Translate this page into:
Foetal stem cell derivation & characterization for osteogenic lineage
Reprint requests: Dr A. Mangala Gowri, Professor, Department of Animal Biotechnology, Madras Veterinary College, Vepery, Chennai 600 007, India e-mail: gowrivalavan@hotmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Mesencymal stem cells (MSCs) derived from foetal tissues present a multipotent progenitor cell source for application in tissue engineering and regenerative medicine. The present study was carried out to derive foetal mesenchymal stem cells from ovine source and analyze their differentiation to osteogenic linage to serve as an animal model to predict human applications.
Methods:
Isolation and culture of sheep foetal bone marrow cells were done and uniform clonally derived MSC population was collected. The cells were characterized using cytochemical, immunophenotyping, biochemical and molecular analyses. The cells with defined characteristics were differentiated into osteogenic lineages and analysis for differentiated cell types was done. The cells were analyzed for cell surface marker expression and the gene expression in undifferentiated and differentiated osteoblast was checked by reverse transcriptase PCR (RT PCR) analysis and confirmed by sequencing using genetic analyzer.
Results:
Ovine foetal samples were processed to obtain mononuclear (MNC) cells which on culture showed spindle morphology, a characteristic oval body with the flattened ends. MSC population CD45-/CD14- was cultured by limiting dilution to arrive at uniform spindle morphology cells and colony forming units. The cells were shown to be positive for surface markers such as CD44, CD54, integrinβ1, and intracellular collagen type I/III and fibronectin. The osteogenically induced MSCs were analyzed for alkaline phosphatase (ALP) activity and mineral deposition. The undifferentiated MSCs expressed RAB3B, candidate marker for stemness in MSCs. The osteogenically induced and uninduced MSCs expressed collagen type I and MMP13 gene in osteogenic induced cells.
Interpretation & conclusions:
The protocol for isolation of ovine foetal bone marrow derived MSCs was simple to perform, and the cultural method of obtaining pure spindle morphology cells was established. Criteria proposed for defining MSCs by this study includes the cell adherence to culture plates, specific surface protein profiles and differentiation to osteogenic lineage. The MSCs and osteogenic differentiated cells in this ovine animal model may serve as a large source for stem cell applications in regenerative medical therapies.
Keywords
Bone marrow
characterization
differentiation
foetal
MACS
mesenchymal stem cells
multilineage
multipotent
osteogenic
Stem cells are defined as immature cells capable of self replication and have intrinsic ability to generate various mature cell types that have remarkable viability and proliferative capacity. Stem cells in adult tissues, involved in tissue replacement and repair, usually give rise only to cell types already present in the surrounding tissue from which these are derived and hence are regarded as ‘multipotent’. Among all known adult stem or progenitor cells, cell populations from bone marrow (BM) have shown the highest potential for multilineage differentiation and functional engraftment into the host animal1.
Bone marrow mesenchymal stromal cells or marrow stromal cells (BMMSCs), are self renewing and expandable stem cells23 and are an ideal source of mesenchymal stem cells which exists as rare subset on non-haematopoietic cells in the bone marrow. These represent about 0.001 per cent of nucleated cells, about 10 fold less abundant than haematopoietic stem cells (HSC). MSCs are expandable in culture and are multipotent, capable of differentiating into several cell types3. These cells exhibit multilineage differentiation capacity, capable of giving rise to diverse tissues, including bone, cartilage, adipose tissue, tendon and muscle. MSCs seem to be reservoirs of reparative cells without tissue specific characteristics and are an ideal source for regeneration of damaged tissues in clinical application due to their ability to self-renew and differentiate into multiple tissues4.
To compare the study outcomes from different research groups, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) proposed minimal criteria to define human MSCs such as, plastic-adherence when maintained in standard culture conditions using tissue culture flasks, expression and lack of expression of specific surface antigens and lineage differentiation12. But the Society has not proposed any criteria for defining animal source MSCs and stated that there is a lack of definite markers. Hence, the present study was carried out with the objectives to isolate foetal mesenchymal stem cells showing specific defined characteristics from sheep (Ovine), a well known animal model for human for cell based engineering and therapies.
Material & methods
Sample collection: The sheep foetuses were collected aseptically from the abattoir (Chennai) and were transferred to the laboratory in sterile Dulbecco's phosphate buffered saline (DPBS) with antibiotics. The bone tissue collection and cell separation process was done as per the approved standard protocols of Instititutional Animal Ethics and Biosafety Committee (IAEC, 2007/10, 318/DFBS/ IBSC /2008). The study was carried out during 2007-2010 in the department of Animal Biotechnology, Madras Veterinary College, Chennai.
Culture isolation of bone marrow stromal cells: Isolation and culture of the MSCs were carried out as described earlier56 with minor modifications. The marrow aspirates were washed twice with phosphate buffered saline (PBS, pH 7.2). The mononuclear cells (MNC) were separated by Histopaque (Sigma, USA) density gradient centrifugation, and counted with nuclear stain (0.1% methyl violet). The cells were cultured in T75 flask (Nunc. Fisher Scientific, USA) at 37°C in a humidified atmosphere containing 5 per cent CO2. The floating haematopoietic cells were removed on observation carefully leaving adherent cells during 24 to 48 h of in vitro culture. The culture medium was changed after every 24 h daily until confluence was reached. The cells were subjected to limiting dilution and colonies reaching confluence were detached by 0.25 per cent trypsin (Gibco, USA) expanded in vitro and cells were used for Magnetic activated cell sorting (MACS)separation.
