Translate this page into:
Genetic variability & chemotoxicity of 5-fluorouracil & cisplatin in head & neck cancer patients: a preliminary study
Reprint requests: Dr Harish Padh, B.V. Patel Pharmaceutical Education & Research Development (PERD) Centre, Thaltej-Gandhinagar Highway, Thaltej, Ahmedabad 380 054, India e-mail: perd@perdcentre.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The efficacy and toxicity of a given chemotherapy regimen varies widely among patients due to the inherited variability of genes that are involved in drug metabolism. There are several crucial enzymes identified involving metabolism of 5-fluorouracil (5-FU) and cisplatin, which are polymorphic. We studied head and neck cancer patients (n=23) on 5-FU and cisplatin combination therapy attending a tertiary care cancer research institute in Gujarat, India, to understand the effect of a particular genotype on toxicity.
Methods:
The patients were genotyped for dihydropyrimidine (DPYD) (85T>C, IVS14+1G>A, 2846A>T, 2194G>A), thymidylate synthase (TYMS) [28bp tandem repeat in the promoter enhancer region (TSER)], methylenetetrahydrofolate reductase (MTHFR) (677C>T, 1298A>C), glutathione S-transferase P1(GSTP1) (Ile105Val), glutathione S-transferase T1 (GSTT1) (null allele) and glutathione S-transferase M1 (GSTM1) (null allele) by multiplex allele-specific PCR and long range PCR.
Results:
Of the 23 (19 males 4 females, age range 18-16 yr) patients, two had grade 3 and 4 toxicity while the remaining 21 had 0 to 2 grade toxicity after treatment with 5-FU and cisplatin combination therapy. An association between the genotype of GSTM1 (+/- and -/-) and the toxicity of cisplatin (P=0.043) was observed.
Interpretation & conclusions:
The findings of this preliminary study suggested an association between the variants of GSTM1 and toxicity observed due to cisplatin. Well planned studies on a large sample of head and neck cancer patients need to be conducted to understand the effects of these genetic variants on toxicity and efficacy of anticancer drugs.
Keywords
Cisplatin
5-fluorouracil
genetic variations
head and neck cancer
toxicity
5-Fluorouracil (5-FU) remains one of the most frequently prescribed chemotherapeutic drugs for the treatment of head and neck, breast and gastrointestinal cancer3. Although this antimetabolite is toxic, its efficacy makes it one of the most widely used agents against solid tumours. 5-Fluorouracil is generally prescribed as a combination with other drugs like cisplatin. 5-FU is metabolized via two routes: the anabolic route which gives rise to active metabolites and the catabolic route which inactivates 5-FU and leads to the elimination of the drug from the system1. Most of the administered dose (80%) of 5-FU is converted to inactive metabolites. The 5-fluorouracil pathway is affected by a number of genes that are known to be polymorphic, for example: dihydropyrimidine dehydrogenase (DPYD), thymidylate synthase (TYMS) and methylenetetrahydrofolate reductase (MTHFR). The cisplatin pathway is also affected by the genes of the glutathione S-transferase family (GSTP1, GSTT1 and GSTM1)2. In this preliminary study we aimed to establish an association between the genetic variability and the toxicity observed in the head and neck cancer patients on a combination of 5-FU and cisplatin chemotherapy regimen.
Material & Methods
Patient recruitment: This prospective pilot study was conducted on 40 head and neck cancer patients during mid 2007 to mid 2009, however clinical data were available only for 23 patients (19 males and 4 females age range 18-60 yr), on 5-fluorouracil and cisplatin combination chemotherapy regime (5-FU: 750mg/m2 day 1 to 4; Cisplatin: 100mg/m2 day 1. Therapy was repeated every 21 days for 2/4-6 cycles). All patients gave a written informed consent and the study was conducted as per the guidelines of the Institutional Ethics Committee, India and was approved by Ethics Committee of Gujarat Cancer Research Institute, Ahmedabad. Patients attending the Medical Oncology OPD at Gujarat Cancer Research Institute, Ahmedbad, Gujarat, India, meeting the inclusion and exclusion criteria were screened and included in the study. The sites of cancer mainly included base of tongue, left lip, tongue and right retromolar trigone (RMT).
