Translate this page into:
Folic acid, one-carbon metabolism & childhood cancer
Reprint requests: Dr. Archana Kumar, Department of Pediatrics, Division of Pediatric Hematology-Oncology, King George's Medical University, Lucknow 226 003, Uttar Pradesh, India e-mail: archanakumar53@yahoo.co.in
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Folate has been studied in relation to many diseases, especially cancer. Although it has been postulated to exert a dual effect on development of cancer, its role remains to be clearly defined. Its effect on cancer is the result of gene-nutrient interaction between the genes in folate metabolic pathway and dietary folate availability; mutations in genes of folate metabolism have been shown to alter individual susceptibility to certain childhood cancers as well as response to cancer chemotherapy. Although mandatory fortification of food items with folate has been initiated in some countries, many countries are yet to adopt this due to concerns about undesired adverse effects of high folate levels on health, especially cancer. However, initial reports suggest that folate fortification has led to reduction in incidence of certain childhood cancers such as neuroblastoma, wilms tumour and leukaemias. Despite studies showing folate depletion during antifolate chemotherapy and higher toxicity of chemotherapy in folate-depleted individuals, folate supplementation during cancer chemotherapy is not routinely recommended. Studies investigating the precise effect of folate supplementation during chemotherapy on both short- and long-term outcomes of cancer are needed to arrive at a consensus guideline.
Keywords
Bioavailability
childhood cancer
dietary sources
folic acid
MTHFR polymorphism
one-carbon metabolism
Introduction
Since its discovery in the 1940s, the importance of folic acid in health and disease is being increasingly recognized. Folate is essential for cell multiplication and homoeostasis due to the role of folate-containing coenzymes in nucleic acid synthesis, methionine regeneration and shuttling; oxidation and reduction of one-carbon compounds are essential for cellular metabolism. Progress in basic, translational and clinical sciences has led to unfolding of knowledge about the complex relationship between folic acid, genes encoding various enzymes related to folate metabolism and cancer12. The present review aims to address the importance of folates in childhood cancers, including their association with the genes in folate metabolic pathway and the proposed role of folates in carcinogenesis as also the effect of folic acid fortification of food on paediatric cancer epidemiology. It also covers in short the sources, bioavailability, absorption and metabolism of folates along with the clinical aspects of folate deficiency.
Folic acid - historical perspective
The correction of macrocytic anaemia in pregnant women in Bombay3 (now Mumbai) by yeast extract prompted the discovery of a new nutrient which was finally extracted from spinach in 19414 and named as folic acid. Pure crystalline form of folic acid was synthesized in 1943 combining a pteridine ring, paraminobenzoic acid and glutamic acid together named as ‘pteroylglutamic acid’5. The term ‘folates’ refers to a large group of compounds including natural folates and folic acid which take part in one-carbon metabolism. The term ‘folic acid’ is in turn used to denote the fully oxidized compound which is hardly ever found in natural food items. Soon after its discovery, folic acid was observed to enhance the growth of cancer leading to the use of folate antagonist 4-aminopteroyl glutamic acid (aminopterin) in the treatment of childhood acute lymphoblastic leukaemia (ALL), thus establishing the link between folate metabolism and cancer for the first time6. Later in the 1990s, the inverse relationship between colon cancer incidence and folate intake was shown in clinical studies7. The beneficial role of folate fortification of food on the incidence of certain childhood cancers has also been reported8.
Dietary sources and bioavailability of folate
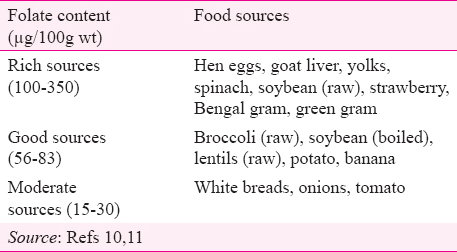
Folates are present in a wide variety of food items, though in a relatively low density, except in the liver9. Diets containing adequate amounts of fresh green vegetables are good folate sources10. Important food sources according to their folate content are shown in Table I. Although folate content is high in foods from animal sources, but cereals, pulses and green leafy vegetables (GLVs) constitute the major sources in vegetarian diets11.
The bioavailability of folic acid is determined mainly by two factors, dietary source of folates and host factors which are discussed below:
Food sources
Bioavailability of folates depends on pre-consumption processing of food and food products. Although folates from animal sources (e.g. beef) have been found to be stable even after prolonged cooking, the method and duration of cooking have marked effects on the folate retention of green vegetables. Accurate estimation of dietary folate availability is still a challenge, and studies designed to investigate the impact of different cooking methods and duration on folate content of food are limited12.
Host factors
Intestinal folic acid absorption occurs through a carrier-mediated process in the proximal small intestine which acts optimally at low pH. Genetic association studies have identified an intestinal folate transporter called the human proton-coupled folate transporter (SLC46A1). Mutations leading to loss of function of this transporter were associated with hereditary folate malabsorption13. Other diseases causing malabsorption including coeliac disease, Crohn's disease and ulcerative colitis were also found to be commonly associated with impaired folate absorption14.
Folate absorption and metabolism
After ingestion, many labile forms of folate get destroyed in the acidic environment of stomach in the absence of protective factors such as ascorbic acid or thiols. The dietary folate which is mostly in the form of polyglutamate has to be reduced to absorbable monoglutamates, a reaction catalyzed by folate conjugase, a rate limiting step in folate absorption. Passive diffusion of folates also occurs in the intestine but only with very high doses15. Majority of folate is absorbed in the duodenum and jejunum and a small amount from the colon. The absorbed monoglutamates are taken up by the liver and reconverted to polyglutamates for storage in the liver itself or release into the blood. Liver stores about half of the total body folate, a part of which is secreted into the bile and undergoes enterohepatic circulation. Most of this is reabsorbed, ‘supposedly to moderate between-meal fluctuations’ in serum folate levels16. In the plasma, folate is bound primarily to albumin and transported to the cells through a number of folate transport systems11.