Magnetic activated cell sorting (MACS) separation: Cells from confluent plates were harvested using trypsin 0.25 per cent and spin down to pellet the cells. Primary antibodies (mouse anti CD45 and CD14 in the ratio of 1:1000 and 1:100, respectively) were added and incubated for 20 min. The cells were washed with PBS to remove unbound antibodies. To the cell pellet fluorescein isothiocyanate (FITC) conjugated secondary antibodies (Miletenyi Biotech Inc, Germany) in the ratio of 1:1000 dilution were added and incubated for 15 min, there were further incubated with MACS anti-FITC micro beads for 15 min and washed with PBS. The cells were resuspended in MACS buffer and passed through the MACS column placed in the magnetic field. The magnetically labelled antibody bound cells were retained and the unbound fraction containing CD45-/CD14- cells were collected aseptically. The sorted cells were cultured to confluency and passaged in T25 flask in the split ratio of 1:4. The third passage cells were used for analysis.
Immunophenotyping: When confluent, the cells were analyzed by incubating with FITC conjugated marker proteins CD45 and CD14 and CD44. The expression of these antigens was determined with flow cytometer (Becton & Dickinson, USA) as described7.
Cell cycle analysis: Cells (1×106) cells were aliquoted in each microfuge tube. The samples were spinned down at 252 × g for 10 min and the supernatant was removed as completely as possible without disturbing the pellet. DNA staining buffer (1 ml) (sodium citrate 0.25g, triton X100-0.75 ml, propidium iodide 1.0 mg/ml stock and ribonuclease A 0.005g) was added to the pellet and mixed well. The samples were incubated at 4°C and protected from light for 30 min before analysis on flow cytometer.
Characterization of cultured cells: The characteristics of pure adherent homogenous populations of cells obtained after culturing the MACS selected CD45-/CD14- cells were analyzed using MSC characterizing kit (Chemicon, USA). Cells were fixed in 4 per cent PBS buffered paraformaldehyde for 30 min and washed three times with PBS, then incubated with 5 per cent BSA and washed three times. The cells were incubated with primary antibodies (CD44), 1:500 (Invitrogen, USA), mouse anti-CD45, CD54 1;100, mouse anti-fibronectin 1:500, mouse anti-integrin β (CD29) and rabbit anti-collagen typeI/III 1:500 (Millipore, USA) were used for immunostaining. Mouse IgG and rabbit IgG were used as controls. Immunoreactivity was demonstrated for CD 45 and fibronectin antigens using horse radish peroxidase conjugate and 3’-3’-diaminobenzidine immunohistochemical staining kit (R&D Systems, Inc) followed by counterstaining with hematoxylin, dehydration. Immunoteactivity for collagen type I/III, CD44 and anti-integrin β (CD29) were analysed using anti-mouse anti-rabbit antibodies conjugated to FITC molecules for 1 h at room temperature in dark and examined after washing.
In vitro differentiation: The BMMSCs were subjected to osteogenic lineage differentiation for three weeks in standard osteogenic medium. Cells were enzymatically detatched from culture dishes and pelleted at a density of 50,000 cells /cm2 in 24 well culture plates. Once confluent, cells were stimulated with osteogenic medium (2×10-4M ascorbic acid, 7×10-3M β-glycerophosphate, 1×10-8M dexamethasone in DMEM with 10% FBS and 1% antibiotic/antimycotic solution). The differentiation profiles were recorded.
Cytochemical analysis of differentiation: Differentiation induced cells were fixed with 4 per cent para-formaldehyde (Sigma, USA) for 10 min at room temperature. Osteogenic differentiation induced MSCs were subjected to alkaline phosphatase staining following kit protocol (86 R Sigma-aldrich, USA).
In vitro mineralization: Cells were seeded at 5×103 in 96 well plates and incubated for 24 h at 37°C in a humidified, 5 per cent CO2 atmosphere in normal culture medium. The medium was replaced by differentiation medium and changed every alternate days for four weeks and analysed for mineralization by Von Kossa staining8 and Masson-Trichrome staining following the kit protocol (Sigma-aldrich, USA) for detecting collagen deposition. Experiment was repeated three times to confirm the changes on differentiation induction.
Alkaline phosphatase (ALP) activity in osteogenic cells: Cells were seeded at 5×103 in 96 well plates and incubated for 24 h at 37° C in a humidified, 5 per cent CO2 atmosphere in normal culture medium. The medium was replaced by differentiation medium and changed every alternate days for two weeks. Analysis for ALP activity was done during different culture days from day 0 to 14. Measurements were performed in triplicates for each time point. ALP activity was determined using p-nitrophenol as substrate N1891 (Sigma Aldrich, USA).
