multiple factors such as ecology of the area, population density, feeding pattern of mosquitoes, temporal and spatial presence of amplifying host (birds), immunological status and activities of people facilitating interaction with vector mosquitoes38. These features make WNV outbreaks sporadic in nature and highly variable among different areas. Environmental factors, precipitation, temperature and landscape use/management parameters have direct influence on the pathogen by modulating extrinsic incubation period and on vector population by affecting anthropogenic behaviours39.
Heavy rainfall and warm and dry temperatures during summer are optimal for breeding of Cx. spp. population and subsequent risk for human infection between mid and late summer40. There are other groups of mosquitoes, e.g. Aedes (Ae) albopictus and Ae. vexans, which may also serve as bridge vectors41. In Africa and the Middle East, WNV has been most frequently isolated from Cx. univitattus134243. During the North American outbreak, members of the Cx. pipiens complex were considered the primary epizootic vectors. Cx. pipiens is a primary vector in northeastern America and Cx. quinquefasciatus is important in southeastern America. In Central and Western America, Cx. tarsalis is the principal vector, while in southern regions of America, Cx. quinquefasciatus is the most important vector. In Europe, Cx. pipiens is considered as primary vector followed by Cx. modestus. In Australia, Cx. annulirostrisis is considered as the primary vector4144. Vector competence studies also played an important part in establishing a species transmission capability41. In a study on Cx. pipiens experimentally infected with WNV genotype, WN02 and NY99, temperature showed a direct relationship in influencing transmission by increased viral replication. WN02 was found to be better adapted to rising temperature than NY9945. Another study with NY99 showed that at a mean temperature of 14°C, Cx. tarsalis mosquitoes got infected and transmitted WNV infection46. In the same study, it was also found that at 10°C, mosquitoes failed to get infected despite feeding on viraemic donor birds46.
Studies indicated birds of the order Passeriformes, Charadriiformes, and Falconiformes to be major amplifying hosts38. In avians, WNV infection usually persists for a week, but in some birds, mostly wild, the presence of virus was recorded for several weeks. They vary in susceptibility with 0-100 per cent mortality recorded. In Europe, Goshawks (Accipiter gentilis) was reported with WNV infection47. In Africa, there was no such description of fatal incidence of birds due to WNV infection, but the virus was isolated from egrets (genus Egretta) and indigenous parrots (Coracopsis vasa) in Madagascar48. Equine WNV encephalitis cases have increased significantly in the new world49. In Europe during 2019 alone, 77 WNV outbreaks in equines were reported21. In South Africa, 2-13 per cent of neurological cases in horses per annum were due to WNV with around 30 per cent mortality rate48. In Australia, a large number of horses suffered from WNV encephalitis and a native Australian WNV virulent strain (WNVNSW2011) was isolated from them50. Other mammalian species such as bats carry WNV, but their role in transmission or maintenance of the virus is not established51. WNV antibodies were found in some tree squirrel species [e.g., fox squirrel and eastern grey squirrel (Sciurus carolinensis)]; they were also found to develop viremia sufficient to infect mosquitoes51. Experimental infection of young alligators (Alligator mississippiensis) and lake frog (Rana ridibunda) produced viremia level sufficient to contribute in transmission51. In addition to natural cycle, reports of WNV transmission through blood transfusion have been published. The first such report came into picture during 2002 in the United States (US)52. Infection rates of 14.9 and 4.4 cases/100,000 blood donations were reported in 2003 and 2004 WNV epidemic53 in the US. During 2012 WNV epidemic in the US, an estimated 12.9 infections/100,000 blood donations were recorded54. Other risk factors include organ donation, infection during pregnancy and breast feeding5556.
In India, WNV has been isolated from Cx. vishnui, Cx. quinquefasciatus and Cx. tritaeniorhynchus30. During 2014-2015, six species of mosquitoes, viz., Mansonia (Ma.) uniformis, Cx. vishnui, Cx. tritaeniorhynchus, Cx. quinquefasciatus, Cx. pseudovishnui and Cx. whitmorei, were incriminated with WNV lineage V virus57. Culex genera have been well established for their role in WNV transmission. Studies in India demonstrated efficient horizontal WNV transmission by Cx. quinquefasciatus, but vertical transmission could not be established58. Cx. quinquefasciatus and Cx. pipiens collected from California were established for vertical transmission of WNV59. Earlier, our group has studied WNV seroconversion using sentinel chicken and found a peak seroconversion corroborated with abundance of incriminated WNV vectors in the study region57. Sentinel chicken could be used to monitor WNV activity in India. In America, sentinel chicken has been used to provide evidence of WNV activity and to assess risk to surrounding animal and human population60. WNV antibodies have been reported in wild migratory water-frequenting birds, the Purple heron and Ruff, the major winter visitors to India61. These birds mostly migrate from sub-Saharan and Serbian countries, where lineage I and II are predominant causing human infection; therefore, they may be an unlikely source of WNV emergence in the north eastern region (NER) of India where lineage V circulates. However, their possible role in southern India cannot be ruled out, where a lineage I strain of WNV is circulating. WNV and JEV antibodies were detected in native domestic ducks (Anas platyrhynchos var. domesticus) in Kuttanad region of Kerala, southern India62. WNV antibodies were also detected in equines from five States of northern India during 201063. There were no reports of mortality or neurological involvement of WNV in equine from India. Perhaps, equine studies need more attention and can potentially be undertaken under one health surveillance network.