Folate in one-carbon metabolism and its genetic regulation
A simplified account of folate metabolism, its role in nucleotide (purine/pyrimidine) synthesis, conversion of homocysteine to methionine and methylation of DNA is shown in Fig. 1. The relationship between folate levels and risk of cancer and response to chemotherapy is variably modified by the polymorphisms in key folate metabolizing enzymes.
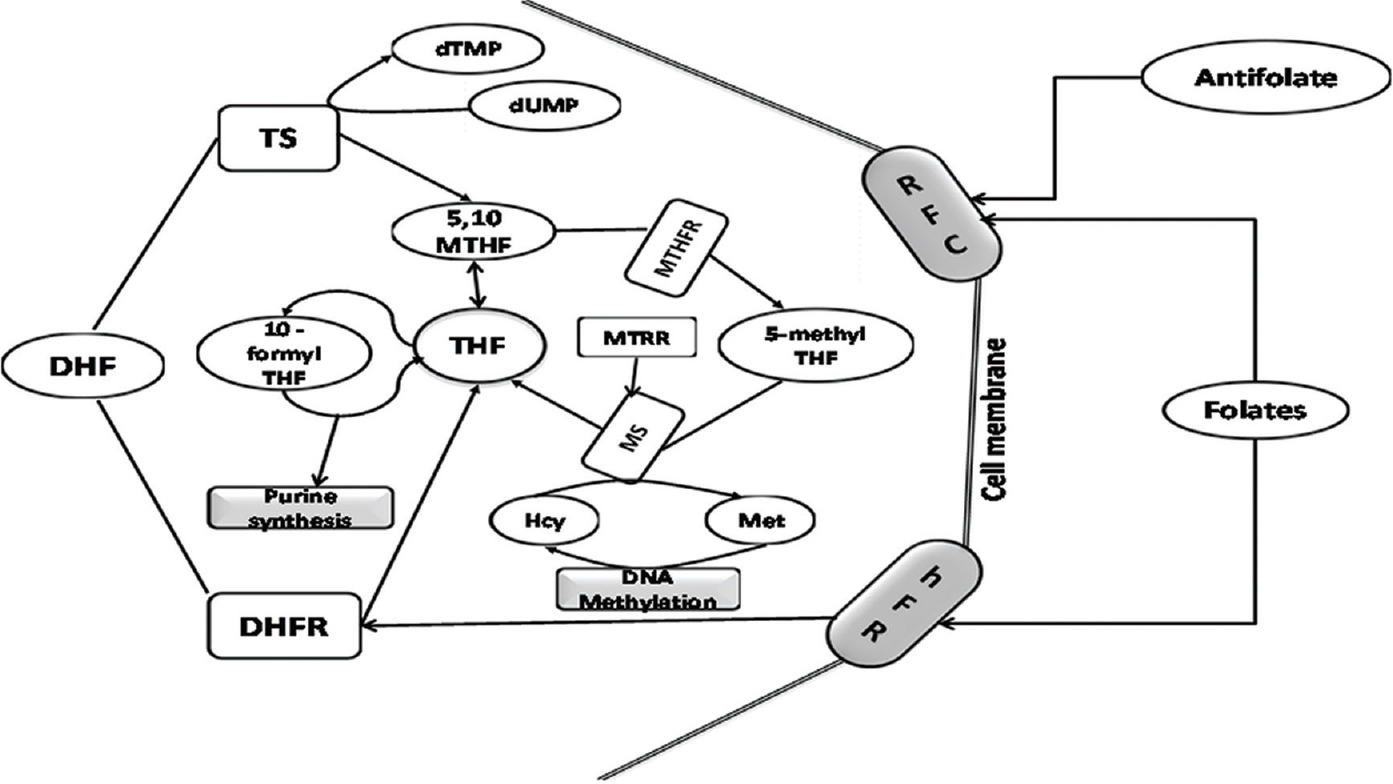
- Simplified scheme for one-carbon metabolism. THF, tetrahydrofolate; DHF, dihydrofolate; RFC, reduced folate carrier; hFR, human folate receptor; MTHFR, 5,10-methylenetetrahydrofolate reductase; DHFR, dihydrofolate reductase; Met, methionine; Hcy, homocysteine; SHMT, serine-hydroxy-methyltransferase; MS, methionine synthase; TS, thymidylate synthase; MT, methyltransferases (enzymes of this pathway are depicted as unshaded boxes, substrates are shown in unshaded ovoid shapes whereas shaded boxes represent biosynthetic/biochemical pathways and shaded cylinders stand for membrane receptors/transporters).
Alteration in folate metabolism can occur due to altered activity/availability of folate pathway enzymes, which in turn depends on the polymorphisms in their coding genes. These polymorphisms result in decreased folate availability at the site of reaction, leading to hyperhomocysteinaemia and modulate the risk of certain cancers17 through epigenetic influences such as DNA methylation, uracil misincorporation and altered purine synthesis (vide section on folate and carcinogenesis). The toxicity of antifolate agents used in the treatment of cancer is also influenced by the alteration of genes encoding the proteins of the folate pathway, namely the carrier protein reduced folate carrier (RFC) and enzymes such as thymidylate synthase (TS), 5,10-methylenetetrahydrofolate reductase (MTHFR), methionine synthase reductase (MTRR) and methionine synthase (MS)1718192021.
Reduced folate carrier (RFC)
Folate being a water soluble vitamin is highly lipophobic and hardly crosses the plasma membrane by passive diffusion22. Reduced-folate carrier (RFC; additionally known as RFC-1, FOLT, RFT-1 or SLC19A1) is a 60 KDa transport protein with 12 membrane-spanning domains involved in transportation of reduced folates as well as classical antifolates such as methotrexate (MTX) into the cell2324. RFC is located in brush-border membrane of small intestine, colon, basolateral membrane of the renal tubular epithelium, hepatocytes and the retinal pigment epithelium25.
Loss of RFC expression or function may have important implications in cancer biology, including response to antifolates24. Among nearly seven single-nucleotide polymorphisms (SNPs) in its encoding gene, only 80G→A translocation is significant as it causes amino acid sequence alteration, whereas others are silent26. This polymorphism alone or in combination with others in the folate pathway may significantly alter folate and homocysteine status27. Reduced expression of RFC has been associated with inferior outcome in childhood ALL probably due to intrinsic MTX resistance in these children2829.