Inclusion criteria: The head and neck cancer patients were selected based on the following inclusion criteria: (i) Male/female, age ≥18 yr; (ii) Histopathologically proven head and neck cancer; (iii) Chemotherapy and hormone therapy naïve; (iv)Must not have received prior radiotherapy; (v) Normal bone marrow, liver, kidney and cardiac function; (vi) Evaluable disease either clinically, endoscopically or radiologically by simple imaging (except mediastinal involvement, pleural or pericardial effusion or bone as only site of evaluable disease). Oral cavity, esophagus and laryngeal cancer were taken if having exophytic growth); (vii) Not taking medicines likely to alter enzymes concerned; and (viii) For women negative pregnancy test and adequate birth control measures.
Exclusion criteria: Patients were excluded from the study according to the following exclusion criteria: (i) Infections like tuberculosis (TB), human immuno-deficiency virus (HIV) and non resolving active bacterial infections; (ii) Pregnant woman; (iii) Drug hypersensitivity; and (iv) Uncontrolled diabetes, cardiac failure, myocardial infarction in recent past, psychiatric illness and any other condition.
Genotyping: Blood sample (5 ml) was collected using ethylene diamine tetra-acetic acid (EDTA) as an anticoagulant from each patient on day one before chemotherapy. DNA was extracted using phenol-chloroform extraction method4. The first step involved rupturing of RBCs using the lysis buffer and the WBC pellet was then subjected to lysis by digestion buffer. The proteins were degraded using proteinase K and the sample was subjected to phenol: chloroform: isoamyl alcohol mixture for purification of the DNA and removal of proteins and lipids. The DNA was precipitated with absolute ethanol and washed with 70 per cent alcohol and then subsequently the DNA pellet was dried and redissolved in appropriate amount of TE buffer4. The patients were genotyped for DPYD (85T>C, IVS14+1G>A, 2846A>T, 2194G>A), TYMS [28bp tandem repeat in the promoter enhancer region (TSER)], MTHFR (677C>T, 1298A>C), GSTP1 (Ile105Val), GSTT1 (null allele) and GSTM1 (null allele) using multiplex allele-specific PCR and long range PCR5. The allele-specific multiplex PCR utilizes the multiplex, amplification refractory mutation system (ARMS)6. Here, two sets of primers were used for the detection of single nucleotide polymorphism (SNPs). The SNP was the part of the primer and was located at the 3’ end of the primer sequence. One of the primers did not have the SNP, which was termed as the wild type primer while the other primer had the SNP, which was termed the mutant primer. Both these primers were added alternatively in two different reactions, ARMS1 and ARMS2. The presence or absence of the SNP was interpreted by the presence or absence of the band from the ARMS1 and ARMS2 reactions on agarose gels.
Toxicity assessment: Side-effects that are typically associated with 5-fluorouracil and cisplatin treatment, like haematological anaemia, neutropenia, thrombocytopenia, mucositis and cardiac toxicity were documented within the first 3 cycles of the therapy. The performance status of the patients was between 0, 1 or 2 according to the scale of the ECOG Performance Status (which assesses how a patient's disease is progressing and how the disease affects the daily living abilities of the patient, and determines appropriate treatment and prognosis). The serum albumin levels ranged between 1.18 to 1.64 g. The toxicity assessment was based on the National Cancer Institute Common Toxicity Criteria Adverse Event reporting guidelines (NCI-CTCAE, version 3.0) (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/doc/ctcaev3.pdf) and was done without the knowledge of the genotyping results.
Statistical analysis: Relationships between the variables collected and measured in the study were assessed by using statistical tests. For normally distributed discrete data, Chi square (χ2) test was used. The population studied was checked for Hardy-Weinberg Equilibrium by using Chi square (χ2) test.