Genetic diversity
WNV strains are classified into eight putative genetic lineages (Fig. 3) although prior studies indicated only two prime lineages (I and II). However, with isolation of new variants of WNV and genome studies, additional lineages have been accepted64. Lineage I is distributed throughout much of the world (Fig. 1A) and has been further sub-divided into several clades2465. Lineage II was detected mainly in sub-Saharan Africa, until outbreaks were seen during 2004 in Western and Eastern Europe and endemic cycles were established in Spain and Greece666768. Lineage III, also known as Rabensburg virus was isolated from the Czech Republic in 1997 and 1999 from Cx. pipiens mosquitoes and in 2006 from a pool of Ae. rossicus6869. Lineage IV was found in numerous isolates from Russia, first detected in 1988 from a Dermacentor tick and subsequently isolated from mosquitoes and frogs in 2002 and 200566. Lineage V comprised WNV isolates from India that previously were grouped within lineage 1c and were isolated from humans and Cx. spp.7071. Another lineage reclassified as lineage VI was proposed for the Sarawak Kunjin virus strain, which was distinguished from Kunjin viruses endemic to Australia. Furthermore, lineage VII was proposed for the African Koutango virus which was closely related to WNV7273. Lineage VIII was proposed for WNV isolated from Cx. pipiens mosquitoes in Southern Spain64. In 2014, WNV-Uu-LN-AT-2013 strain, detected in Uranotaenia unguiculata mosquitoes, was proposed to be lineage IX or was grouped into lineage 4 as sub-lineage 4c74. Among all the lineages detected or proposed, only three, I, II and V are known to cause human infection.
![Molecular phylogenetic analysis of West Nile virus lineages by Maximum Likelihood method (timetree): The tree was generated using the RelTime method. Relative divergence times for all branching points in the topology were calculated based on the General Time Reversible model. A discrete Gamma distribution was used to model evolutionary rate differences among sites [5 categories (+G, parameter=1.8515)]. The rate variation model allowed for some sites to be evolutionarily invariable [(+I), 7.0510% sites]. The tree is drawn to scale, with branch lengths measured in the relative number of substitutions per site. Evolutionary analyses were conducted in MEGA6 (Pennsylvania State University, PA, USA) with JEV strain GP-78 as an outgroup.](/content/175/2021/154/1/img/IJMR-154-36-g003.png)
- Molecular phylogenetic analysis of West Nile virus lineages by Maximum Likelihood method (timetree): The tree was generated using the RelTime method. Relative divergence times for all branching points in the topology were calculated based on the General Time Reversible model. A discrete Gamma distribution was used to model evolutionary rate differences among sites [5 categories (+G, parameter=1.8515)]. The rate variation model allowed for some sites to be evolutionarily invariable [(+I), 7.0510% sites]. The tree is drawn to scale, with branch lengths measured in the relative number of substitutions per site. Evolutionary analyses were conducted in MEGA6 (Pennsylvania State University, PA, USA) with JEV strain GP-78 as an outgroup.
The distinct nature of the Indian WNV isolates was first observed with the complete genome sequence of Indian strains, G22886 (GenBank accession no. AY944241; isolated from mosquito; Cx. vishnui in 1955 from Saduperi, Karnataka, a South Indian State) compared to WNV lineage I and II strains70. Subsequently, a systematic study based on partial and complete genome sequence analysis of WNV isolates in India over a period of 27 yr (1955-1982) suggested re-classification of WNV isolated worldwide into five lineages, with the Indian virus strains forming a distinct lineage V70. Partial sequence analysis of a 921 nucleotide fragment of 13 Indian WNV isolates spanning the C–prM–E region of WNV genome showed 21.0-26.5 per cent nucleotide difference (ND) with other lineages70. When compared with the whole-genome sequence of WNV strain 804994, the WNV isolates were distinctly classified into five genetic lineages, similar to the analysis based on partial genomic sequence analysis with 21.0-24.76 per cent ND70. Two Indian strains, WNI672698H (GenBank Accession No. AY944238; isolated from human serum in 1967 from Kasoudi, South India) and WNI68856B (GenBank Accession No. AY944239; isolated from bat Rousettus leschenaultii in 1968 from Horabail, South India) were grouped within the lineage I viruses, indicating co-circulation of lineage I and V strains during the late 1960s70. This co-circulation continued till the present decade; lineage V circulates in North-East India and lineage I circulates in South India337175. Phylogenetic analysis revealed variations among North-East lineage V strains, forming a sub-clade within the lineage V, mostly isolated from Southern India71. Partial sequence analysis showed substitutions in A81T and A84P in the capsid region among the two isolates obtained from NER, India71. The mutated strains may have evolved independently from the undetected circulating strains or previously circulating strain either in the NER area or beyond. Whole-genome analysis may be helpful to elucidate this aspect. However, phylogenetic analysis assigned the two isolates (WNIRGC07; GenBank Accession No. HQ246154 and WNIRTC08; GenBank Accession No. JQ037832) within lineage V forming the presence of sub-clades. Pathogenicity studies using mouse models of each phylogenetically depicted lineage V strains demonstrated higher virulence as compared to archival isolated strains71. These findings correspond to the increased mortality recorded due to WNV infection in the region71.