Thymidylate synthase (TS)
TS, catalyzing the methylation of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP), is one of the key enzymes in the de novo synthesis of dTMP, an essential precursor of DNA30. Being a rate limiting step in DNA synthesis, it has been used as a potential target of many anticancer drugs. The promoter enhancer region of the TS gene contains polymorphism of a double (2R) or triple (3R) 28-base pairs (bp) tandem repeat31. The triple repeat results in increased TS gene expression, whereas the double repeat is associated with decreased TS gene expression. Increased synthesis of dTMP due to higher expression of TS enzyme reduces rate of uracil misincorporation, thereby protecting against oncogenesis31. The 3R polymorphism of the promoter region has been shown to be protective for adult ALL and lymphoma3233 and improves the outcome of ALL in children34, whereas the 2R polymorphism has been associated with poor outcome in childhood ALL34.
5,10-methylenetetrahydrofolate reductase (MTHFR)
MTHFR, one of the most well-studied enzymes in the folate pathway, is a 77 kDa protein encoded by a gene located on the short arm of chromosome 1 (1p36.3)35. It catalyzes the step producing 5-methyltetrahydrofolate (5-MTHF), the circulating form of folic acid which is also a methyl donor for the conversion of homocysteine to methionine36 (Fig. 1). Methionine, when converted back to homocysteine, causes methylation of DNA. SNPs in the MTHFR gene lead to alteration in enzyme activity by affecting its thermal stability or affinity towards coenzyme, thereby reducing 5-MTHF production. Although many SNPs in the MTHFR gene have been described, till date two of these (at loci 677 and 1298) have been found to be of clinical significance36.
C677T polymorphism
A common variant of MTHFR involves a cytosine (C) to thymine (T) transition at position 677 within exon 4 of the gene, resulting in an alanine to valine amino acid substitution in the protein, reducing MTHFR enzyme activity to 65 and 30 per cent of the CC genotype in the CT heterozygote and TT homozygote variants, respectively37. Similar findings have also been documented in a meta-analysis where folate levels were found to significantly differ across 677 genotypes (CC>CT>TT), the difference being most significant between the wild-mutant (CC) and homozygous-mutant (TT) variants38. This untoward effect of a mutated genotype can be circumvented by adequate dietary folate39. However, in the absence of sufficient folic acid availability, intracellular homocysteine accumulates, methionine resynthesis is decreased and essential methylation reactions are hampered40.
A1298C polymorphism
An A-to-C transition at locus 1298 within exon 7 results in a change from amino acid glutamate to alanine, reducing MTHFR activity to approximately 60 per cent of the wild state in the homozygous mutated state. The effect of polymorphism at 1298 locus is less pronounced than that at the 677 locus. The enzyme activity is further reduced in the compound heterozygote state (677CT and 1298AC) than in either of the two mutations alone4142.
MTHFR gene variants have been proposed to modulate risk of childhood cancer. Most of the case-control studies investigating the association between MTHFR polymorphisms and childhood ALL have reported protective effect of 677T allele4344 whereas some of these reported no effect45 and some showed increased risk46. 1298C allele was however, not found to have any effect on the risk of childhood ALL4246, except in a few studies which reported reduced risk45. These disagreements have been attributed to lack of adequate power47 of the individual studies due to inadequate sample size. In an attempt to circumvent this problem, many meta-analyses4748495051525354 have been performed (Table II). Though most of these meta-analyses reported a protective effect of 677T variant on childhood ALL, it was significant in only three of these505254. When the studies were grouped according to the local folate fortification guidelines, the protective effect was only seen in population covered under mandatory food fortification by folic acid further highlighting the importance of gene-environment interaction, where availability of folic acid enhances the benefit of a favourable genotype54. Conversely, the 1298C variant was not seen to alter the risk of childhood ALL in all these meta-analyses except two which showed it to marginally increase the risk5054 (Table II).
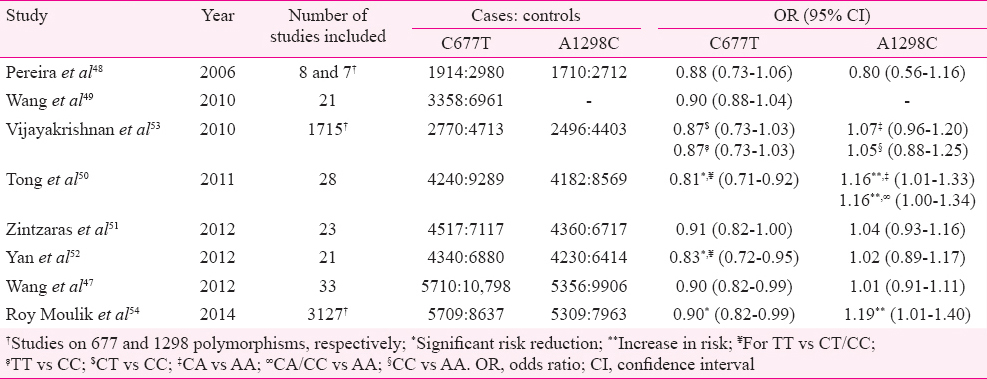
The protection conferred by 677T allele may be due to inhibition of hypermethylation of CpG islands leading to increased expression of certain tumour suppressor genes conferring protection against leukemogenesis54. The lack of association between 1298C and risk of childhood ALL may be largely due to the less significant effect of this polymorphism on the MTHFR enzyme level54.