Results
Based on common toxicity criteria guidelines (NCI-CTCAE, version 3.0), two of 23 cancer patients presented with grade 3 and 4 toxicity, while the remaining 21 had grade 0 to 2 toxicity after treatment with 5-FU and cisplatin. In the majority of patients the combination chemotherapy was well tolerated or caused only mild toxicity. The patient characteristics according to the toxicity classification are shown in Table I.
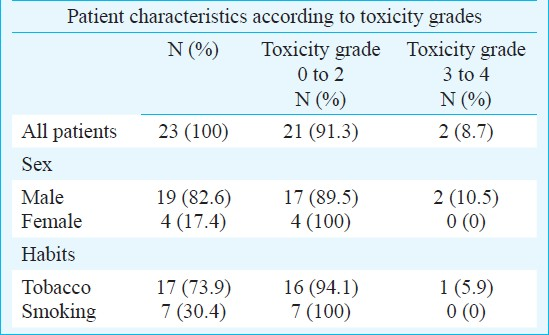
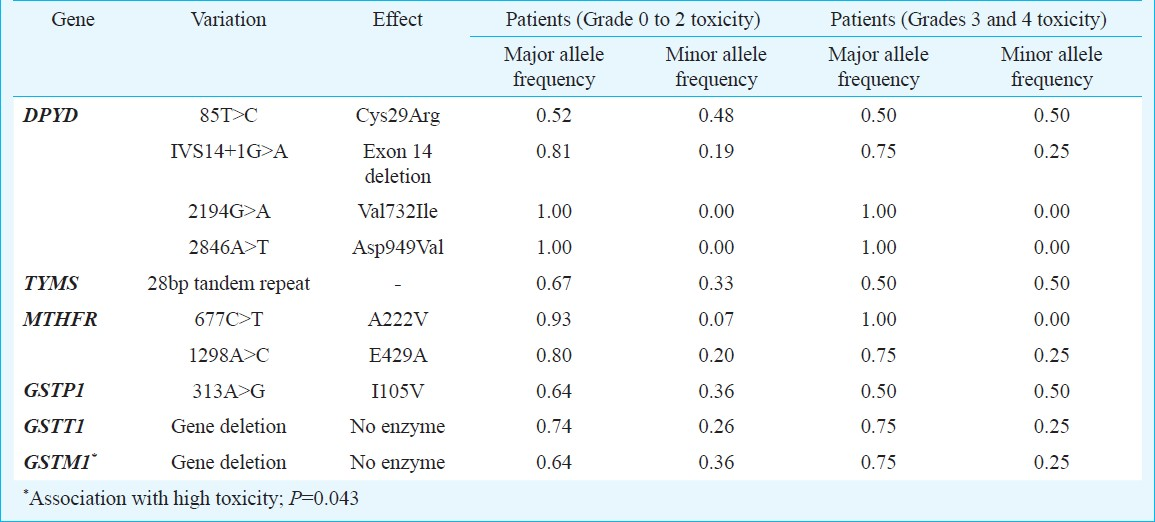
For 5-FU toxicity the association between genotypes of DPYD, TYMS and MTHFR and two groups of toxicity grades was studied. Similarly for cisplatin the genotypes of GSTP1, GSTT1 and GSTM1 were associated with the two groups of toxicity classification. A significant association was observed between the variants of GSTM1 and the toxicity due to cisplatin (P=0.043) (Table II). Due to small sample size, it was not possible to analyse combination of the different genotypes with the toxicity observed in the patients.