Clinical presentation
The majority (~75-80%) of humans infected with WNV remain asymptomatic or present with mild febrile illness, about 20 per cent of infected persons present with febrile or flu-like illness and <1 per cent present with neuroinvasive disease7677. As per the CDC data, about one in five individuals infected with WNV develop fever and about one in 150 individuals develop neurological symptoms78. The incubation period of WNV in human is 2-14 days, but extended incubation period of up to 21 days have been observed among immunocompromised patients79. Uncomplicated symptomatic WNV infection begins with sudden onset of fever (usually >39°C), headache and myalgia, often accompanied by gastrointestinal symptoms. More acute cases with neurological complications are classified as encephalitis (or meningoencephalitis), with characteristic features of fever, headache, altered mental status and vomiting188081. Associated abnormalities may include depressed deep tendon reflexes and flaccid paralysis. Respiratory muscles are also involved, resulting in acute respiratory failure8283. In half of febrile patients, generalized roseolar or maculopapular rashes have also been reported which may last up to a week. However, these symptoms were more common during earlier epidemics8485. In contrast, in the new world epidemics, less than 22 per cent of patients have skin rash and only around five per cent presented with lymphadenopathy178086. Rare neurological manifestations of WNV infection include myelitis, optic neuritis, rhombencephalitis and polyradiculitis878889. Myocarditis, pancreatitis and fulminant hepatitis were reported as rare extraneurological manifestations9091. A patient with asymptomatic WNV infection was reported to develop fever, altered mental status and temporary vision loss after elective multilevel spine fusion surgery92. In Sri Lanka, WNV infection was reported in three patients, two with asthenia, polyarthralgia and photophobia presenting with atypical encephalitis symptoms28. Generally, WNV disease is diagnosed most frequently in the adult segment of populations. Lower incidence of WNV illness in children could be due to lack of surveillance for WNV in young children9394. In some studies, age >15 yr was considered an inclusion criterion for WNV case definition in surveillance systems959697.
In India, during 2006, 12 paediatric AES patients confirmed to be WNV infected were hospitalized in Assam. Six months follow up of the patients showed impaired memory (6 patients), irritable behaviour (5 patients), impaired hearing (3 patients), incoherent speech and disorientation (1 patient), breathing difficulty (1 patient), impaired speech (1 patient) and quadriparesis in one patient32. During 2015, paediatric age group patients presented with AES were found to be positive for WNV in Madhya Pradesh, Central India37. Seizure and altered consciousness were the most common symptoms observed followed by fever, headache, altered sensorium and vomiting37. All the WNV-positive patients had recovered fully, and no neurological sequelae were observed during discharge37. These differences in the clinical scenario of WNV infection in the paediatric population can be attributed to strain variations and host susceptibility to the virus. The clinical presentation of patients infected with lineage V in North-East India was distinguishable from lineage I WNV in southern India (Table I). Published data on details of WNV lineage-associated clinical spectrum are rare and clinical features are used to be reported as AES or WN encephalitis. During 2009-2010, patients residing in the coastal areas of Tamil Nadu developed signs of acute posterior uveitis and febrile illness which later were diagnosed to be due to infection with West Nile lineage I virus33. In 2011, lineage I WNV was detected in AES cases from Kerala; of whom, 66.82 per cent were adults and 33 per cent comprised paediatric age group35.
| WNV lineage | General clinical observation | Clinical features |
| Lineage I (South India) | Acute posterior uveitis with febrile illness | Fever |
| Neuroretinitis | ||
| Retinal vasculitis | ||
| Retinitis | ||
| Vascular occlusion | ||
| Bilateral combined vascular occlusion | ||
| Foveolitis | ||
| Lineage V (North-East India) | Acute encephalitis syndrome | Fever |
| Headache | ||
| Convulsions | ||
| Altered sensorium | ||
| Neck rigidity | ||
| Seizure | ||
| Vomiting |
Laboratory diagnosis
Serology is the cornerstone of WNV diagnosis, detection of antibody in serum or cerebrospinal fluid (CSF) using the IgM-ELISA–based assay has been the prime tool in most clinical settings. Generally, detection of IgM antibodies signifies recent infection. Its detection in CSF indicates CNS infection. However, there are reports of IgM antibodies persisting until 500 days post-WNV infection98. Hence, diagnosis with IgM antibodies has to be associated with clinical presentation and duration between onset of symptoms and sample collection. Other factors to consider include the presence of other flaviviruses in the region which may cross-react in serological diagnosis. Therefore, a virus-specific neutralizing antibody assay demonstrating a >four-fold rise in antibody titre between acute and convalescent phases of illness typically by plaque-reduction neutralization assay remains the gold standard for serological confirmation of WNV infection99. For neutralization assay, the ideal acute-convalescent phase specimen collection duration would be the initial days of illness followed by a blood sample collected 21 days post-infection. However, cross-reactivity between flaviviruses continues to be a serious drawback of serological diagnosis in areas where more than one flavivirus co-exists. Neutralization assays are not specific enough to distinguish between primary and secondary flavivirus infection. These diagnostic challenges should be taken into account in areas experiencing co-circulation of more than one flavivirus100.
WNV infection can also be confirmed with detection of viral RNA in clinical samples, viz. serum, CSF, plasma and urine99. Detection of WNV RNA is difficult due to the persistence of non-infectious RNA and short viraemia101. Studies showed persistence of WNV RNA in the CNS and kidney of mice and hamsters, respectively, after several weeks of acute infection102. Low level of WNV RNA was detected in naturally and experimentally infected avain hosts up to 36 wk post-infection102. However, follow up in human studies failed to document the same. In a study, evaluation of nucleic acid amplification testing (NAAT) for WNV diagnosis was done in plasma derived from symptomatic patients. Among the 191 patients tested by both serology and NAAT, 45.0, 58.1 and 94.2 per cent were detected by NAAT, serology and a combination of two, respectively103. Urine samples have also been used for detection of WNV RNA104. WNV RNA can be detected in urine within 20 days or longer post-onset of the disease, which is comparatively longer duration than in blood or CSF in WNV-infected individuals105. The virus has also been isolated in cell culture from urine specimens of patients with acute infection106. However, NAAT will be more useful in immunocompromised patients where antibody development is delayed or absent. Another important strategy is the use of qRT-PCR platform for NAAT-based diagnosis. Different chemistry-based application of qPCR led to sensitive, easy identification, genotyping and quantification of viral targets in single, rapid reactions107.