5,10-methylenetetrahydrofolate reductase (MTHFR) polymorphisms, outcome and toxicity of cancer chemotherapy
Polymorphisms in MTHFR (C677T and T677T) have been reported to increase relapse rate of childhood ALL when controlled for other risk factors55. MTHFR677TT genotype has been associated with higher MTX toxicity as compared to others56. Children having genotypes with reduced enzyme activity were found to be at enhanced risk of thrombocytopenia and deranged creatinine in response to high-dose MTX in a study; MTX toxicity was considerably ameliorated when the doses were adjusted depending on MTHFR genotype57. An increased risk of hepatic, bone marrow, mucocutaneous toxicity with MTHFR677CT genotype and a decreased risk of skin toxicity of MTX with MTHFR1298AC genotype were also reported in a meta-analysis58, but these findings did not compare favourably with another meta-analysis59 which failed to show any role of genotype on the severity of MTX toxicity. Though the adverse effects of MTHFR polymorphism on the toxicity of MTX and the outcome of childhood ALL are most likely due to alteration in the folate metabolism, none of these studies accounted for the folate levels of the patients. It is possible that the differences in clinical outcomes could be due to the interaction with other genes as well as nutrients and not the effect of the MTHFR genotype alone.
Methionine synthase reductase (MTRR), methionine synthase (MS)
Synthesis of methionine by methylation of homocysteine is mediated by two enzymes MTRR and MS which are located on chromosomes 5p15.3-p15.2 and 1q43, respectively. MS is maintained in its active form by MTRR60. The A→G polymorphism at locus 2756 in the protein binding region of MS substitutes aspartate with glycine61. Polymorphism in this gene may cause elevation of homocysteine, but its significance and effect on folate status are uncertain due to conflicting reports6263.
The A66G polymorphism in the MTRR gene leads to substitution of isoleucine with methionine at codon 2260. Individuals homozygous for the common allele (AA) had higher homocysteine levels compared to those with other genotypes6263; its effect on folate status is also not clear. In adults, MS and MTRR polymorphisms have been shown to be associated with a reduced risk for ALL64; however, paediatric data are lacking20.
Folate - its dual role in cancer
The role of folate in cancer is paradoxical65. Studies, mostly based on laboratory models, show that folate supplementation protects against certain cancers while hastening the progression of some pre-malignant lesions6566. Folate is critically important for cell division because of its role in de novo purine and pyrimidine synthesis and also in the DNA repair mechanism. Cancer cells need more substrates for DNA synthesis due to their rapid turnover and thus have higher folate requirement. Folate deficient states or blockade of folate metabolism by antifolates lead to arrest of cell proliferation. Paradoxically, folate supplementation was found to be protective against development of certain cancers due to its crucial role in maintaining the genomic integrity67. Folate deficiency may lead to DNA strand breaks, impaired repair, increased mutations and abnormal methylation of DNA, leading to carcinogenesis6668. The proposed paradoxical role of folate in oncogenesis269 is depicted in Fig. 2. Though much light on this issue has been shed by epigenetic studies in animals, extrapolation to human system is subject to usual caveats more so due to conflicting evidence from randomized studies in human beings27071.
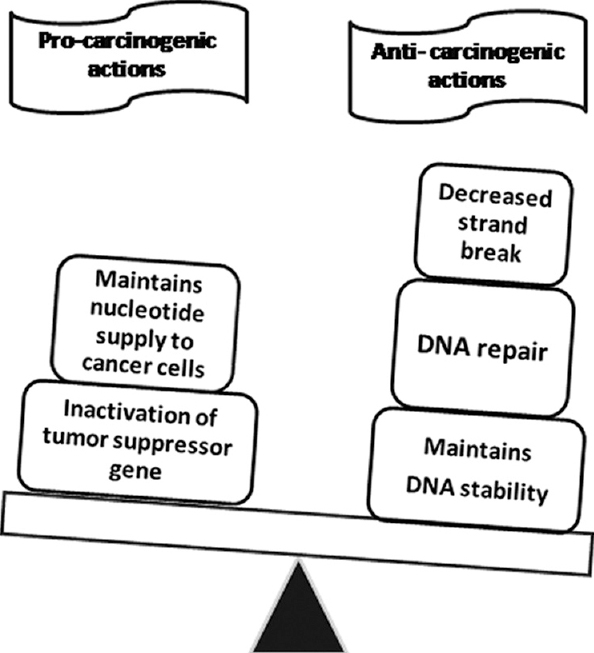
- Proposed dual role of folate in oncogenesis. Source: Refs 2,69
Folic acid fortification of food - its impact on paediatric cancers
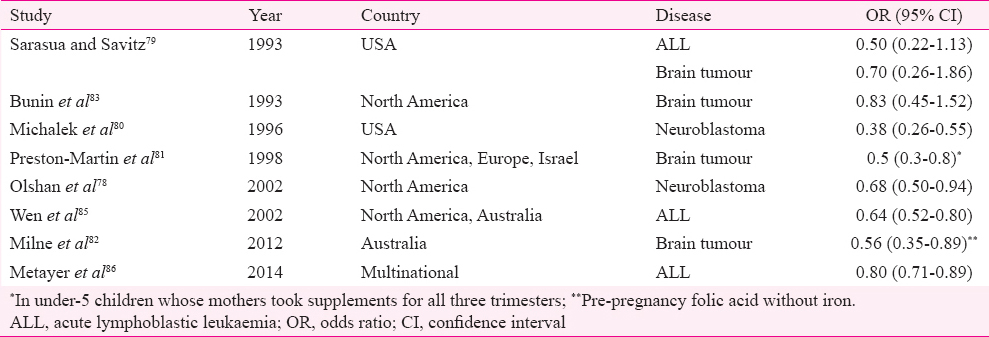
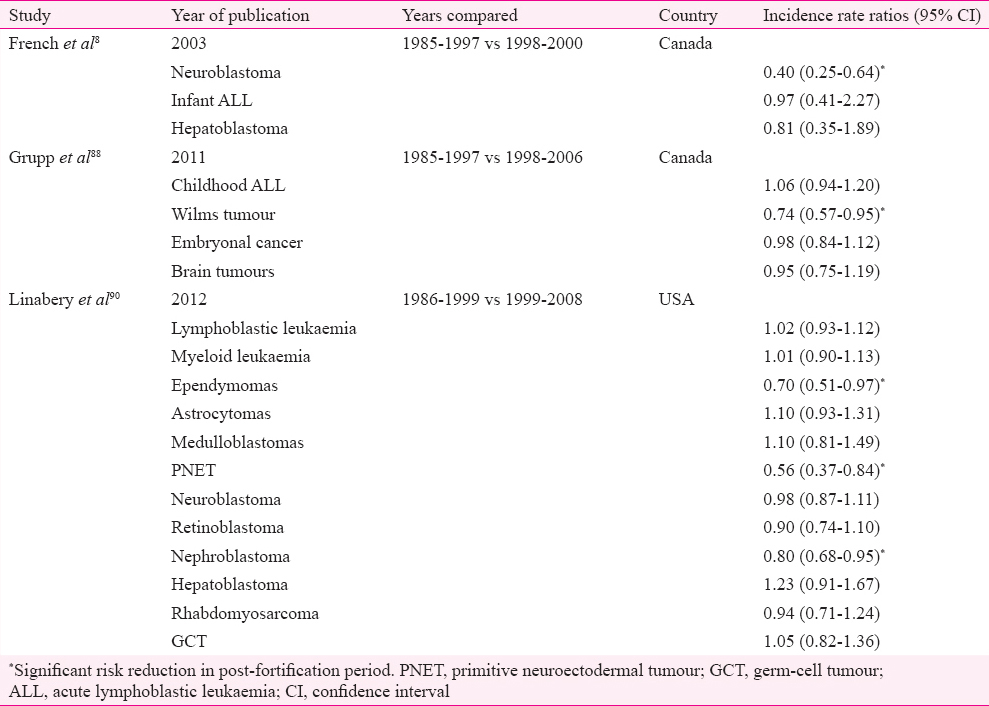
In response to the overwhelming evidence in favour of periconceptional supplementation of folic acid in preventing serious birth defects such as neural tube defect (NTD)272, the United States and Canada initiated mandatory fortification of food products with folic acid from mid-1990s7374. Though this trend was followed by many other countries7576, more than 70 per cent of the world population remains uncovered as many countries including those under the European Union opted against fortification due to safety concerns. The concerns were acceleration of cognitive decline with age and reduction of the efficacy of antifolate drugs such as MTX used in cancer chemotherapy/rheumatic disorders and antiepileptics. The dual role of folates in cancer was another important concern77. While being remarkably successful in achieving its primary objective of reducing NTDs by 50 per cent78, mandatory fortification also provided an opportunity to assess unrelated effects of folates in otherwise healthy