Translate this page into:
Fidaxomicin - the new drug for Clostridium difficile infection
Reprint requests: Dr C. Vaishnavi, Department of Gastroenterology, Postgraduate Institute of Medical Education & Research Chandigarh 160 012, India e-mail: cvaishnavi@rediffmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Clostridium difficile is one of the many aetiological agents of antibiotic associated diarrhoea and is implicated in 15-25 per cent of the cases. The organism is also involved in the exacearbation of inflammatory bowel disease and extracolonic manifestations. Due to increase in the incidence of C. difficile infection (CDI), emergence of hypervirulent strains, and increased frequency of recurrence, the clinical management of the disease has become important. The management of CDI is based on disease severity, and current antibiotic treatment options are limited to vancomycin or metronidazole in the developing countries. this review article briefly describes important aspects of CDI, and the new drug, fidaxomicin, for its treatment. Fidaxomicin is particularly active against C. difficile and acts by inhibition of RNA synthesis. Clinical trials done to compare the efficacy and safety of fidaxomicin with that of vancomycin in treating CDI concluded that fidaxomicin was non-inferior to vancomycin for treatment of CDI and that there was a significant reduction in recurrences. The bactericidal properties of fidaxomicin make it an ideal alternative for CDI treatment. However, fidaxomicin use should be considered taking into account the potential benefits of the drug, along with the medical requirements of the patient, the risks of treatment and the high cost of fidaxomicin compared to other treatment regimens.
Keywords
Clinical trials
Clostridium difficile
cost
diarrhoea
fidaxomicin
hypervirulent strain
recurrences
vancomycin
Introduction
Clostridium difficile, a Gram-positive bacterium is one of the many aetiological agents of antibiotic associated diarrhoea (AAD) implicated in 10-25 per cent of AAD, 50-75 per cent of those with antibiotic associated colitis (AAC) and 90-100 per cent of those with antibiotic-associated pseudomembranous colitis1. Hospitalized patients are at an increased risk for acquiring C. difficile spores from contaminated surfaces23, which germinate into vegetative forms, colonize the large intestine and produce toxins. Clostridium difficile exists as multiple strains, inclusive of non-toxinogenic ones. However, the hypervirulent North American Pulsed-field-gel-electrophoresis type 1 (NAP1/B1/027) strain is known to be associated with severe diarrhoea and colitis45. Exacerbation of inflammatory bowel disease6 and extracolonic manifestations like small bowel involvement7 and bacteraemia89 are also becoming common. With an initial increase in the NAP1/B1/027 strain, an increased mortality associated with it was found. However, now the ribotype 027 in C. difficile infection (CDI) has declined and ribotype 078, a strain frequently isolated from pigs and calves10 and equally virulent is on the rise1112.
When the patient is on antibiotic therapy, disruption of the human gut flora leads to an overgrowth of C. difficile13. However, CDI can also occur without exposure to antimicrobials particularly in elderly patients or young immunocompromised persons1415. CDI is also increasingly affecting people considered to be at low risk such as pregnant women16 and children17 and is now moving into the community affecting otherwise healthy adults who have no history of hospital admission or recent antibiotic exposure18.
The disease incidence due to C. difficile infection is increasing worldwide. Between 1999 and 2004, the mortality rate due to CDI increased from 5.7 to 23.7 deaths per million people in the United States19. Subsequently, data comparing the burdens of hospital onset healthcare facility for C. difficile and methicillin-resistant Staphylococcus aureus suggest that the former has surpassed the latter as the leading cause of hospital acquired infection20. The frequency of CDI in US has been estimated to be 420,000-700,000 cases annually21. In United States, CDI accounts for health care expenditure between 1.122 and 3.223 billion dollars annually. Even though CDI is prevalent in India24, it is not widely recognized and the extent of the disease is not known. Widespread unregulated and inappropriate prescribing of antibiotics in the country indicates that the CDI could be prevalent even in areas where surveillance for the disease is absent.
Due to an increase in the incidence of CDI, emergence of hypervirulent strains and increased frequency of recurrence, the clinical management of the disease has become important24. In the early part of current decade, a new antibiotic, fidaxomicin, was reported to cause less disruption of the gut microbiota25 and lower rates of recurrence compared to vancomycin26. In this review after briefly describing important aspects of CDI, the new drug, fidaxomicin for the treatment of CDI is discussed.
Spectrum of C. difficile infection
The spectrum of CDI may be mild to severe and sometimes fatal27. CDI patients with leukocytosis <15,000 cells/µl and serum creatinine <1.5 times of basal level are described as having mild to moderate disease. The course of disease begins with mild diarrhoea and abdominal cramps. However, in severe CDI, fever may be >38.3°C with signs of peritonitis, ileus, leukocytosis, serum creatinine >50 per cent baseline, elevated serum lactate levels, pseudomembranous colitis, toxic megacolon, thick colonic wall and ascites. In some of the cases it may lead to colonic perforations and sepsis, followed by death. The morbidity and mortality in CDI range from dehydration to gastrointestinal haemorrhage. There is a need for intensive care in 2-3 per cent patients leading to emergency bowel resection and colectomy in atleast 1 per cent of them28. The death rate attributed to CDI is up to 6.9 per cent in outbreaks29, and up to 25 per cent frail elderly patients are involved30.