In patients with WN encephalitis, computer- assisted tomography (CT) scan often revealed pre-existing lesions and chronic changes in brain tissue but rarely showed signs of CNS inflammation108. In a 34 yr old woman diagnosed with WN encephalitis, electromyography and magnetic resonance imaging (MRI) revealed acute transverse myelitis109. CSF findings in WN meningoencephalitis showed mild pleocytosis (30-100 cells/µl; range 0-1800 cells/µl) with lymphocytes predominant, elevated protein concentration of 80-105 mg/dl (rarely up to 1900 mg/dl) and normal glucose concentration. Leucocytosis and leucopenia usually were observed in 50 and 15 per cent of patients, respectively. Other laboratory findings include mild anaemia110. In some patients, intermittent fever is observed. On examination, neck rigidity and a positive Kernig’s sign are often found. Several patients in the 1999 New York outbreak had marked flaccid paralysis with mild or no encephalopathy, leading to a provisional diagnosis of Guillain–Barré syndrome. Nerve conduction studies revealed reduced motor amplitudes with normal sensory potentials18. However, these symptomatic observations were based on limited studies within specific demographic areas. More studies on clinical parameters are needed to provide conclusive evidence of differences related to WNV lineages in different demographic settings. It is also paramount to document differences of clinical manifestation and biochemical parameters between closely related flaviviruses such as JEV and WNV. In one such study, front temporal hyper-intense signals in JE encephalitis patients were observed which distinguished it from herpes simplex encephalitis111. Greater awareness and more neurological associated evidence are needed to emphasize the threat evoked by a neurotropic virus to the brain112.
Vaccine, clinical management and control strategies
At present, no WNV vaccine or treatment has been licensed for use in humans. Four equine vaccines are licensed in the market; three are whole-inactivated virus (WN Innovator™, Vetera WNV, and Prestige® WNV) and one live chimeric virus expressing prM/E gene in Canarypox virus (Recombitek™ Equine WNV). Each of these vaccines is based on NY99 strain except Vetera WNV, which is based on E159 WNV isolate from equine. All the vaccines demonstrate protective immune response in horses that lasts for one year113. Several human vaccines have undergone preclinical studies and a few are in phase II trials (Table II)113. These vaccines have shown promising results in terms of safety and elicitation of antiviral immunity114. However, phase III efficacy trials have not been conducted because of doubts on market potential of a WNV human vaccine and due to difficulties in conducting phase III clinical trials for this sporadic and widely dispersed disease115. Other issues include the cost-effectiveness for a WNV vaccine and the fact that humans are dead-end hosts for the virus114. In addition to the present vaccine concept, alternatively, a vaccine against proteins of mosquito saliva associated with virus transmission is under development. This concept, if developed, will be useful in providing protection to many mosquito-borne pathogens113. Flavivirus infection providing cross-reactive antibodies to closely related virus sero-complex group is well known. This generates an assumption whether flavivirus vaccination programme will provide immunity to heterologous flavivirus. In one such study, it was found that JE vaccination with SA14-14-2 (live-attenuated JE vaccine) failed to elicit protective neutralizing antibodies in vaccinated human study group against WNV116. In another vaccine study done with inactivated JE vaccine (JEVAX®) showed similar results failing to elicit cross-neutralizing antibody against WNV117. Our study also supported these findings, where SA14-14-2 provided no protection to mice when infected with circulating WNV strain, WNIRTC08 and trivial protection against another circulating strain, WNIRGC07118. However, confounding results were observed when JE-VAX was co-delivered with (live) yellow fever 17D vaccine, eliciting effective levels of cross-neutralizing antibody against WNV117. This was perhaps due to an increase in vaccine immunogenicity by co-delivering with yellow fever vaccine. Future vaccine development against WNV is imminent due to increase in incidence of cases, mortality recorded and emergence of the virus in new geographical areas. Possible strategies may be to improve the surveillance network worldwide, planning trials where the incidence of WNV is high and most importantly adoption of uniform method for neutralization to test the efficacy during different clinical trials for prolong periods.
| Vaccine | Type | Strain | Clinical trial phase | Seroconversion rate (time post-vaccination) | Neutralization titre range© |
|---|---|---|---|---|---|
| VRC 303 | CMV/R promoter | NY99 | Phase I (2006) | 97 per cent (12 wk) | ~20-10,000 |
| WN-80E | Recombinant E protein | NY99 | Phase I (2008) | 100 per cent (2 wk) | ~50-100 |
| WN/DEN4D30 | Chimeric, live virus with WNV prM/E and DENV-4 non-structural genes with a 30 nt deletion | NY99 | Phase I (2004) Phase I (2007) Phase I (2014) | 75 per cent for NY99 (180 days) | ≤50-232 |
| Hydrovax-001 | Hydrogen peroxide-inactivated whole virus | Kunjin | Phase I (2015) | 31 per cent (15 days) | 9.8 (GMT)* |
| Formalin- inactivated WNV | Formalin-inactivated whole virus | NY99 | Phase I/II | ND | 140 (GMT)* |
| Chimerivax- WN02 | Chimeric, live virus with WNV prM/E and YFV 17D non-structural genes with three site-directed mutations in the E protein | NY99 | Phase 1 Phase II (2005) Phase II (2008) | 96 per cent (28 days) | 3309 (GMT)* |
Source: Ref 111. *Values represent in GMT; ©Neutralization titre range is shown as the reciprocal serum dilution. ND, no data; WNV, West Nile virus; DEN, dengue; CMV, cytomegalovirus; DENV, dengue virus; prM/E, premembrane envelope; YFV, yellow fever virus; GMT, geometric mean titre
The current therapeutic approach against WN encephalitis includes administration of corticosteroids, immune γ-globulin, monoclonal antibodies, interferon α-2b, antisense oligomers and anticonvulsants or osmotic agents115119. However, controlled studies have not been undertaken to document the therapeutic efficacy of these agents115. Several antiviral agents have been either studied in WNV-infected cell lines in vitro or in vivo studies with laboratory animals. Natural compounds such as isoflavone were also found to exhibit strong antiviral activity against a range of RNA viruses120. Anti-flavivirus activities of antibiotics have been demonstrated in vitro121. In an experimental study, oral dosage of vancomycin, neomycin, ampicillin and metronidazole in mice was found to exacerbate the disease severity of multiple flavivirus infections122. Further research is warranted for controlled group studies.