population without any ethical concerns. As a sequel to this, many studies were conducted to compare the incidence of childhood cancers between the pre- and post-fortification periods. Even before post-fortification data were available, many case-control studies indicated a possible protective role of maternal supplementation with certain vitamins (mostly vitamins B and folic acid) for childhood cancers such as ALL7779, neuroblastoma7880, brain tumours818283, germ cell tumour84 and primitive neuroectodermal tumours83 (Table III). This observation was further strengthened by a meta-analysis, wherein maternal ingestion of prenatal multivitamins was associated with decreased risk for paediatric brain tumours, leukaemia and neuroblastoma, without any specifications as to which component of multivitamins was responsible for this87. Another case-control study involving a large number of childhood leukaemia cases documented a protective role of folic acid on childhood leukaemia86. A decline in incidence rate ratios of many childhood cancers was demonstrated by a number of studies done in the post-fortification era with inconsistent findings between studies885888990 (Table IV). The mechanism through which cancer risk in the offspring is modulated by maternal folic acid supplementation is speculative and is hypothesized to be similar to its role in adult cancers87 despite the fact that origin of cancer in children and adults is not identical. This rapidly growing body of knowledge is yet to generate definite evidence in favour of folic acid in the absence of randomized trials in children87; though many randomized studies on folate supplementation in adults exist, showing conflicting results7071; therefore, the facts from the post-fortification cohort need to be interpreted with caution before more long-term data are available.
Folates and children on cancer chemotherapy
Folate deficiency is known to manifest as disordered haematopoiesis and bone marrow dysfunction, similar to that produced by the antifolate chemotherapeutic agents in haematological malignancies, by limiting intracellular folate availability and blockade of folate dependent one-carbon metabolism. Thus, MTX forms an important part of chemotherapeutic protocols for childhood ALL and non-Hodgkin's lymphoma5556. Non-antifolate chemotherapeutic agents also cause variable degree of myelosuppression. Bone marrow recovery following chemotherapy is at least partly dependent on the folate status of the patients; therefore, folate supplementation may be an effective intervention to reduce the myelotoxicity of chemotherapeutic agents91. However, the idea of recommending folate supplementation to patients on cancer chemotherapy needs exploration as it has not been adequately studied91 probably due to the concern that folates may interfere with the efficacy of antifolate agents and thus support tumour growth, similar to observations made in studies on clinical response of patients on MTX for rheumatic disorders92. Excess of folates may also lead to resistance to antifolates; higher doses of folinic acid rescue following high-dose MTX have also been correlated with increased chances of relapse in paediatric ALL93.
Though the non-malignant cells are rescued from the toxicity of high-dose MTX by folinic acid, in the absence of any such rescue, the normal cells have to rely on the intrinsic folate status to recover following lower doses of MTX, which might vary among individuals as well as between populations due to dietary differences as well as differences in folate fortification recommendations across regions. Therefore, promoting folate restriction uniformly to all patients on antifolates needs to be carefully reconsidered. This is more relevant for the developing countries as folate deficiency might be one of the contributors for the higher incidence of toxic deaths during chemotherapy seen in these countries91. More studies on this aspect from various regions of the world are warranted to arrive at a proper consensus guideline91.
Folate status of Indian children with cancer
Though nutritional anaemia is common in Indian children stemming from nutritional deficiencies of iron, folic acid and vitamin B12, Indian data on prevalence of folate deficiency in children are incomplete due to lack of national data or multicentric surveys. Most of the surveys conducted have been limited to children in large cities. In a study on toddlers and preschool children from Delhi, about 15 per cent children were found to be having folate deficiency94 whereas another study from the same region showed over 40 per cent children between five and 11 yr and nearly one-third of those between 12 and 18 yr to be folate deficient95. A study on urban healthy children from southern India found folate deficiency in nearly all the children studied96.
