Translate this page into:
Field performance of malaria rapid diagnostic test for the detection of Plasmodium falciparum infection in Odisha State, India
Reprint requests: Dr K. Gunasekaran, Vector Control Research Centre (ICMR), Medical Complex, Indira Nagar, Puducherry 605 006, India e-mail: k_guna@yahoo.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Rapid diagnostic tests (RDTs) have become an essential surveillance tool in the malaria control programme in India. The current study aimed to assess the performance of ParaHIT-f, a rapid test in diagnosis of Plasmodium falciparum infection through detecting its specific antigen, histidine rich protein 2 (PfHRP-2), in Odisha State, India.
Methods:
The study was undertaken in eight falciparum malaria endemic southern districts of Odisha State. Febrile patients included through active case detection, were diagnosed by Accredited Social Health Activists (ASHAs) for P. falciparum infection using the RDT, ParaHIT-f. The performance of ParaHIT-f was evaluated using microscopy as the gold standard.
Results:
A total of 1030 febrile patients were screened by both microscopy and the RDT for P. falciparum infection. The sensitivity of ParaHIT-f was 63.6% (95% CI: 56.0-70.6) and specificity was 98.9% (95% CI: 97.9-99.5), with positive and negative predictive values (PPV and NPV) of 92.6% (95% CI: 86.0-96.3) and 93.0% (95% CI: 91.0-94.5), respectively. When related to parasitaemia, the RDT sensitivity was 47.8% at the low parasitaemia of 4 to 40 parasites/μl of blood.
Interpretation & conclusions:
The results showed that the performance of the RDT, ParaHIT-f, was not as sensitive as microscopy in detecting true falciparum infections; a high specificity presented a low frequency of false-positive RDT results. The sensitivity of ParaHIT-f was around 60 per cent. It is, therefore, essential to improve the efficiency (sensitivity) of the kit so that the true falciparum infections will not be missed especially in areas where P. falciparum has been the predominant species causing cerebral malaria.
Keywords
Microscopy
Odisha
ParaHIT-f
Plasmodium falciparum
P. vivax
rapid diagnostic test
Malaria presents a diagnostic challenge to laboratories in most countries. An accurate species-specific diagnosis is becoming increasingly important, in view of the rising problem of drug resistance in Plasmodium falciparum, the parasite species causing cerebral malaria. Microscopy of stained thick and thin blood smears remains the gold standard for confirmation of diagnosis of malaria. However, microscopy requires a functioning laboratory, significant skills, and time that limit the access to the facility and thereby causing therapeutic delays. Where diagnosis by microscopy is not possible, an alternative is the use of a rapid diagnostic test (RDT) which is simple to perform, requires no equipment or electricity and gives a result within 15 to 20 min. Further, the use of RDTs will minimize delays in treating malaria cases, especially P. falciparum infections that are known to cause complications. There are currently over 200 rapid diagnostic test products commercially available1. WHO and the Foundation for Innovative New Diagnostics (FIND) evaluated the sensitivity of 168 RDTs for the diagnosis of P. falciparum and P. vivax malaria1. The threshold of detection by these rapid diagnostic tests is in the range of 100 parasites/ μl of blood compared to 5 to 10 parasites by thick film microscopy234. RDTs that are based on recognition of the circulating parasite antigens, detect a variety of proteins, including P. falciparum histidine-rich protein 2 (PfHRP2) and P. falciparum lactate dehydrogenase (PfLDH), and also Plasmodium LDH (pLDH) and aldolase, enzymes shared by the five human pathogenic Plasmodium species3. Since 2009, the National Vector Borne Disease Control Programme (NVBDCP) in India has been supplying ParaHIT-f, a rapid diagnostic test that detects PfHRP2, to Odisha State for diagnosis of P. falciparum infection in areas, where microscopy results are not obtainable within 24 h of blood smear collection5. RDTs are manufactured by different companies and hence there may be differences in their accuracy in diagnosing malaria infections. To date, no information on the performance of RDTs supplied by the NVBDCP to the Accredited Social Health Activists (ASHAs) in falciparum endemic villages of Odisha State is available. Therefore, the current study was carried out with an aim to find out the accuracy of the RDT, ParaHIT-f by comparing its diagnosis results with a high quality microscopy in eight southern districts of Odisha State, inhabited predominantly by tribes and are hyperendemic for falciparum malaria since many decades6.