Prevention strategies are the only direct established method to control WNV infection. Development of comprehensive early warning tools and vector control programmes is an important measure to limit spill-over transmission to humans123. Therefore, it is important to implement effective surveillance programmes for vectors in an area to indicate an impending human outbreak. The immediate goal should be to control the vector density by widespread application of organophosphate or synthetic pyrethroid insecticides. In recent times, ‘one health’ approach is gaining popularity for timely control of WNV incidence. In Europe, this approach led to an increase in the timeliness of blood safety measures. Studies also described it to be a key factor in timely implementation of control measures124.
Knowledge gap and research priorities
WNV is a globally emerging virus, but unlike Zika and Ebola WNV outbreaks are usually localized and sporadic83. Yet, the virus continues to be an important cause of encephalitis worldwide32104115. In India, currently circulating WNV strains are more pathogenic than those reported earlier71. In 2019, a seven year old boy from Malappuram district of Kerala died of WNV infection. It was assumed that WNV infection affected the boys’ nervous system. However, conclusive knowledge on whether higher pathogenicity has resulted from characteristic virulence potential of the circulating strain or due to host factors is limited. Therefore, more information on the virus strains circulating currently in India and elsewhere is warranted. In addition, it is also important to interrogate the ecological factors and transmission dynamics of the virus. Host competence studies should be done on each perceived reservoir host and vectors for better understanding of the basic enzootic cycle. However, given the heterogeneity of the circulating strains of WNV, these experiments would require an extensive collaborative approach. Role of environmental factors on virus transmission also needs to be established. These eco-epidemiological studies must be done with one health approach124, which will help in building predictive models of WNV risk, similar to the JEV early warning system developed for JE-endemic areas in Assam, India125. An effective epidemiological model requires reliable epidemiological information; a classic example is database-integrated model developed in California (http://www.westnile.ca.gov/).
From an epidemiological point of view, the actual infection rate with WNV or similar pathogens remains under-represented due to diagnostic challenges and case selection. Only hospitalized encephalitis patients are referred for laboratory diagnosis for viral infection. Community-based surveillance could provide a better picture of disease epidemiology. The dilemma in WNV diagnosis is cross-reactivity between co-circulating flaviviruses. Therefore, new generation diagnostic assays are needed to overcome cross-reactions. Effort must also be taken to develop and implement point-care diagnostics in public health centres or State government hospitals. This requires a considerable effort and investment from the research and development sector. The Government of India under Department of Health Research (DHR) has established a network of viral research diagnostic laboratories (VRDL) across the country (https://dhr.gov.in/schemes/establishment-network-laboratories-managing-epidemics-and-natural-calamities). Similarly, Integrated Diseases Surveillance Programme (IDSP) under the National Health Mission (NHM) is working on reporting and surveillance of various diseases in the community126. National Vector Borne Disease Control Programme (NVBDCP) is another important part of NHM responsible for framing policies and providing technical and financial support to the States for control and management of vector-borne diseases (https://nvbdcp.gov.in/). Operational guidelines formulated by the NVBDCP is an important step towards this direction127. Another important knowledge gap is the impact of other co-circulating flaviviruses like JEV could have on WNV epidemiology. Further assessment of consequences of viral interference like JE vaccination in flaviviruses co-endemic areas on the immune response in the population will be interesting to study. At present, in India, SA14-14-2 JE vaccine has been in use for vaccination campaign of both adult and paediatric populations32128. Studies must be undertaken to assess development or presence of co-neutralizing antibodies in vaccinated population against WNV.
Conclusion
WNV continues to pose an emerging threat to public health worldwide. The distinction of WNV epidemic into old and new provides a clear understanding on its epidemiology. This review highlights the importance of continuing need for careful monitoring of disease epidemiology, implement interdisciplinary approach to surveillance and research programmes in parallel to management of cases. WNV as an emerging global pathogen can be a model for international public health community to further strengthen the line of communication for all the infectious diseases and for better preparedness in worst case scenario.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Changing patterns of West Nile virus transmission:Altered vector competence and host susceptibility. Vet Res. 2009;40:43.
- [Google Scholar]
- A neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med Hyg. 1940;20:471-3.
- [Google Scholar]
- West Nile virus population genetics and evolution. Infect Genet Evol. 2012;12:181-90.
- [Google Scholar]
- A KDEL retrieval system for ER-Golgi transport of Japanese encephalitis viral particles. Viruses. 2016;8:44.
- [Google Scholar]
- Pathogenesis of West Nile virus infection:A balance between virulence, innate and adaptive immunity, and viral evasion. J Virol. 2006;80:9349-60.