Gene expression analysis: The gene expression profiles of undifferentiated and differentiated cells were analyzed. Gene expressions of collagen type I, matrix metalloprotease-13, RAB3B and osteopontin genes were analyzed by reverse transcriptase (RT)-PCR analysis using the primes synthesized by Integrated DNA Technologies 1 DT, USA. Total RNA was extracted from undifferentiated passage 3 (5×106) cells by Trizol (Invitrogen) and the cDNA was synthesized (Fermentas cDNA synthesis kit, USA). The PCR reaction was carried out in 25 μl volume containing 10 μl of PCR master mix (Amplicon), 1 μl each (50 picomoles) forward and reverse primer, 2 μl of template cDNA. Specific primers for collagen type I (FP ‘CAAGGAAGTCTGCATGTCTGAGTG3 RP 5’ GTCCCTGGAACCCTTGTGG 3), Osteopontin (FP 5’ TGCCTCCGCCCTTCCAGTTA 3’RP 5’ACCTCGGCCATCATTTGTGCT 3’), MMP13 (FP 5’ATGCCATTACCAGTCTCC 3’ RP 5’TAGCCTTTATCCATCACAT 3’), RAB3B (FP5’GATTCCTTCACGCCTGCTTTTGTC 3’RP 5’GGCGCCTGCTGGTCCGTGAG 3’) genes were used for PCR reaction. The PCR products were purified from gel using gel elution kit (Merk Biosciences, USA) as per the manufacturer's protocol. The purified amplicons were subjected to sequencing and sequence homology was compared with adult ovine nucleotide sequences in Gen bank (www.ncbi.nlm.nih.gov).
Results
MSCs were isolated based on the ability to form adherent monolayer in culture and the concomitant lack of adherence of other cells in the bone marrow stroma such as HSCs, adipocytes and macrophages. Ovine foetal samples were processed and the number of MNCs recovered per bone marrow aspirates was 3.4 ± 0.606 ×108 cells. The MACS selected CD45-/CD14- population was 6.3 ± 0.802 ×104 cells. During the initial days of plating, cells were found to be round and glistening (Fig. 1) which slowly assumed spindle morphology, a characteristic oval body with the flattened ends. Selective removal of cells in suspension allowed the growth of adherent uniform spindle morphology cells forming colony forming units (cfu) which fused to form the confluent monolayer. Upon reaching 80-90 per cent confluence, the MSCs were subjected to passaging and re-seeded at the density of 1x103 cells/cm2 at the split ratio of 1:4. The immunophenotypic analysis confirmed the surface protein profiles of MACS selected cells (Fig. 2). The cells subjected to cell cycle analysis on day 3 of seeding showed the cell cycle phases (M2: G0-G1; M3: S; M4: G2-M) M2 65.13 ± 2.53, whereas M3/M4 17.42 ± 3.43/14.97 ± 2.45 (Fig. 3). The ovine foetal BMMSC showed surface markers CD44, CD54, integrin β1 (CD29) and intracellular collagen type I/III, fibronectin (Fig. 4) positive and were found negative for CD45 surface antigen. The osteogenic induction showed increasing ALP enzymatic activity and cells stained positively for ALP staining. The ALP activity was present at baseline levels in undifferentiated cells and at increased levels in different days of osteoblasts induced cells (Fig. 5), which was found at its peak during 14th day of culture. In the osteogenic induced MSCs the deposition of hydroxy apatite could be visualized by Von Kossa staining. The increased deposition of collagen was detected by Masson-Trichrome staining (Fig. 6).
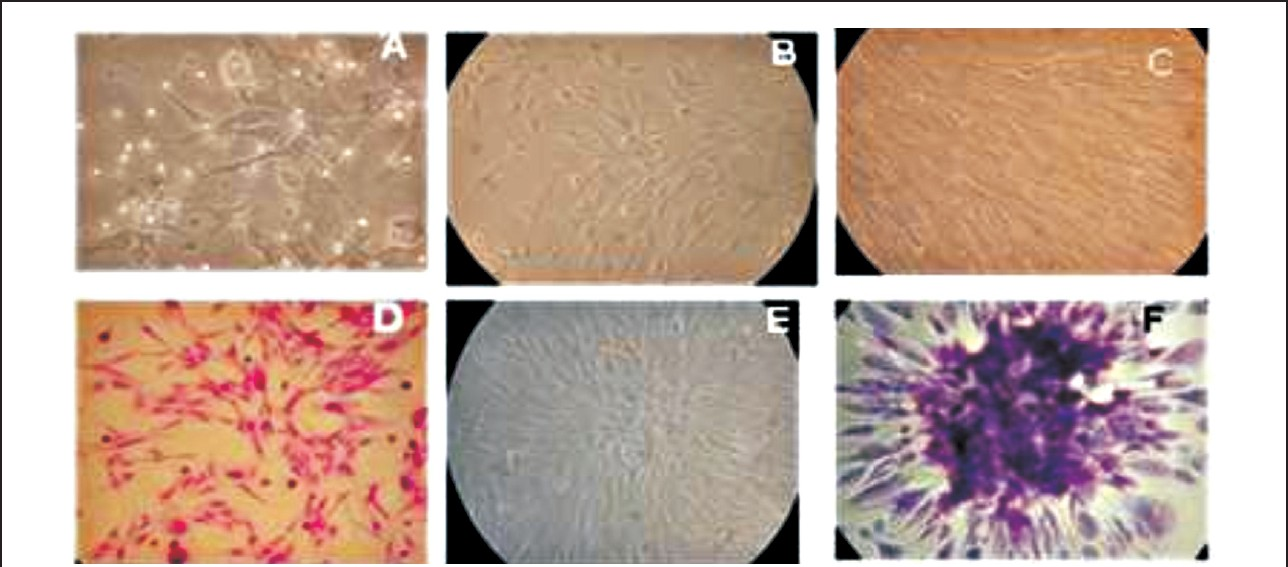
- Sheep fetal mesenchymal cells in culture. A. Glistening heterogenous population of MSCs after 24 h (200X). B. MSCs reached 60-70 per cent confluency at 4th day (200X). C. MSCs showing <90 per cent confluency at the 9th day (200X). D. MSCs stained with Hematoxylin and Eosin (H&E) Staining (200X). E. A single colony forming unit (cfu) of MSC (200X). F. A single cfu stained with H&E (200X).