Material & Methods
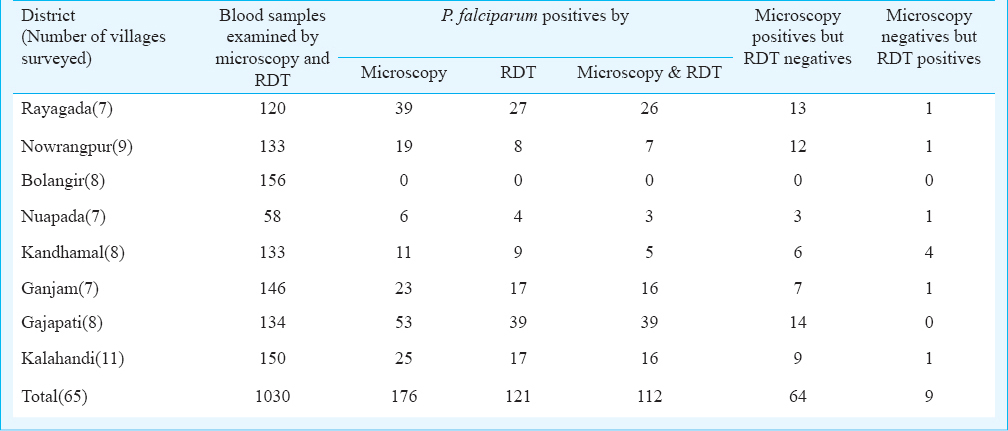
Study area: The performance of the RDT, ParaHIT f was assessed during September 2010 to August 2011 in the eight southern districts viz. Rayagada, Nowrangpur, Kalahandi, Nuapada, Bolangir, Kandhamal, Gajapati and Ganjam of Odisha State, as a part of a study on vector bionomics in relation to transmission of malaria and its control. Most of these districts are located in hilly and forested areas6. Malaria has been endemic in these districts with two transmission peaks in a year; one during post rainy months (October to November) and the other during summer (March to May)6. The annual parasite incidence (API) in the districts during 2009 ranged from 4.2 (Ganjam district) to 49.6 (Kandhamal district) with 89 reported malaria deaths7. Among the total malaria cases, infection due to P. falciparum accounted for >90 per cent in this region, with the remainder being predominantly P. vivax6.
Selection of villages: For this study, 0.5 per cent of the villages in each of the eight districts were selected, giving a total of 65 villages. Selection of villages from all the blocks (sub-districts) of each district was not possible due to practical constraints. Therefore, two blocks were randomly selected in each district, with all villages of the selected block then divided into three categories according to ecotype (hill-top, foot-hill and plain)6. Finally, 0.5 per cent of the villages in each district were randomly selected proportionate to the total number of villages present under each ecotype-category in the two selected blocks.
Fever study: Three fever surveys, one during each of the three prevailing seasons; summer, rainy and winter, were carried out in the 65 selected villages, covering a population of 19,615. The fever surveillance using the RDT kit, ParaHIT f was done in the morning hours by the ASHAs of the respective villages in the presence of research team from Vector Control Research Centre, (VCRC), Puducherry, which ensured the quality of testing by ASHAs. Every member of household present at the time of survey was covered.