Discussion
More than 80 per cent of 5-FU is inactivated by dihydropyrimidine dehydrogenase (DPD). Decreased DPD activity has been associated with more than four-fold risk of severe or fatal toxicity from standard doses of 5-FU7. In this study, we did not observe a significant association between the variants and toxicity due to 5-FU. The allele DPYD*2A, a IVS14 + 1 G>A splice site transition that causes skipping of exon 14, has been found in up to 40 to 50 per cent of patients who developed grade 4 neutropenia, and was associated with DPD deficiency8. The patients who are heterozygous for this polymorphism have low DPD activity hence more amount of drug remains in the system which leads to toxicity to 5-FU9. Thymidylate synthase (TS) is the main target for 5-FU. The overexpression of TS has been correlated with the resistance to 5-FU10. We have studied a polymorphic 28bp tandem repeat in the promoter enhancer region (TSER) for all the individuals and observed a significant association (P=0.065) between the variants (TSER*2/*3 and *3/*3) and toxicity due to 5-FU. TYMS polymorphism has been observed in a large prospective study in Germany conducted in 683 patients, in whom the TSER*2/*2 genotype was found to increase the risk of toxicity 1.56 fold11 compared with the findings of another study of 90 patients, in whom the TSER*2/*2 genotype was found to have a grade 3 or 4 toxicity rate of 43 per cent12. Overexpression of TS is associated with poor prognosis1314 and resistance to TS targeted chemotherapy agents1315.
MTHFR catalyses the irreversible conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate16. In this study, no association was observed between variants of C677T and A1298C and toxicity due to 5-FU. The C677T variant (Ala222Val, rs1801133) is known to be associated with a decreased activity of MTHFR, an increased level of homocysteine and an altered distribution of folate17–19. The A1298C variant (Glu429Ala, rs1801131) has also been related to a reduced MTHFR activity, but at a lower degree compared to C677T20–22. GSTP1 is known to detoxify platinum compounds like cisplatin and oxaliplatin, which are generally used in combination with 5-FU for the treatment of a wide range of tumours. We did not observe a significant association between the variants and toxicity observed due to cisplatin. In a study of 107 patients with advanced colo-rectal cancer treated with a combination of 5-FU and oxaliplatin,patients homozygous for the valine allele had a median of 24.9 months survival, compared to 7.9 months for patients homozygous for the isoleucine allele23.
GSTT1 and GSTM1 polymorphisms have not been widely studied as a response biomarker for xenobiotics. In the present study, we genotyped all the individuals to identify GSTT1 and GSTM1 gene deletion. There was no significant association between the GSTT1 variants and toxicity observed due to cisplatin. However, there was a significant association between the variants of GSTM1 and toxicity observed due to cisplatin. Oldenburg and colleagues19 did not observe any significant correlation between GSTT1 deletions and chemotherapy-induced toxicities in testicular cancer survivors; however, GSTM1 deletion protected against hearing impairment significantly24. Also, another study on head and neck squamous cell carcinoma patients reported no significant difference in the prevalence of GSTT1 or GSTM1 variants and response to chemotherapy25. There are reports of borderline significance between GSTT1 deletion polymorphism and progression-free survival26. GSTs have been mainly studied for susceptibility to various types of cancer.
There were several limitations to this study. Low serum albumin levels generally lead to increased free drug which might lead to toxicity; however, this scenario was not seen in our study. The smaller sample size of the patient population limited us in studying the correlation with a combination of variants in terms of toxicity. Also the clinical data obtained for phenotype were inadequate, and data on efficacy could not be obtained. Therefore, the genetic variants could not be correlated with the effectiveness of the drugs.
In conclusion, this preliminary study on a small sample size suggested an association between the genotype and phenotype in case of head and neck cancer patients on 5-FU and cisplatin chemotherapy regimen. Further studies with a larger sample size are warranted to establish the effect of these genetic variants on toxicity and/or efficacy. When more association studies linking particular genotype variants with either efficacy or toxicity are reported and validated in other populations, our knowledge base will permit us to utilize genotype screening techniques to avoid adverse drug reactions and improve drug efficacy.
References
- The oral fluoropyrimidines in cancer chemotherapy. Clin Cancer Res. 1999;5:2289-96.
- [Google Scholar]
- The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445-600.
- [Google Scholar]
- Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur J Cancer. 2004;40:939-50.
- [Google Scholar]
- Molecular cloning: A laboratory manual. New York: Cold Spring Harbor Laboratory Press; 1989.