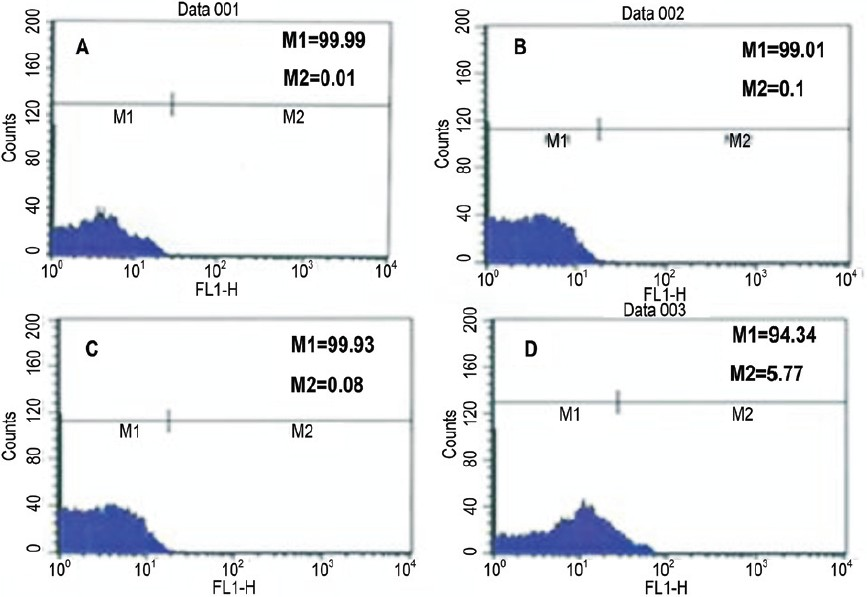
- Immunophenotypic profiles of third passage cells on Day 3. A. Control B. CD45 showing negative phenotype C. CD14 showing negative phenotype and D. CD44 showing positive phenotype (M1/M2/M3/M4) are cell cycle phases (M2: G0-G1; M3: S; M4: G2-M.
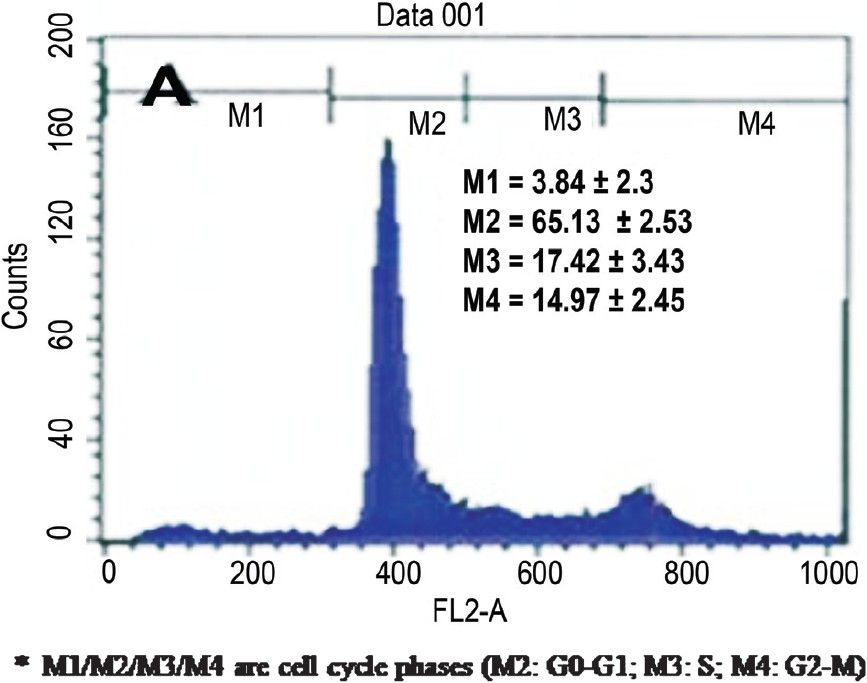
- Cell cycle analysis of BMMSC culture on third day of culture.