- [Google Scholar]
- Isolation from human sera in Egypt of a virus apparently identical to West Nile virus. Proc Soc Exp Biol Med. 1951;77:661-5.
- [Google Scholar]
- Isolation of West Nile virus from hooded crow and rock pigeon in the Nile delta. Proc Soc Exp Biol Med. 1953;84:719-22.
- [Google Scholar]
- Clinical and laboratory observations in an outbreak of West Nile fever in Israel in 1957. Harefuah. 1958;54:275-80.
- [Google Scholar]
- Epidémiologie du virus West Nile:Étude d'un foyer en Camargue. III. Les maladies humaines. Ann Inst Pasteur. 1968;115:435-45.
- [Google Scholar]
- Epidemics of West Nile and Sindbis viruses in South Africa with Culex univittatus theobald as vector. S Afr J Sci. 1976;72:295-300.
- [Google Scholar]
- Epidemic West Nile encephalitis in Romania:Waiting for history to repeat itself. Ann N Y Acad Sci. 2001;951:94-101.
- [Google Scholar]
- West Nile fever –A re-emerging mosquito borne viral disease in Europe. Emerg Infect Dis. 1999;5:643-50.
- [Google Scholar]
- West Nile encephalitis in Russia 1999-2001:Were we ready?Are we ready? Ann N Y Acad Sci. 2001;951:102-16.
- [Google Scholar]
- The outbreak of West Nile virus infection, New York City area, 1999. N Engl J Med. 2001;344:1807-14.
- [Google Scholar]
- West Nile fever outbreak, Israel, 2000:Epidemiologic aspects. Emerg Infect Dis. 2001;7:686-91.
- [Google Scholar]
- West Nile virus:Statistics and Maps. Available from: https://www.cdc.gov/westnile/statsmaps/index.html
- West Nile Virus Infection. Available from: https://www.ecdc.europa.eu/en/west-nile-virus-infection
- West Nile in Europe:An increasing public health problem. J Travel Med. 2018;25:1-2.
- [Google Scholar]
- West nile virus infection in Xinjiang, China. Vector Borne Zoonotic Dis. 2013;13:131-3.
- [Google Scholar]
- Virulence and evolution of West Nile Virus, Australia, 1960-2012. Emerg Infect Dis. 2016;22:1353-62.
- [Google Scholar]
- West Nile Virus in the New World:Trends in the spread and proliferation of West Nile Virus in the Western Hemisphere. Zoonoses Public Health. 2009;56:357-69.
- [Google Scholar]
- West Nile virus disease outbreak in Pakistan, 2015-2016. Front Public Health. 2018;6:20.
- [Google Scholar]
- West Nile virus in Pakistan. 1. Sero-epidemiological studies in Punjab Province. Trans R Soc Trop Med Hyg. 1982;76:431-6.
- [Google Scholar]
- Emergence of human West Nile Virus infection in Sri Lanka. BMC Infect Dis. 2015;15:305.
- [Google Scholar]
- Preliminary observations on antibody patterns against certain viruses among inhabitants of Bombay city. Indian J Med Sci. 1952;6:733-66.
- [Google Scholar]
- Transmission dynamics and changing epidemiology of West Nile virus. Anim Health Res Rev. 2008;9:71-86.
- [Google Scholar]
- Molecular detection and characterization of West Nile virus associated with multifocal retinitis in patients from southern India. Int J Infect Dis. 2012;16:e53-9.
- [Google Scholar]
- Acute flaccid paralysis due to West Nile virus infection in adults:A paradigm shift entity. Ann Indian Acad Neurol. 2014;17:85-8.
- [Google Scholar]
- West Nile encephalitis outbreak in Kerala, India, 2011. J Clin Virol. 2014;61:152-5.
- [Google Scholar]
- Emergence of West Nile virus in West Bengal, India:A new report. Trans R Soc Trop Med Hyg. 2017;111:178-84.
- [Google Scholar]
- Emergence of human West Nile virus infection among paediatric population in Madhya Pradesh, India. J Med Virol. 2019;91:493-97.
- [Google Scholar]
- Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167-73.
- [Google Scholar]
- Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera:Culicidae) J Med Entomol. 2014;43:309-17.
- [Google Scholar]
- Environmental drivers of West Nile fever epidemiology in Europe and Western Asia –A review. Int J Environ Res Public Health. 2013;10:3543-62.
- [Google Scholar]
- Vector competence of European mosquitoes for West Nile virus. Emerg Microbes Infect. 2017;6:e96.
- [Google Scholar]
- First field evidence for natural vertical transmission of West Nile virus in Culex univittatus complex mosquitoes from Rift Valley province, Kenya. Am J Trop Med Hyg. 2000;62:240-6.
- [Google Scholar]
- A study of the ecology of West Nile virus in Egypt. Am J Trop Med Hyg. 1956;5:579-620.
- [Google Scholar]
- The global ecology and epidemiology of West Nile virus. Biomed Res Int. 2015;2015:376230.
- [Google Scholar]
- Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008;4:e1000092.
- [Google Scholar]
- Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera:Culicidae) J Med Entomol. 2006;43:309-17.
- [Google Scholar]
- Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Vet Microbiol. 2013;165:61-70.
- [Google Scholar]
- Epidemiology and ecology of West Nile virus in sub-Saharan Africa. Parasit Vectors. 2018;11:414.
- [Google Scholar]
- West Nile virus. Available from: https://aaep.org/guidelines/vaccination-guidelines/core-vaccination-guidelines/west-nile-virus
- Characterization of virulent West Nile virus Kunjin strain, Australia, 2011. Emerg Infect Dis. 2012;18:792-800.