Microscopic examination: Thick and thin smears were prepared by ASHAs on clean slides using the finger prick blood samples obtained from any household member with an axillary temperature of >37.5°C. All blood smears were stained with 1:10 Giemsa diluted in pH 7.2 phosphate buffer and examined for the presence of malaria parasites at 5×100 magnification with oil immersion lens searching 100 fields (0.25 μl of blood) in each thick smear. Blood smears were examined in the field-camp laboratory by an experienced microscopist of the research team to whom the results of the RDTs were blinded. Smears were considered negative, if no parasite was seen in 100 oil immersion fields on a thick blood smear. To indicate the level of parasitaemia or parasite density in the positive cases, the number of parasites in thick smears was graded using the plus system scale: + (1 to 10 parasites per 100 thick film fields); ++ (11 to 100 parasites per 100 thick film fields); +++ (1 to 10 parasites per one thick film field); ++++ (>10 parasites per one thick film field)8. Using these scores, the parasite density was estimated: + = 4 to 40 parasites/μl; ++> 40 to 400 parasites/μl; +++> 400 to 4,000 parasites/μl; ++++> 4,000 parasites/μl.
Quality check: As a standard quality control procedure6, all positive blood smears and 10 per cent of randomly selected negative blood smears were cross-examined. The results of the slides selected for cross-checking were blinded to the microscopist who cross-examined the slides independently and the results were obtained. In case of discrepancies, the investigator re-examined the slides and confirmed the results.
RDT for malaria diagnosis: The RDTs used by the ASHAs in the field were procured and supplied by the NVBDCP. Prior to initiation of this study, the ASHAs were trained by the Medical Officers of the respective Community Health Centres (CHCs). Moreover, in the current study, the ASHAs conducted the rapid diagnostic tests in the presence of the research team. Concurrent with collection of blood smears from the patients with fever, RDTs were performed by ASHAs in the presence of the research team on the same finger prick blood samples, transferring them directly to the sample pad using the sample applicator. All RDTs were labelled with patient ID and results were recorded 15 min after adding four drops (300 μl) of clearing buffer. Appearance of both control and test bands indicated a P. falciparum infection and presence of only the control band indicated a negative result. In cases, where the control band did not appear, the results were considered invalid and the tests repeated. During the study, different lots/batches of RDTs were used. Internal quality check included an immediate second reading of all RDTs by a different person to whom the results of the first reading were blinded. In case of discordance, one of the trained technicians, who did entomological collections simultaneously in the village re-examined independently the RDT results9. Also, the results of the RDTs were blinded to the microscopist who examined the corresponding blood smears of the fever patients.
The fever patients diagnosed with P. falciparum infection using ParaHIT-f were administered with artemisinin combination therapy (ACT) [Artesunate 4mg/kg body weight for 3 days plus sulphadoxine (25 mg/kg body weight) and pyrimethamine (1.25 mg/kg bodyweight) on the first day and primaquine (0.75 mg/kg body weight) on second day] by ASHAs according to the national guidelines10. Further, on the basis of microscopy results, the P. falciparum infections that were not detected by the RDTs, were administered with ACT and P. vivax cases with radical treatment (Chloroquine 25 mg/kg body weight divided over three days i.e. 10 mg/kg body weight on day 1, 10 mg/kg body weight on day 2 and 5mg/kg body weight on day 3 and Primaquine 0.25mg/kg body weight daily for 14 days)10 by ASHAs. All P. vivax cases were removed from the data-set for further analysis. The mixed infections of P. falciparum and P. vivax were treated as P. falciparum infections for the analysis purpose. The isolated presence of P. falciparum gametocytes was not considered indicative of acute malaria.