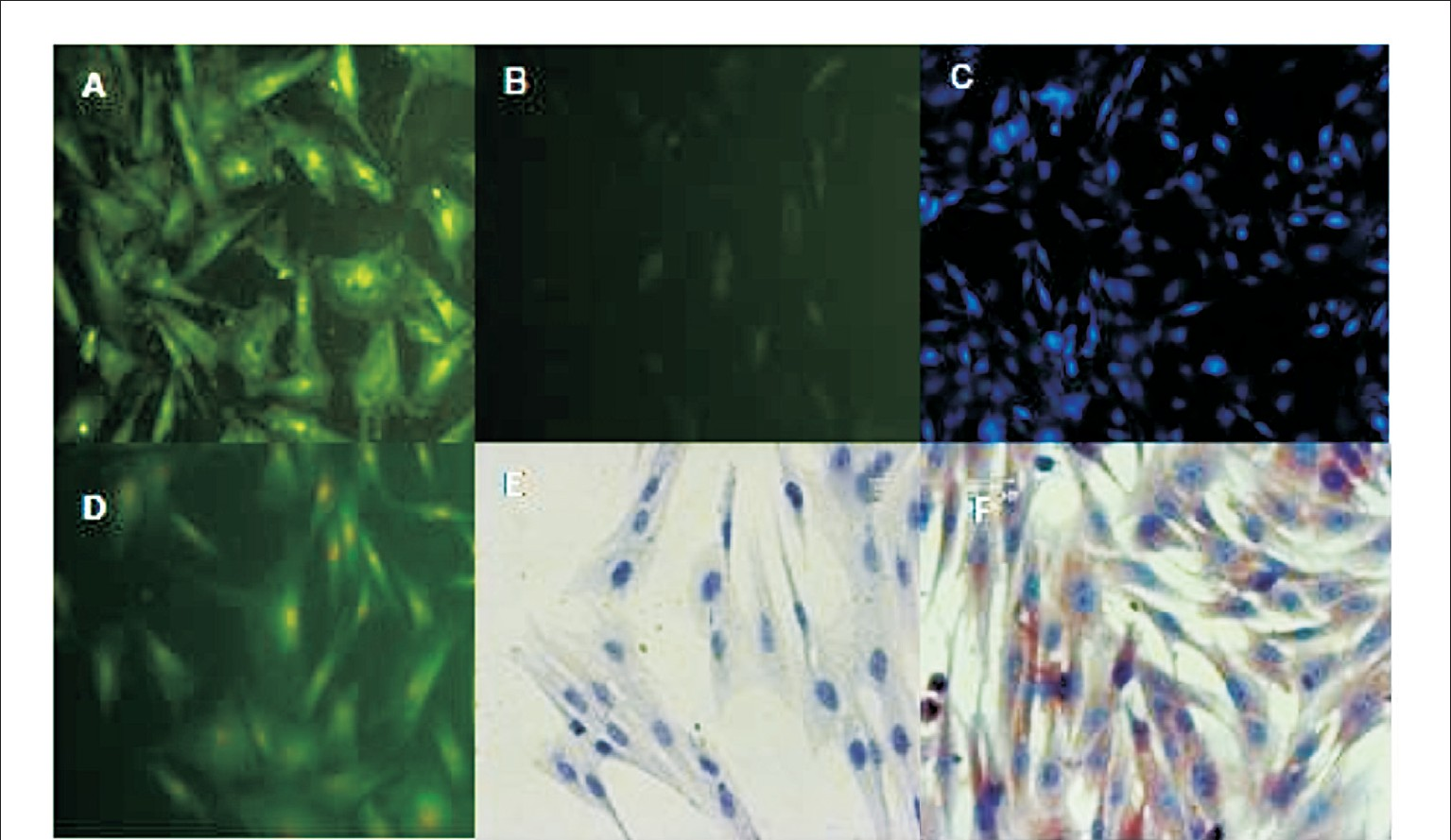
- Cellular antigenic characterization of Sheep fetal Mesenchynial Cells-Immunostaining (200 ×). A. Fetal MSCs stained positive for intra-cellular collagen type I/III expression (FITC), B. Lack of fluorescence in negative controls (FITC), C. MSCs stained positive for CD 44 (Immuno fluorescence, DAPI stained (100×) D. MSCs stained positive for integrinβ1 (FITC), E. MSCs stained negative immunocytochemistry for CD 45 F. MSCs stained positive immunocytochemistry for fibronectin.