With the available data on folate deficiency in Indian children, the documented prevalence has been clearly higher than in children from many developed countries such as the USA or European countries according to a review comparing folate and vitamin B12 deficiencies across the globe97. Deficiency of folate in healthy Indian children continues to be a common problem due to lack of dietary folate, unlike in developed countries where it has significantly declined after initiation of food fortification.
The data on folate status of Indian children with cancer are also sparse. A case-control study from southern India demonstrated significantly lower levels of folate in children with ALL98. Two more studies from north India also showed a high baseline prevalence of folate deficiency in children with newly diagnosed ALL as well as decline in folate levels with chemotherapy99100. Furthermore, folate deficiency in these children was associated with adverse outcome during their initial phases of treatment99100.
Future directions
Recent evidence suggests an important role of folates in relation to cancer in both adults and children. Epidemiologic data from countries with mandatory folic acid fortification of food recording decline in incidence of certain paediatric cancers need to be confirmed to generate robust evidence in favour of fortification. Therefore, more epidemiological and basic research is warranted in this field to establish the role of folic acid in cancer prevention, epidemiologically and mechanistically, respectively. In addition, more studies are needed to elucidate the association between folate deficiency and toxicities encountered in children undergoing cancer chemotherapy taking into consideration nutrition and other related confounders. Based on such findings, randomized trials assessing the efficacy and safety of folic acid supplementation in folate deficient children undergoing chemotherapy are needed to generate convincing evidence. This is particularly important for population with high prevalence of folate deficiency as in India where folic acid fortification is not mandatory.
Acknowledgment
The first author (NRM) was supported by a post-doctoral fellowship by the Indian Council of Medical Research (ICMR), New Delhi. This work was carried out as part of an ICMR sponsored MD-PhD project at the King George's Medical University, Lucknow, India.
Conflicts of Interest: None.
References
- Physiology of folate and Vitamin B12 in health and disease. Nutr Rev. 2004;62(6 Pt 2):S3-12.
- [Google Scholar]
- Nutrition Classics. British Medical Journal 1:1059-64, 1931. Treatment of “pernicious anaemia of pregnancy” and “tropical anaemia” with special reference to yeast extract as a curative agent. By Lucy Wills. Nutr Rev. 1978;36:149-51.
- [Google Scholar]
- Journal of the American Chemical Society, Vol 63, 1941: The concentration of “folic acid” by Herschel K. Mitchell, Esmond E. Snell, and Roger J. Williams. Nutr Rev. 1988;46:324-5.
- [Google Scholar]
- Synthesis of a compound identical with the L. casei factor isolated from liver. Science. 1945;102:227-8.
- [Google Scholar]
- Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med. 1948;238:787-93.
- [Google Scholar]
- Multivitamin use, folate, and colon cancer in women in the Nurses’ Health Study. Ann Intern Med. 1998;129:517-24.
- [Google Scholar]
- Folic acid food fortification is associated with a decline in neuroblastoma. Clin Pharmacol Ther. 2003;74:288-94.
- [Google Scholar]
- The megaloblastic anaemias (3rd ed). Chicago, IL: Blackwell Scientific Publications; 1990.
- Folate content and retention in selected raw and processed foods. Arch Latinoam Nutr. 2010;60:298-305.
- [Google Scholar]
- Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127:917-28.
- [Google Scholar]
- Folate deficiency in chronic inflammatory bowel diseases. Scand J Gastroenterol. 1979;14:1019-24.
- [Google Scholar]
- Membrane transporters and folate homeostasis: Intestinal absorption and transport into systemic compartments and tissues. Expert Rev Mol Med. 2009;11:e4.
- [Google Scholar]
- 5, 10-Methylenetetrahydrofolate reductase polymorphisms and leukemia risk: A HuGE minireview. Am J Epidemiol. 2003;157:571-82.
- [Google Scholar]
- Folate cycle gene variants and chemotherapy toxicity in pediatric patients with acute lymphoblastic leukemia. Haematologica. 2006;91:1113-6.
- [Google Scholar]
- Genetic polymorphisms associated with adverse events and elimination of methotrexate in childhood acute lymphoblastic leukemia and malignant lymphoma. J Hum Genet. 2007;52:166-71.
- [Google Scholar]
- Gene-gene interactions in the folate metabolic pathway influence the risk for acute lymphoblastic leukemia in children. Leuk Lymphoma. 2007;48:786-92.
- [Google Scholar]
- Functional loss of the reduced folate carrier enhances the antitumor activities of novel antifolates with selective uptake by the proton-coupled folate transporter. Mol Pharmacol. 2012;82:591-600.
- [Google Scholar]
- Interaction between anions and the reduced folate/methotrexate transport system in L1210 cell plasma membrane vesicles: Directional symmetry and anion specificity for differential mobility of loaded and unloaded carrier. J Membr Biol. 1984;79:285-92.
- [Google Scholar]
- Reduced-folate carrier (RFC) is expressed in placenta and yolk sac, as well as in cells of the developing forebrain, hindbrain, neural tube, craniofacial region, eye, limb buds and heart. BMC Dev Biol. 2003;3:6.
- [Google Scholar]
- Human reduced folate carrier: Translation of basic biology to cancer etiology and therapy. Cancer Metastasis Rev. 2007;26:111-28.
- [Google Scholar]
- Expression and differential polarization of the reduced-folate transporter-1 and the folate receptor alpha in mammalian retinal pigment epithelium. J Biol Chem. 2000;275:20676-84.
- [Google Scholar]
- Single nucleotide polymorphisms in the human reduced folate carrier: Characterization of a high-frequency G/A variant at position 80 and transport properties of the His(27) and Arg(27) carriers. Clin Cancer Res. 2001;7:3416-22.
- [Google Scholar]
- A polymorphism (80G->A) in the reduced folate carrier gene and its associations with folate status and homocysteinemia. Mol Genet Metab. 2000;70:310-5.
- [Google Scholar]
- Reduced folate carrier and dihydrofolate reductase expression in acute lymphocytic leukemia may predict outcome: A Children's Cancer Group Study. J Pediatr Hematol Oncol. 2003;25:688-95.
- [Google Scholar]
- Polymorphism G80A in the reduced folate carrier gene and its relationship to methotrexate plasma levels and outcome of childhood acute lymphoblastic leukemia. Blood. 2002;100:3832-4.