- [Google Scholar]
- Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003;349:1236-45.
- [Google Scholar]
- West Nile virus among blood donors in the United States, 2003 and 2004. N Engl J Med. 2005;353:451-9.
- [Google Scholar]
- Correlation of West Nile Virus incidence in donated blood with West Nile neuroinvasive disease rates, United States, 2010-2012. Emerg Infect Dis. 2017;23:212-9.
- [Google Scholar]
- Donor-derived West Nile virus infection in solid organ transplant recipients:Report of four additional cases and review of clinical, diagnostic and therapeutic features. Transplantation. 2014;97:881-9.
- [Google Scholar]
- Transmission of West Nile virus through human breast milk seems to be rare. Pediatrics. 2007;119:e666-71.
- [Google Scholar]
- Detection of West Nile virus in six mosquito species in synchrony with seroconversion among sentinel chickens in India. Parasit Vectors. 2017;10:13.
- [Google Scholar]
- Vector competence of two Indian population of Culex quinquefasciatus (Diptera:Culicidae) mosquitoes to three West Nile virus strains. J Vector Borne Dis. 2015;52:185-92.
- [Google Scholar]
- Vertical transmission of West Nile Virus by three California Culex (Diptera:Culicidae) species. J Med Entomol. 2003;40:743-6.
- [Google Scholar]
- Sentinel chicken seroconversions track tangential transmission of West Nile virus to humans in the greater Los Angeles area of California. Am J Trop Med Hyg. 2010;83:1137-45.
- [Google Scholar]
- Serological evidence of West Nile virus infection in wild migratory and resident water birds in Eastern and Northern India. Comp Immunol Microbiol Infect Dis. 2012;35:591-8.
- [Google Scholar]
- Serological evidence of widespread West Nile virus and Japanese encephalitis virus infection in native domestic ducks (Anas platyrhynchos var domesticus) in Kuttanad region, Kerala, India. Comp Immunol Microbiol Infect Dis. 2016;48:61-8.
- [Google Scholar]
- West Nile virus infection among animals and humans in India. Adv Anim Vet Sci. 2014;2:17-23.
- [Google Scholar]
- Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333-7.
- [Google Scholar]
- Phylogeography of West Nile virus:From the cradle of evolution in Africa to Eurasia, Australia, and the Americas. J Virol. 2011;85:2964-74.
- [Google Scholar]
- Ongoing outbreak of West Nile virus infections in humans in Greece, July-August 2010. Euro Surveill. 2010;15:19644.
- [Google Scholar]
- Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg Infect Dis. 2006;12:618-23.
- [Google Scholar]
- Novel flavivirus or new lineage of West Nile virus, central Europe. Emerg Infect Dis. 2005;11:225-31.
- [Google Scholar]
- West Nile virus isolates from India:Evidence for a distinct genetic lineage. J Gen Virol. 2007;88:875-84.
- [Google Scholar]
- Characterization of West Nile virus isolates from Assam, India:Insight into the circulating WNV in Northeast India. Comp Immunol Microbiol Infect Dis. 2014;37:39-47.
- [Google Scholar]
- The zoonotic flaviviruses of southern, south-eastern and eastern Asia, and Australasia:The potential for emergent viruses. Zoonoses Public Health. 2009;56:338-56.
- [Google Scholar]
- Virus Taxonomy:Ninth report of the international committee on taxonomy of viruses. San Diego: Academic Press; 2011.
- [Google Scholar]
- Putative new West Nile virus lineage in Uranotaenia unguiculata mosquitoes, Austria, 2013. Emerg Infect Dis. 2014;20:2119-22.
- [Google Scholar]
- Complete genome sequence of West Nile virus isolated from Alappuzha district, Kerala, India. Genome Announc. 2013;1:e00230-13.
- [Google Scholar]
- Clinical manifestations and outcomes of West Nile virus infection. Viruses. 2014;6:606-23.
- [Google Scholar]
- West Nile virus:Symptoms, Diagnosis &Treatment. Available from: https://www.cdc.gov/westnile/symptoms/index.html
- West Nile virus encephalitis acquired via liver transplantation and clinical response to intravenous immunoglobulin:Case report and review of the literature. Transpl Infect Dis. 2011;13:312-7.
- [Google Scholar]
- The West Nile Virus outbreak of 1999 in New York:The Flushing Hospital experience. Clin Infect Dis. 2000;30:413-8.
- [Google Scholar]
- Clinical characteristics and functional outcomes of West Nile Fever. Ann Intern Med. 2004;141:360-5.
- [Google Scholar]
- Clinical findings of West Nile virus infection in hospitalized patients, New York and New Jersey, 2000. Emerg Infect Dis. 2001;7:654-8.
- [Google Scholar]
- The long-term outcomes of human West Nile virus infection. Clin Infect Dis. 2007;44:1617-24.
- [Google Scholar]
- West Nile fever;the clinical features of the disease and the isolation of West Nile virus from the blood of nine human cases. Am J Hyg. 1954;59:89-103.
- [Google Scholar]
- The natural history of West Nile Fever. I. Clinical observations during an epidemic in Israel. Am J Hyg. 1956;64:259-69.
- [Google Scholar]
- Clinical characteristics of the West Nile fever outbreak, Israel, 2000. Emerg Infect Dis. 2001;7:675-8.
- [Google Scholar]
- West Nile virus meningoencephalitis with optic neuritis. Arch Intern Med. 2002;162:606-7.
- [Google Scholar]
- Clinical studies of viruses as antineoplastic agents with particular reference to Egypt 101 virus. Cancer. 1952;5:1025-34.