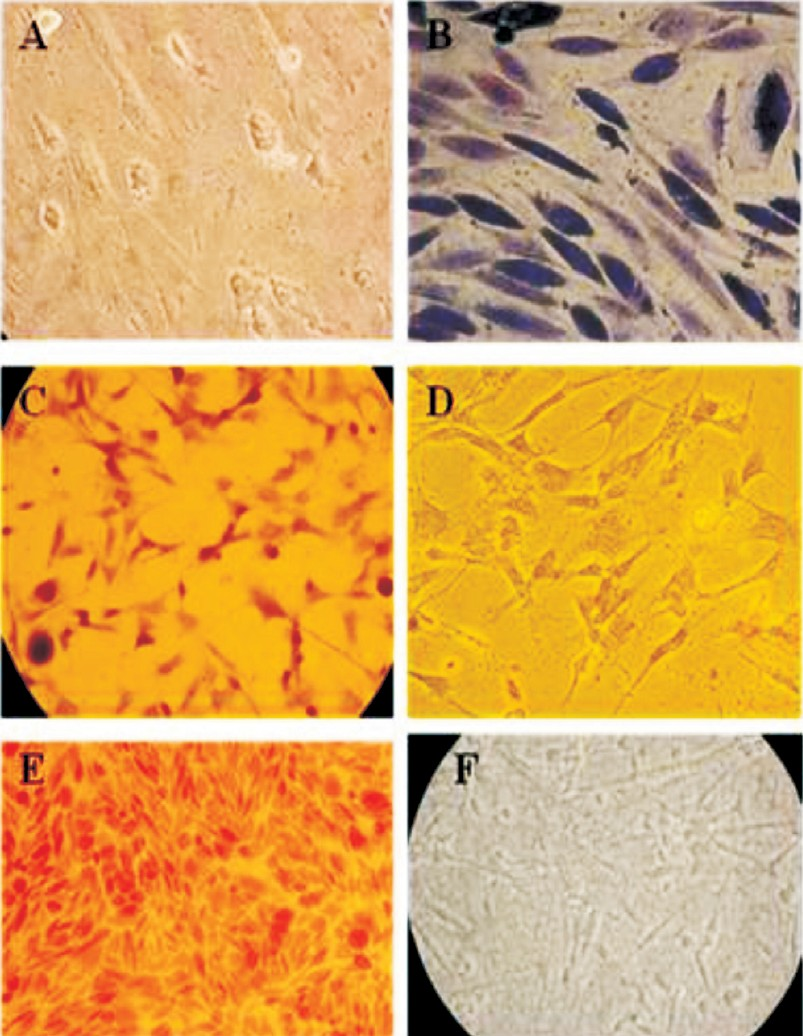
- Differentiation of ovine fetal mesenchymal stem cells-special staining. A. Differentiation of MSCs showing flattened and polygonal shapes (200×). B. Masson trichrome staining (200×). C. Alkaline phosphatase staining (100×). D. Negative staining for Alkaline phosphatase (100×). E. Von Kossa staining showing positive for calcium deposition (100×). F. Von Kossa staining showing negative for calcium deposition in undifferentiated MSCs (200×).
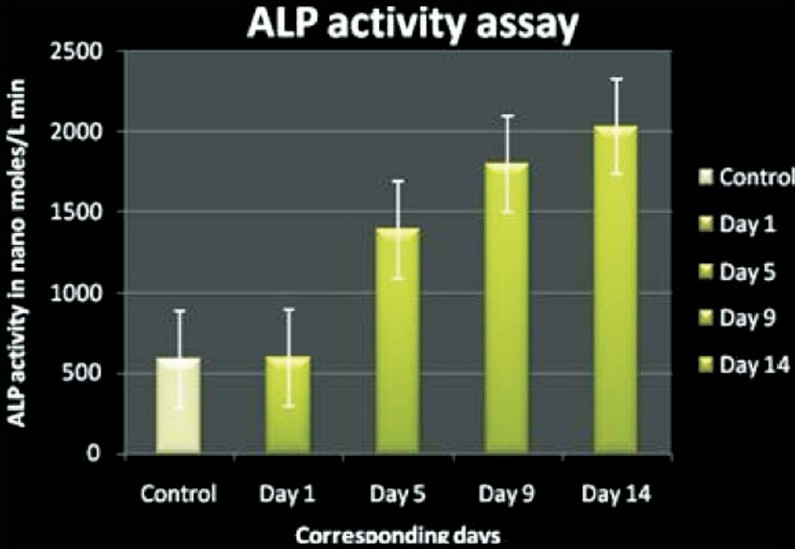
- Alkaline phosphatase activity in cultured cells. Alkaline phosphatase activity was determined by absorbanoe spectroscopy and is represented as mean standard deviation with N=3. Groups are significantly different (P<0.05) in the enzyme activity during different culture days (X axis- Days, Yaxis- ALP during different log or time points during culture days).
The expression of RAB3B gene product (506 bp) in undifferentiated MSCs was found to be one of the candidate marker genes which defined the stemness status in the MSCs. The induced and un induced MSCs expressed collagen type I (370 bp amplicon) and MMP13 gene was found to be expressed in osteogenic induced cells (376 bp) which has been reported to be specific for osteogenic lineage (Fig. 7). The expression was confirmed by amplification of collagen gene by RT-PCR using primers specific for ovine collagen type I and by sequencing. The sequence homology was compared with ovine nucleotide sequence in Genbank (NCBI tools) and found to have 91 per cent homology with ovine nucleotide sequence NCBI BLAST analysis tool. The osteogenic induced MSCs expressed both the collagen type I and osteopontin transcripts whereas the undifferentiated MSCs expressed only collagen type I transcripts hence osteopontin transcripts were more specific to differentiated MSCs (Fig. 7). The osteopontin gene expression was confirmed by the presence of 421 bp amplicon on RT-PCR and it was purified and subjected to sequencing (Applied Biosystems, USA). The sequence homology was compared with ovine nucleotide sequence in Genbank. A 97 per cent homology was shown in BLAST analysis and submitted to Gen bank (Gene accession number HQ013316).