- [Google Scholar]
- Mechanism of interaction of thymidylate synthetase with 5-fluorodeoxyuridylate. Biochemistry. 1974;13:471-81.
- [Google Scholar]
- Functional analysis and DNA polymorphism of the tandemly repeated sequences in the 5’-terminal regulatory region of the human gene for thymidylate synthase. Cell Struct Funct. 1995;20:191-7.
- [Google Scholar]
- Polymorphisms in the thymidylate synthase and serine hydroxymethyltransferase genes and risk of adult acute lymphocytic leukemia. Blood. 2002;99:3786-91.
- [Google Scholar]
- Associations between polymorphisms in the thymidylate synthase and serine hydroxymethyltransferase genes and susceptibility to malignant lymphoma. Haematologica. 2003;88:159-66.
- [Google Scholar]
- Polymorphism of the thymidylate synthase gene and outcome of acute lymphoblastic leukaemia. Lancet. 2002;359:1033-4.
- [Google Scholar]
- Human methylenetetrahydrofolate reductase: Isolation of cDNA, mapping and mutation identification. Nat Genet. 1994;7:195-200.
- [Google Scholar]
- Characterization of mutations in severe methylenetetrahydrofolate reductase deficiency reveals an FAD-responsive mutation. Hum Mutat. 2003;21:509-20.
- [Google Scholar]
- Abnormal folate metabolism and mutation in the methylenetetrahydrofolate reductase gene may be maternal risk factors for Down syndrome. Am J Clin Nutr. 1999;70:495-501.
- [Google Scholar]
- Assessing the association between the methylenetetrahydrofolate reductase (MTHFR) 677C>T polymorphism and blood folate concentrations: A systematic review and meta-analysis of trials and observational studies. Am J Clin Nutr. 2015;101:1286-94.
- [Google Scholar]
- The relation between erythrocyte volume and folate levels is influenced by a common mutation in the methylenetetrahydrofolate reductase (MTHFR) gene (C677T) J Investig Med. 2000;48:14-20.
- [Google Scholar]
- A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111-3.
- [Google Scholar]
- A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med (Berl). 2001;79:522-8.
- [Google Scholar]
- Methylenetetrahydrofolate reductase (MTHFR) polymorphisms and risk of molecularly defined subtypes of childhood acute leukemia. Proc Natl Acad Sci U S A. 2001;98:4004-9.
- [Google Scholar]
- Role of MTHFR genetic polymorphisms in the susceptibility to childhood acute lymphoblastic leukemia. Blood. 2004;103:252-7.
- [Google Scholar]
- The methylenetetrahydrofolate reductase C677T gene polymorphism decreases the risk of childhood acute lymphocytic leukaemia. Br J Haematol. 2001;115:616-8.
- [Google Scholar]
- Genetic variation in the folate metabolic pathway and risk of childhood leukemia. Blood. 2010;115:3923-9.
- [Google Scholar]
- Methylenetetrahydrofolate reductase gene polymorphisms: Association with risk for pediatric acute lymphoblastic leukemia in north Indians. Leuk Lymphoma. 2010;51:928-32.
- [Google Scholar]
- Methylenetetrahydrofolate reductase polymorphisms and risk of acute lymphoblastic leukemia-evidence from an updated meta-analysis including 35 studies. BMC Med Genet. 2012;13:77.
- [Google Scholar]
- 5,10-Methylenetetrahydrofolate reductase polymorphisms and acute lymphoblastic leukemia risk: A meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1956-63.
- [Google Scholar]
- MTHFR C677T polymorphisms and childhood acute lymphoblastic leukemia: A meta-analysis. Leuk Res. 2010;34:1596-600.
- [Google Scholar]
- Methylenetetrahydrofolate reductase gene polymorphisms and acute lymphoblastic leukemia risk: A meta-analysis based on 28 case-control studies. Leuk Lymphoma. 2011;52:1949-60.
- [Google Scholar]
- Variants of the MTHFR gene and susceptibility to acute lymphoblastic leukemia in children: A synthesis of genetic association studies. Cancer Epidemiol. 2012;36:169-76.
- [Google Scholar]
- A meta-analysis of MTHFR C677T and A1298C polymorphisms and risk of acute lymphoblastic leukemia in children. Pediatr Blood Cancer. 2012;58:513-8.
- [Google Scholar]
- Candidate gene association studies and risk of childhood acute lymphoblastic leukemia: A systematic review and meta-analysis. Haematologica. 2010;95:1405-14.
- [Google Scholar]
- MTHFR gene polymorphism in acute lymphoblastic leukemia among North Indian children: A case-control study and meta-analysis updated from 2011. J Hum Genet. 2014;59:397-404.
- [Google Scholar]
- Germline genetic variation and treatment response on CCG-1891. Pediatr Blood Cancer. 2012;58:695-700.
- [Google Scholar]
- Methylenetetrahydrofolate reductase C677T and A1298C gene polymorphisms and therapy-related toxicity in children treated for acute lymphoblastic leukemia and non-Hodgkin lymphoma. Leuk Lymphoma. 2009;50:912-7.
- [Google Scholar]
- Methotrexate consolidation treatment according to pharmacogenetics of MTHFR ameliorates event-free survival in childhood acute lymphoblastic leukaemia. Pharmacogenomics J. 2012;12:379-85.
- [Google Scholar]
- Impact of methylenetetrahydrofolate reductase (MTHFR) polymorphisms on methotrexate-induced toxicities in acute lymphoblastic leukemia: A meta-analysis. Tumour Biol. 2012;33:1445-54.
- [Google Scholar]
- A systematic review and meta-analysis of MTHFR polymorphisms in methotrexate toxicity prediction in pediatric acute lymphoblastic leukemia. Pharmacogenomics J. 2013;13:498-506.
- [Google Scholar]
- Human methionine synthase: cDNA cloning and identification of mutations in patients of the cblG complementation group of folate/cobalamin disorders. Hum Mol Genet. 1996;5:1867-74.