- [Google Scholar]
- West Nile virus infection and postoperative neurological symptoms:A case report and review of the literature. J Clin Anesth. 2014;26:410-3.
- [Google Scholar]
- West Nile virus infection in the pediatric population. Pediatr Infect Dis J. 2006;25:75-8.
- [Google Scholar]
- Completeness of West Nile virus testing in patients with meningitis and encephalitis during an outbreak in Arizona, USA. Epidemiol Infect. 2012;140:1632-6.
- [Google Scholar]
- West Nile virus infection in Veneto region, Italy, 2008-2009. Euro Surveill. 2009;14:19289.
- [Google Scholar]
- West Nile virus transmission with human cases in Italy, August - September 2009. Euro Surveill. 2009;14:19353.
- [Google Scholar]
- Outbreak of West Nile virus infection in humans, Romania, July to October 2010. Euro Surveill. 2011;16:19762.
- [Google Scholar]
- Persistence of virus-reactive serum immunoglobulin m antibody in confirmed West Nile virus encephalitis cases. Emerg Infect Dis. 2003;9:376-9.
- [Google Scholar]
- Diagnosis of West Nile virus human infections:Overview and proposal of diagnostic protocols considering the results of external quality assessment studies. Viruses. 2013;5:2329-48.
- [Google Scholar]
- Finding the signal in the noise in the serologic diagnosis of flavivirus infections. J Infect Dis. 2018;218:516-8.
- [Google Scholar]
- Superiority of West Nile virus RNA detection in whole blood for diagnosis of acute infection. J Clin Microbiol. 2016;54:2294-7.
- [Google Scholar]
- Persistent RNA virus infections:Do PAMPS drive chronic disease? Curr Opin Virol. 2017;23:8-15.
- [Google Scholar]
- Nucleic acid testing for West Nile virus RNA in plasma enhances rapid diagnosis of acute infection in symptomatic patients. J Infect Dis. 2006;193:1361-4.
- [Google Scholar]
- Fatal case of West Nile neuroinvasive disease in Bulgaria. Emerg Infect Dis. 2016;22:2203-4.
- [Google Scholar]
- Persistent infection with West Nile virus years after initial infection. J Infect Dis. 2010;201:2-4.
- [Google Scholar]
- Excretion of West Nile virus in urine during acute infection. J Infect Dis. 2013;208:1086-92.
- [Google Scholar]
- West Nile viral encephalitis in an HIV-positive woman in New York. N Engl J Med. 2000;342:59-60.
- [Google Scholar]
- Myelopathy in West Nile virus encephalitis:Report of a case and review of literature. J Spinal Cord Med. 2020;43:444-8.
- [Google Scholar]
- Clinical manifestations in the West Nile virus outbreak. Rom J Virol. 1997;48:3-11.
- [Google Scholar]
- Role of imaging in herpes and Japanese encephalitis –Two cases and review of literature. J Indian Acad Clin Med. 2012;13:338-43.
- [Google Scholar]
- Emerging viral infections in sub-Saharan Africa and the developing nervous system:A mini review. Front Neurol. 2018;9:82.
- [Google Scholar]
- Twenty years of progress towards West Nile virus vaccine development. Viruses. 2019;11:823.
- [Google Scholar]
- Current trends in West Nile virus vaccine development. Expert Rev Vaccines. 2014;13:589-608.
- [Google Scholar]
- Failure of Japanese encephalitis vaccine and infection in inducing neutralizing antibodies against West Nile virus, People's Republic of China. Am J Trop Med Hyg. 2008;78:999-1001.
- [Google Scholar]
- Feasibility of cross-protective vaccination against flaviviruses of the Japanese encephalitis sero complex. Expert Rev Vaccines. 2012;11:177-87.
- [Google Scholar]
- Cross protective immunity against circulating Japanese Encephalitis virus and West Nile virus by live attenuated Japanese encephalitis vaccine SA-14-14-2. Inter J Infect Dis. 2016;45:434.
- [Google Scholar]
- Vaccines and immunotherapeutics for the prevention and treatment of infections with West Nile virus. Immunotherapy. 2011;3:269-85.
- [Google Scholar]
- Isoflavone agonists of IRF-3 dependent signaling have antiviral activity against RNA viruses. J Virol. 2012;86:7334-44.
- [Google Scholar]
- Pharmacodynamics of aminoglycosides and tetracycline derivatives against Japanese encephalitis virus. Asian Pac J Trop Med. 2016;9:241-6.
- [Google Scholar]
- Oral antibiotic treatment of mice exacerbates the disease severity of multiple flavivirus infections. Cell Rep. 2018;22:3440-53.e6.
- [Google Scholar]
- West Nile virus in the United States:Guidelines for detection, prevention, and control. Viral Immunol. 2000;13:469-75.
- [Google Scholar]
- One Health approach for West Nile virus surveillance in the European Union:Relevance of equine data for blood safety. Euro Surveill. 2019;24:1800349.
- [Google Scholar]
- Forecasting spatial distribution of Japanese encephalitis occurrence –A remote sensing and GIS based study in Assam, India. Int J Geoinform. 2014;10:1-7.
- [Google Scholar]
- Available from: http://idsp.nic.in/index4.php?lang=1&level=0&linkid=406&lid=3689
- Available from: https://nvbdcp.gov.in/Doc/JE-AES-Prevention-Control(NPPCJA).pdf
- Effectiveness of single dose of Japanese encephalitis vaccines among adults, Assam, India, 2012-2018. Vaccine. 2021;39:4973-8.
- [Google Scholar]