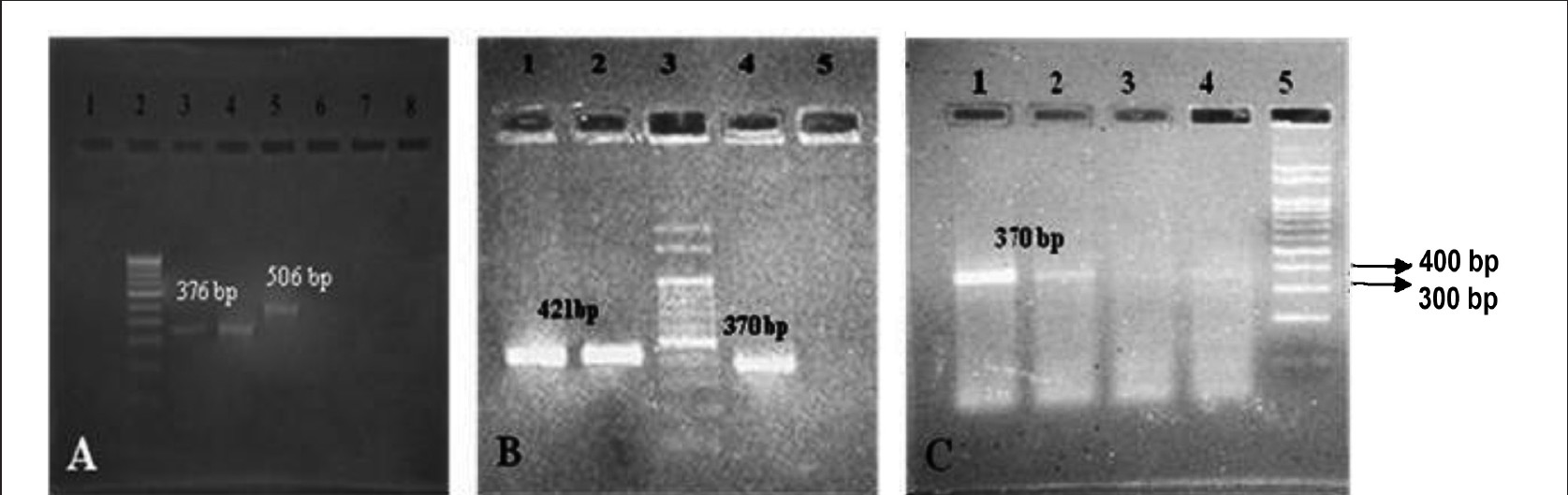
- Gene expression analysis of MSC induced for osteogenic lineage and un-induced MSCs. 100 bp Marker- A lane 2, B lane 3, and C lane 5. RAB3 gene- 506 bp, Osteopontin- 421 bp, MMP13- 1376 bp, and Collagen type 1-370 bp. Supernatant containing floating cells processed for gene expression analysis were loaded in the Lane 7, 8 in A, Lane 5 in B and Lane 3, 4 in C showed negative expression for the MSC and MSC derived osteogenic lineage genes.
Discussion
The stem cells are isolated and cultured for use in repair of damaged tissues, and in replacement therapies and to treat debilitating diseases. MSCs have been isolated based on the ability of these cells to form adherent monolayer in culture and the concomitant lack of adherence of other cells in the bone marrow stroma such as HSCs, adipocytes and macrophages37. While this procedure results in enriched populations of MSCs the resulting BM derived cell populations are heterogenous comprised not only of MSCs but also of concomitant lineage restricted progenitors. MACS selection was followed in the present study to obtain cells to derive pure populations of MSCs.
The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT)16 proposed minimal criteria to define human MSCs such as, MSC must be plastic-adherent when maintained in standard culture conditions using tissue culture flasks, MSC population must express specific surface antigens and must lack expression (<2% positive) of CD45, CD34, CD14, or CD11b. In the present study, the isolated MSCs were found to be adherent, lack haematopoietic markers such as CD14 and CD45 but expressed several surface proteins including CD29, CD44, CD54, intracellular collagen typeI/III and fibronectin and differentiate to other lineages.
MSC population was isolated utilizing the physical property of plastic adherence similar to that originally used by Friedenstein and his team910 and popularized by Caplan11. This methodology was applied in the present study where the cells prepared from bone marrow were selectively isolated and the plastic adherent spindle shaped cells obtained were of similar morphology as reported earlier1012. Several researchers12–15 have stated that isolation of MSCs was based initially on their ability to adhere to plastic which apparently resulted in morphologically, phenotypically and functionally heterogeneous population of cells including reticular cells, fibroblasts, adipocytes and osteogenic precursor cells. MACS separation was done for the selection of CD45-/CD14- population, which satisfied the second criteria and the lineage differentiation analysis confirmed the third criteria of ISCT position statement for defining mesenchymal stem cells16. The cell cycle analysis of cultured adherent cells on different days showed that most of the cells were initially in G0/G1 quiescent state in suspension before adherence to culture flask. After adherence, day 3 cultured cells on analysis showed that most of the cells were engaged in S phase which were ready to proliferate17.