- Molecular biomarkers for cancer chemotherapy in the Indian population. In: Ph.D. thesis. Ahmedabad, India: Nirma University; 2010.
- [Google Scholar]
- Analysis of any point mutation in DNA.The amplification refractory mutation system (ARMS) Nucleic Acids Res. 1989;17:2503-16.
- [Google Scholar]
- High prevalence of the IVS14+1G>A mutation in the dihydropyrimidine dehydrogenase gene of patients with severe 5-fluorouracil-associated toxicity. Pharmacogenetics. 2002;12:555-8.
- [Google Scholar]
- Increased risk of grade IV neutropenia after administration of 5-fluorouracil due to a dihydropyrimidine dehydrogenase deficiency: high prevalence of the IVS14+1g>a mutation. Int J Cancer. 2002;101:253-8.
- [Google Scholar]
- Molecular basis of the human dihydropyrimidine dehydrogenase deficiency and 5-fluorouracil toxicity. J Clin Invest. 1996;98:610-5.
- [Google Scholar]
- Thymidylate synthase gene amplification in human colon cancer cell lines resistant to 5-Fluorouracil. Biochem Pharmacol. 1995;49:1419-26.
- [Google Scholar]
- Role of genetic and nongenetic factors for fluorouracil treatment-related severe toxicity: a prospective clinical trial by the German 5-FU Toxicity Study Group. J Clin Oncol. 2008;26:2131-8.
- [Google Scholar]
- Thymidylate synthase gene polymorphism predicts toxicity in colorectal cancer patients receiving 5-fluorouracil-based chemotherapy. Clin Cancer Res. 2004;10:5880-8.
- [Google Scholar]
- Thymidylate synthase mRNA level in adenocarcinoma of the stomach: a predictor for primary tumour response and overall survival. J Clin Oncol. 1996;14:176-82.
- [Google Scholar]
- The role of thymidylate synthase expression in prognosis and outcome of adjuvant chemotherapy in patients with rectal cancer. J Clin Oncol. 1994;12:2640-7.
- [Google Scholar]
- Quantitation of intratumoral thymidylate synthase expression predicts for disseminated colorectal cancer response and resistance to protracted-infusion fluorouracil and weekly leucovorin. J Clin Oncol. 1997;15:3223-9.
- [Google Scholar]
- A common mutation in the methylenetetrahydrofolate reductase gene is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proc Natl Acad Sci USA. 1998;95:13217-20.
- [Google Scholar]
- Common methylenetetrahydrofolate reductase gene mutation leads to hyperhomocysteinemia but not to vascular disease: the result of a meta-analysis. Circulation. 1998;98:2520-6.
- [Google Scholar]
- A canidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111-3.
- [Google Scholar]
- Association of total plasma homocysteine with methylenetetrahydrofolate reductase genotypes 677C>T, 1298A>C, and 1793G>A and the corresponding haplotypes in Swedish children and adolescents. Int J Mol Med. 2007;19:659-65.
- [Google Scholar]
- A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet. 1998;62:1044-51.
- [Google Scholar]
- A second genetic polymorhism in methylenetetrahydrofolate reducatase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64:169-72.
- [Google Scholar]
- Association between glutathione S-transferase P1, T1, and M1 genetic polymorphism and survival of patients with metastatic colorectal cancer. J Natl Cancer Inst. 2002;94:936-42.
- [Google Scholar]
- Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and –M1, a retrospective cross sectional study. J Transl Med. 2007;5:70.
- [Google Scholar]
- Glutathione-associated enzymes in head and neck squamous cell carcinoma and response to cisplatin-based neoadjuvant chemotherapy. Int J Cancer. 2001;93:725-30.
- [Google Scholar]
- Pharmacogenetic analyses of a phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil and leucovorin plus either oxaliplatin or cisplatin: a study of the arbeitsgemeinschaft internistische onkologie. In: J Clin Oncol. Vol 27. 2009. p. :2863-73.
- [Google Scholar]