Statistical analysis: Based on the microscopic results, the RDT results were considered true positive (TP), true negative (TN), false positive (FP), and false negative (FN). Sensitivity and specificity were calculated as TP/(TP+FN) and TN/(TN+FP), respectively. Positive predictive value (PPV) [the proportion of true malaria cases (positive blood smears) among the total number of positive RDTs] and negative predictive value (NPV) [the proportion of true negative malaria cases (negative blood smears) among the total number of negative RDTs] were also calculated. Sensitivity of the RDT was further calculated according to the level of parasitaemia. Sensitivity, specificity and predictive values of ParaHIT-f and their respective 95% confidence intervals (CI) were estimated using microscopy as the reference diagnosis (gold standard)11 using the software EPIINFO 6.0 version (Centre for Disease Control and Prevention, Atlanta Georgia, USA). Season-wise analysis of sensitivity and specificity of the RDT was also done.
Results
A total of 1059 fever patients (534 males and 525 females) of age ranged from 2 months (infants) to 70 yr, were screened through the fever surveys for malaria infection using the RDT kits, ParaHIT-f along with microscopic examination of blood smears. During the survey, only two strips of ParaHIT-f did not show the control band and the tests were repeated with two other valid strips.
The microscopy detected a total of 205 malaria positives and of them, 164 (80.0%), 29 (14.1%) and 12 (5.9%) were P. falciparum, P. vivax and mixed infections with P. falciparum and P. vivax parasites, respectively. Since, the study focused on the accuracy of the monovalent RDT that can detect only P. falciparum, in comparison to microscopy, the 29 fever patients found positive for P. vivax infection were removed and the results of the remaining 1030 fever cases (including the 12 fever cases detected with mixed infections) were considered for further analysis. Of the 176 (164 single infections with only P. falciparum + 12 mixed infections with P. falciparum and P. vivax) P. falciparum positives by microscopy, only in eight cases gametocytes were found along with asexual stages and hence included for the analysis. No blood smear was found positive exclusively for P. falciparum gametocytes.
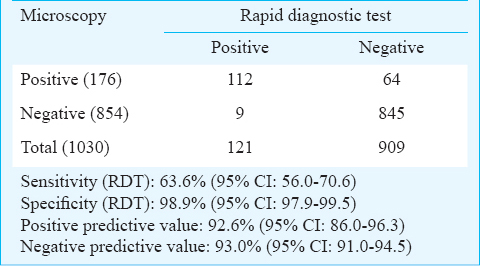
Of the 1030 fever cases screened, 121 (11.7%) and 176 (17.1%) were P. falciparum positives by ParaHIT-f, and by microscopy, respectively (Table I). As shown in Table II, the RDT accurately diagnosed 112/176 true P. falciparum cases, indicating a sensitivity of 63.6 per cent (95% CI, 56.0-70.6%). Of the 854 negatives by microscopy, RDT detected 845 negatives and nine false positives, showing a specificity of 98.9% (95% CI, 97.9-99.5%). The positive predictive (PPV) and the negative predictive values (NPV) were 92.6% (95% CI: 86.0-96.3) and 93.0% (95% CI: 91.0-94.5), respectively. Season-wise analysis showed that the sensitivity of ParaHIT-f was 75.0% (95% CI: 61.7-88.3), 65.7% (95% CI: 53.6-77.8) and 52.5% (95% CI: 39.1-65.8) in summer, rainy and winter seasons, respectively and the corresponding specificity was 97.0 (95% CI: 94.6-99.4), 99.3 (95% CI: 98.2-100) and 100.0% (95% CI: 99.9-100).