- [Google Scholar]
- Sequence analysis of the coding region of human methionine synthase: Relevance to hyperhomocysteinaemia in neural-tube defects and vascular disease. QJM. 1997;90:511-7.
- [Google Scholar]
- Influence of a methionine synthase (D919G) polymorphism on plasma homocysteine and folate levels and relation to risk of myocardial infarction. Atherosclerosis. 2001;154:667-72.
- [Google Scholar]
- Methionine synthase D919G polymorphism is a significant but modest determinant of circulating homocysteine concentrations. Genet Epidemiol. 1999;17:298-309.
- [Google Scholar]
- Common gene polymorphisms in the metabolic folate and methylation pathway and the risk of acute lymphoblastic leukemia and non-Hodgkin's lymphoma in adults. Cancer Epidemiol Biomarkers Prev. 2004;13:787-94.
- [Google Scholar]
- Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr. 2004;80:1123-8.
- [Google Scholar]
- Role of folate in colon cancer development and progression. J Nutr. 2003;133(11 Suppl 1):3731S-9S.
- [Google Scholar]
- Folate and carcinogenesis: Evidence, mechanisms, and implications. J Nutr Biochem. 1999;10:66-88.
- [Google Scholar]
- Folate: A magic bullet or a double edged sword for colorectal cancer prevention? Gut. 2006;55:1387-9.
- [Google Scholar]
- Folic acid supplementation and cancer risk: A meta-analysis of randomized controlled trials. Int J Cancer. 2013;133:1033-41.
- [Google Scholar]
- Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: Meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-36.
- [Google Scholar]
- Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832-5.
- [Google Scholar]
- Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338:131-7.
- [Google Scholar]
- Motherisk alert: Folic acid fortification of flour – Three years later. Can J Clin Pharmacol. 2001;8:91-2.
- [Google Scholar]
- Folic acid and human reproduction-ten important issues for clinicians. J Exp Clin Assist Reprod. 2011;8:2.
- [Google Scholar]
- Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: A children's oncology group study. J Clin Oncol. 2009;27:5175-81.
- [Google Scholar]
- Maternal folate supplementation in pregnancy and protection against acute lymphoblastic leukaemia in childhood: A case-control study. Lancet. 2001;358:1935-40.
- [Google Scholar]
- Maternal vitamin use and reduced risk of neuroblastoma. Epidemiology. 2002;13:575-80.
- [Google Scholar]
- Cured and broiled meat consumption in relation to childhood cancer: Denver, Colorado (United States) Cancer Causes Control. 1994;5:141-8.
- [Google Scholar]
- Gravid health status, medication use, and risk of neuroblastoma. Am J Epidemiol. 1996;143:996-1001.
- [Google Scholar]
- Prenatal vitamin supplementation and risk of childhood brain tumors. Int J Cancer Suppl. 1998;11:17-22.
- [Google Scholar]
- Maternal use of folic acid and other supplements and risk of childhood brain tumors. Cancer Epidemiol Biomarkers Prev. 2012;21:1933-41.
- [Google Scholar]
- Relation between maternal diet and subsequent primitive neuroectodermal brain tumors in young children. N Engl J Med. 1993;329:536-41.
- [Google Scholar]
- Pediatric germ cell tumors and maternal vitamin supplementation: A Children's Oncology Group study. Cancer Epidemiol Biomarkers Prev. 2009;18:2661-4.
- [Google Scholar]
- Parental medication use and risk of childhood acute lymphoblastic leukemia. Cancer. 2002;95:1786-94.
- [Google Scholar]
- Maternal supplementation with folic acid and other vitamins and risk of leukemia in offspring: A Childhood Leukemia International Consortium study. Epidemiology. 2014;25:811-22.
- [Google Scholar]
- Prenatal multivitamin supplementation and rates of pediatric cancers: A meta-analysis. Clin Pharmacol Ther. 2007;81:685-91.
- [Google Scholar]
- Pediatric cancer rates after universal folic acid flour fortification in Ontario. J Clin Pharmacol. 2011;51:60-5.
- [Google Scholar]
- National Cancer Institute. SEER Cancer Statistics Review 1975-2014. Available from: https://seer.cancer.gov/csr/1975_2014/results_merged/sect_28_childhood_cancer.pdf
- [Google Scholar]
- Childhood cancer incidence trends in association with US folic acid fortification (1986-2008) Pediatrics. 2012;129:1125-33.
- [Google Scholar]
- Folate during antifolate chemotherapy: What we know… and do not know. Nutr Clin Pract. 2005;20:411-22.
- [Google Scholar]
- Reduction of the efficacy of methotrexate by the use of folic acid: Post-hoc analysis from two randomized controlled studies. Arthritis Rheum. 2005;52:3030-8.
- [Google Scholar]
- Nordic Society of Paediatric Haematology and Oncology (NOPHO). High leucovorin doses during high-dose methotrexate treatment may reduce the cure rate in childhood acute lymphoblastic leukemia. Leukemia. 2006;20:1955-62.
- [Google Scholar]
- Cobalamin and folate status in infants and young children in a low-to-middle income community in India. Am J Clin Nutr. 2007;86:1302-9.
- [Google Scholar]
- Prevalence of ferritin, folate and vitamin B12 deficiencies amongst children in 5-18 years of age in Delhi. Indian J Pediatr. 2014;81:312.
- [Google Scholar]
- Effect of micronutrient supplement on health and nutritional status of schoolchildren: Biochemical status. Nutrition. 2006;22(1 Suppl):S15-25.
- [Google Scholar]
- Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr Bull. 2008;29(2 Suppl):S38-51.
- [Google Scholar]
- Association between plasma homocysteine and riboflavin status in Acute Lymphoblastic Leukemia in children. Indian J Clin Biochem. 2009;24:257-61.
- [Google Scholar]
- Role of folate status and methylenetetrahydrofolate reductase genotype on the toxicity and outcome of induction chemotherapy in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2015;56:1379-84.
- [Google Scholar]
- Effect of pre-treatment nutritional status, folate and Vitamin B12 levels on induction chemotherapy in children with acute lymphoblastic leukemia. Indian Pediatr. 2015;52:385-9.
- [Google Scholar]