The gene expression studies identified the specific expression of genes such as RAB3B, collagen, osteopontin and other related genes. The function of RAB3B, one of the five genes involved in cell proliferation, survival, and multilineage differentiation has been identified earlier in MSC18 and the present study also confirmed the expression of RAB3B gene product (506 bp) in undifferentiated MSCs. The undifferentiated MSCs which were obtained freshly in suspension of slow cycling cells with low cytoplasmic granularity did not express any differentiation associated markers. MSCs were induced toward osteogenic lineage differentiation in vitro, and confirmed by the ALP staining. ALP activity is often considered as early marker of osteoblasts differentiation, however, in ovines the ALP activity was present at baseline levels in undifferentiated cells and at significantly increased levels in different days of osteoblasts induced cells, which was found at its peak during 14th day of culture, prior to calcium deposition as shown earlier3111519. Later the calcium deposition within the cells was visualized by Von Kossa staining after 21 days of induction with osteogenic induction medium was similar to the findings reported earlier119.
In vitro studies of ovine MSCs revealed that the osteoblast progenitors were expressed positive for type I collagen120, an early marker gene and also positive for the osteopontin expression21. MSCs induced with osteogenic induction medium for 21 days expressed collagen type I, osteopontin, and also ALP activity and matrix metalloproteases-13 (MMP-13)11723. The RT-PCR, done to find the expression of osteopontin gene transcripts revealed the positive expression of 421 bp amplicon and it was eluted further, purified and subjected for sequencing; 97 per cent homology was obtained by BLAST analysis from the ovine nucleotide sequence in Genbank.
The expression of RAB3B gene product in undifferentiated MSCs was found to be one of the candidate marker genes which defined the stemness status in the MSCs. During osteogenesis multipotent MSCs undergo asymmetric division and generate precursors which progress to form osteoprogenitors, preosteoblasts, functional osteoblasts and finally osteocytes. This progression from one differentiation stage to the next is accompanied by the activation and subsequent inactivation of transcription factors and expression of related marker genes i.e. osteopontin, collagen type I, ALP, bone sialoprotein, osteocalcin and MMP13 in osteoblasts. It was also hypothesized22 that when the cell expressed more collagen type I and osteopontin, these cells assume a more mesenchymal phenotype and participate more in epithelial mesenchymal transition (EMT), take part in the tissue repair and tend to have more regenerative potential. In conclusion, the present cell culture system to derive BMMSC and their differentiated cell types may be a remarkable source for EMT studies and to apply the cells for regenerative medical applications in animal models of human diseases.
Acknowledgment
The authors sincerely acknowledge the Department of Biotechnology, Govt. India for funding and Tamil Nadu Veterinary and Animal Sciences University for the facilities provided for the research.
References
- Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen. 2007;15:18-26.
- [Google Scholar]
- Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-7.
- [Google Scholar]
- Mesenchymal stem cells: no longer second class marrow citizens. Nat Med. 1999;5:262-4.
- [Google Scholar]
- Preparation and chracterization of a thermosatble and biodegradable biopolymers using natural cross-linker. Int J Biol Macromol. 2011;48:276-85.
- [Google Scholar]
- Ph. D Thesis. Isolation and characterization of murine embryonic and bone marrow derived stem cells. Tamil Nadu Veterinary and Animal Sciences University 2006
- [Google Scholar]
- PEDF from mouse mesenchymal stem cell secretome attracts fibroblasts. J Cell Biochem. 2008;104:1793-802.
- [Google Scholar]
- Von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int. 2003;72:537-47.
- [Google Scholar]
- The development of fibroblast colonies in monolayer cultures of guinea pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393-403.
- [Google Scholar]
- Fibroblastic precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267-74.
- [Google Scholar]
- Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301-16.
- [Google Scholar]
- Multilineage differentiation capability comparison between MSC and multipotent adult progenitor cells. Adv Studies Biol. 2009;1:25-35.
- [Google Scholar]
- Building a consensus regarding the nature and origin of mesenchymal stem cells. J Cell Biochem. 2002;38(Suppl):7-12.
- [Google Scholar]
- Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy. 2006;8:315-7.
- [Google Scholar]
- Characterization of stromal progenitor cells enriched by flow cytometry. Blood. 1997;90:3471-81.
- [Google Scholar]
- Identification and functional analysis of candidate genes regulating mesenchymal stem cells self-renewal and multipotency. Stem Cells. 2006;24:1707-18.
- [Google Scholar]
- Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20:249-58.
- [Google Scholar]
- Increased expression of G11 alpha in osteoblastic cells enhances parathyroid hormone activation of phospholipase C and AP-1 regulation of matrix metalloproteinase-13 mRNA. J Cell Physiol. 2005;204:336-43.
- [Google Scholar]
- Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2005;116:1827-35.
- [Google Scholar]