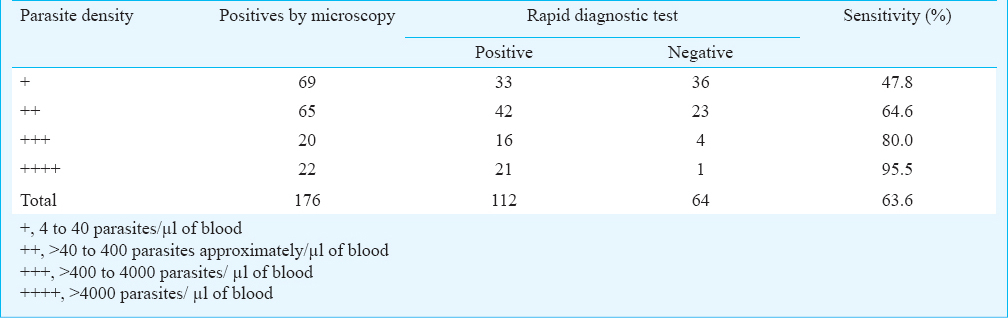
The sensitivity of the RDT, ParaHIT-f in detecting P. falciparum infection in relation to the degree/level of parasitaemia is illustrated in Table III. Among the 176 positives by microscopy, the maximum number (69) was with a parasite density of +, followed by ++ (65); the positive cases with +++ (20) and ++++ (22) density were lower in number. When related to the level of parasitaemia, the sensitivity of the RDT was found to be 47.8% (95% CI: 36.0-59.6), 64.6% (95% CI: 53.0-76.2), 80.0% (95% CI: 62.5-97.5) and 95.5% (95% CI: 84.5-100.0) at the parasite density of +, ++, +++ and ++++, respectively.
Discussion
Malaria continues to be one of the major public health problems in India. The existing and the accepted gold standard technique for malaria diagnosis is the microscopic examination of stained thick and thin blood films. However, in many places there are no laboratory support to provide the malaria microscopy. Wherever it is available, several factors reportedly affect the quality of diagnosis by microscopy1213. In Odisha State, particularly in the southern districts, where high malaria transmission continued to occur, fever is often treated presumptively as malaria, resulting in misdiagnosis and overuse of anti-malarial drugs as reported elsewhere514. Therefore, there is a need for rapid and accurate diagnosis of malaria to ensure rational use of anti-malarial drugs. To offer such diagnostic facility, many rapid diagnostic tests (RDTs) have been developed. One such test kit is ParaHIT-f supplied by the NVBDCP, for the diagnosis of P. falciparum malaria in Odisha State. In Tanzania and other African countries also11, ParaHIT-f is used for malaria diagnosis.
There are 30 districts in Odisha State and among them the 10 districts, situated in the southern part of the State, are highly endemic for falciparum malaria since many decades6. The current study, carried out on the request of the Government of Odisha, generated entomological and epidemiological information in the eight districts to formulate evidence based suitable site specific strategies for the control of malaria. However, restricting only to the endemic districts could be a limitation of the study as continuous exposure to malaria parasites might help in acquiring some kind/amount of immunity against malaria parasites and reduce the parasite load among patients; as a result, the RDTs, which have limitations in detecting malaria positives with low parasitaemia, would not diagnose all true malaria cases in the field and their sensitivity would become lower.
In the current study, the sensitivity of ParaHIT-f in detecting P. falciparum infection was found to be low, but the studies conducted outside India reported a still lower sensitivity; 29.8 in Tanzania15 and 9.6 per cent in Philippines16. Another study conducted in a rural area of Tanzania reported that of the total 743 samples screened, 31.8 per cent (236/743) was found positive for P. falciparum infection by microscopy, while only 3.4 per cent was detected positive by the RDT (ParaHIT-f); showing a sensitivity of 10.7 per cent (95% CI, 6.7-14.7), specificity of 100% (95% CI, 97.4-102), PPV of 100% (95% CI, 99.1-100.2) and NPV of 70.9% (95% CI, 66.9-74.9)9. In our study, nearly 36 per cent of the P. falciparum cases were not correctly diagnosed by the RDT (ParaHIT-f), thereby missing a number of P. falciparum positives. Consequently, such missed positive cases in the field without any treatment may constitute an important reservoir for transmission. The level of agreement between microscopy and the RDT warrants more investigations in different transmission settings and clinical situations. In an earlier study, the performance characteristics of ParaHIT-f was assessed among the ethnic tribes in four districts of Central India with different transmission levels and the overall sensitivity, specificity and accuracy of the RDT were reported to be >90 per cent9. HRP2-based ParaHIT-f RDTs were introduced for routine patient care in Rufiji district, Tanzania, from December 2007 to October 2008, and their performance was assessed; the sensitivity was 90.7 per cent (range 85.7-96.5%) and the specificity was 73.5 per cent (range 50.0-84.3%)17.
Four brands of RDTs, when tested for their field performance in malaria diagnosis in Philippines, showed wide variability in their sensitivity during repeated surveys and such variability was found associated with both high and low parasitaemia16. Some other studies reported a wide variability in sensitivity at lower parasite densities and occurrence of false negative results at higher parasitaemia181920. In the current study, the sensitivity of ParaHIT-f was found to be lower with a higher proportion of false negatives, at lower parasite densities (+ and ++). When the parasitaemia was at +++ and ++++ levels, the sensitivity was also higher. Since, the study was conducted along with the routine malaria control programme in the State and the blood smears were examined in the field-camp laboratory, it was not possible to count the parasites following the standard techniques and hence the + system was used to grade the parasitaemia. A 95 per cent sensitivity at 100 malaria parasites/μl of blood has been recommended as a target for RDT performance by the WHO21. In this context, the results of the current study pointed out that the RDT met the WHO performance criteria only at the parasitaemic level of >4000/μl or at a parasite density of ++++, otherwise its field performance was poor.
Although, all the RDT kits were used before the expiry date, the poor performance of ParaHIT-f recorded in the current study could be due to manufacturing defects of the test strips or problems related to transport, utilization and storage at peripheral level as reported elsewhere1622. The RDT kits were not stored according to the manufacturer's instructions; refrigerators were not available with ASHAs and hence the RDT kits were stored at room temperature. The ambient conditions at the time of testing can also affect the quality of the tests as high temperature and humidity were reported to cause a loss of monoclonal antibodies from the strip23. Although, the southern districts of Odisha State record more than 40 °C during May-June every year6, the seasonal analysis of the results showed that neither temperature nor humidity interfered with the accuracy of ParaHIT-f, as the sensitivity of this RDT was found to be higher in summer than in rainy and winter seasons. It has also been pointed out in Round 3 of the WHO/TDR/FIND/CDC evaluation that this RDT is heat stable and can withstand high temperature (40°C) for a couple of months24. Therefore, the temperature and humidity might not be the cause for the low sensitivity of ParaHIT-f. The other possible reason could be the fact that a major proportion (76.1%) of positive cases had low parasitaemia (+ and ++), perhaps due to intake of drugs by them or a high acquired immunity on continuous exposure to parasites. Yet another limitation of the study was that the history of anti-malarial drugs intake by the patients in the last two weeks was not recorded as the tests were performed by ASHAs in the villages as a part of their routine activities under malaria control programme.
The poor sensitivity of the RDT, ParaHIT-f, compared to microscopy, in detecting P. falciparum infection might hinder its reliability or utilization for malaria diagnosis especially in areas where microscopic facility is poor or not available. There is a need to further improve the quality of ParaHIT-f by enhancing its sensitivity in diagnosing falciparum malaria.
Acknowledgment
Authors acknowledge all the patients for their voluntary participation in the study and the staff of VCRC field station, Koraput, for technical assistance, and the District Malaria Officers and the VBD Consultants of the eight southern districts of Odisha State for their help and cooperation in carrying out this study. Authors thank to Dr M. M. Pradhan, Deputy Director, Malaria, Government of Odisha for his timely help in coordinating the field activities. The current study is a part of a project funded by the National Rural Health Mission (NRHM), Odisha Unit, Bhubaneswar.
Conflicts of Interest: None.
References
- World Health Organization. Malaria rapid diagnostic test performance. Summary results of WHO product testing of malaria RDTs: round 1-4 (2008-2012). Available from: http://www.who.int/malaria/publications/rdtmalaria_summary.pdf
- [Google Scholar]
- ACP Broadsheet no. 148. July 1996. Laboratory diagnosis of malaria. J Clin Pathol. 1996;49:533-8.
- [Google Scholar]
- National Vector Borne Disease Control Programme. Diagnosis and treatment of malaria 2013. National Vector Borne Disease Control Programme, Directorate General of Health Services, Ministry of Health & Family Welfare, Govt. of India. 2013. Available from www.nvbdcp.gov.in
- [Google Scholar]
- Persistent foci of falciparum malaria among tribes over two decades in Koraput district of Odisha State, India. Malar J. 2013;12:72.
- [Google Scholar]
- Annual Report 2011 - 2012. National Vector Borne Disease Control Programme. Ministry of Health & Family Welfare, Government of Odisha. 2013:36.
- [Google Scholar]
- Diagnostic and prognostic utility of an inexpensive rapid on site malaria diagnostic test (ParaHIT f) among ethnic tribal population in areas of high, low and no transmission in central India. BMC Infect Dis. 2005;5:50.
- [Google Scholar]
- Directorate of National Vector Borne Disease Control Programme. National Drug Policy on Malaria (2013) New Delhi: Directorate General of Health Services, Ministry of Health and Family Welfare; 2013. p. :15.
- [Google Scholar]
- Low sensitivity of ParaHIT-f rapid malaria test among patients with fever in rural health centers, Northern Tanzania. J Infect Dev Ctries. 2011;5:204-8.
- [Google Scholar]
- Rapid diagnostic tests for malaria at sites of varying transmission intensity in Uganda. J Infect Dis. 2008;15(197):510-8.
- [Google Scholar]
- Low sensitivity but high specificity of ParaHIT f in diagnosing malaria among children attending outpatient department in Butimba District Hospital, Mwanza, Tanzania. Tanzan J Health Res. 2009;11:97-9.
- [Google Scholar]
- Field evaluation of malaria rapid diagnostic tests for the diagnosis of P. falciparum and non-P. falciparum infections. Southeast Asian J Trop Med Public Health. 2005;36:552-61.
- [Google Scholar]
- Quality assurance of rapid diagnostic tests for malaria in routine patient care in rural, Tanzania. Am J Trop Med Hyg. 2010;82:151-5.
- [Google Scholar]
- Diagnosis of imported malaria by Plasmodium lactate dehydrogenase (pLDH) and histidine-rich protein 2 (PfHRP-2)-based immunocapture assays. Am J Trop Med Hyg. 2001;64:20-3.
- [Google Scholar]
- Comparison of three antigen detection methods for diagnosis and therapeutic monitoring of malaria: a field study from southern Vietnam. Trop Med Int Health. 2002;7:304-8.
- [Google Scholar]
- A comparison of two rapid field immuno-chromatographic tests to expert microscopy in the diagnosis of malaria. Acta Trop. 2002;82:51-9.
- [Google Scholar]
- World Health Organization (WHO). Foundation for Innovative New Diagnostics (FIND)/CDC/TDR. In: Malaria rapid diagnostic test performance: results of WHO product testing of malaria RDTs, Round 4 (2012). Geneva, Switzerland: WHO; 2012.
- [Google Scholar]
- Routine parallel diagnosis of malaria using microscopy and the malaria rapid diagnostic test SD 05FK60: the experience of Médecins Sans Frontières in Myanmar. Malar J. 2013;12:167.
- [Google Scholar]
- World Health Organization (WHO). Malaria rapid diagnosis: making it work (Meeting Report 20-23 January 2003) Manila: WHO Regional Office for the Western Pacific; 2003.
- [Google Scholar]
- World Health Organization (WHO)/FIND/CDC/TDR. Malaria rapid diagnostic test performance, results of WHO product testing of malaria RDTs: Round 3 (2010–2011) Geneva, Switzerland: WHO; 2011.
- [Google Scholar]