Emergence of a hypervirulent strain
The emergence of a hypervirulent BI/NAP1/027 strain during the turn of the century brought about an increased incidence of nosocomial CDI in the West. This fluoroquinolone-resistant epidemic strain was responsible for a marked increase in the severity of CDI cases. The strain is resistant to the newer broad spectrum fluoroquinolones such as moxifloxacin. For the first time in 2007, severe cases of CDI with BI/NAP1/027 strain were detected in Germany and the use of cephalosporins and fluoroquinolones in the three months prior to the onset of symptoms were implicated31. Treatment failures with both metronidazole and vancomycin increased32. Increased cases involving pseudomembranous colitis, toxic megacolon, colectomy and death were reported33. The BI/NAP1/027 strain was identified in eight institutions in six different States of US and more than 80 per cent affected with CDI were aged over 65 yr34. This strain has been reported from United Kingdom, The Netherlands, Belgium, France, Austria, Luxembourg, Poland, Japan, Finland, etc35, but so far it has not been reported from India. Hypervirulent strains of NAP7 and NAP8 ribotype/078, frequently identified in food animals have also been observed to be increasing in Europe36.
The Indian scenario for CDI
The incidence of CDI varies from place to place. From India, Gupta & Jadav37 first reported the organism in 25.3 per cent diarrhoeal patients of all age group. Ayyagari et al38 reported C. difficile in 22.6 per cent stool specimens obtained from cases of AAC with or without pseudomembranes. Niyogi et al39 reported C. difficile in 8.4 per cent and cytotoxin in 7 per cent of faecal samples from children. Another study from the same group40 reported a prevalence of 11 per cent in hospitalized patients with diarrhoea. In an investigation of 233 patients with acute diarrhoea, Bhattacharya et al41 isolated C. difficile as a sole pathogen from 7.3 per cent, of which 82.3 per cent produced cytotoxin. We42 reported a positive C. difficile toxin assay in 30 per cent patients in the antibiotic receiving group compared to only seven per cent in those not receiving the antibiotics. Our group also reported that C. difficile toxin positivity was influenced by antibiotics in the paediatric group of patients43. CDI was found to be more common in the post-bone marrow transplantation period in India than in other developed countries44. Increased prevalence of CDI after antibiotic usage in the ulcerative colitis group45 and increased C. difficile carriage in psoriatic patients given either methotrexate or mesalamine were also reported46. C. difficile was reported as an important pathogen in younger children with AAD47. A decrease in the number of C. difficile positive cases due to stringent surveillance and improved antibiotic policy adopted by the hospital during a five-year study period was reported from north India48.
Current CDI management and recurrences
The management of CDI is based on disease severity. A suspected CDI case is managed clinically firstly by withdrawal of the offending agent. The current antibiotic treatment options are limited to vancomycin or metronidazole in the developing countries. Mild to moderate CDI is treated with oral metronidazole 500 mg given three times daily whereas oral vancomycin 125 mg four times daily for 10-14 days is recommended for severe disease49. For patients who develop ileus, simultaneous intravenous metronidazole 500 mg thrice daily for ten days and intracolonic vancomycin 500 mg in 100-500 ml saline 4-12 hourly may also be given. The line of further management depends on the prognosis and may include surgery, intravenous immunoglobulin treatment or high dose of vancomycin50.
With this CDI treatment schedule, recurrence has become a common complication and may be due to persistently altered faecal flora by repeated antibiotic treatment or due to impaired immune response. Recurrence begins with re-appearance of the symptoms of CDI after successful treatment and is assessed at the end of 10 days of treatment. However, recurrences may occur anytime, usually within four weeks following therapy. Recurrence may vary in severity and continue repeatedly for months and years and the treatment is very difficult. Both metronidazole and vancomycin have an initial recurrence rate between 20-35 per cent despite therapy. Again 45-65 per cent of these patients will have subsequent recurrences5152. Recurrence is serious because it leads to hospitalization and subsequent death.
Vancomycin treatment increases the risk of colonization with vancomycin-resistant-enterococci (VRE), and at times with vancomycin-intermediate S. aureus, because of multiple dosing. The disadvantages of metronidazole is that it has a lower cure rate in severe CDI, compared to vancomycin, and it gets fully absorbed in the gastrointestinal tract and has several adverse effects, including neurotoxicity53. Therefore, it cannot be used for long duration which is required in cases of relapse. Thus, the true cure for CDI is elusive.
Fidaxomicin
In May 2011, the US Food and Drug Administration (FDA) approved fidaxomicin as the first new antibiotic for CDI in the past three decades54. In December 2011, fidaxomicin was approved by the European Medicine Agency. Fidaxomicin is a macrocylic antibiotic derived from the fermentation product of actinomycete, Dactylosporangium aurantiacum and Actinoplanes deccanensis55. The compound found naturally was variously called as lipiarmycin5657, tiacumicin B5859, OPT-806061, PAR-10162 and difimicin63. After oral administration, the parent compound gets converted to OPT-1118, probably via hydrolysis by gastric acid or by enzymatic activity of intestinal microsomes64. More than 92 per cent gets eliminated in the faeces and only a small fraction (0.59%) is eliminated from the urine65.
Fidaxomicin is made up of 18-membered lactone ring55 with a molecular weight of 1058.04 g/mol. The minimum inhibitory concentration (MIC90) for fidaxomicin is four times less than that of metronidazole and vancomycin and is the same for NAP1 and non-NAP1 strains66. Fidaxomicin is bactericidal in activity which is time-dependent not concentration-dependent. It is thus distinct from macrolides and rifamycins. The mechanism of action of fidaxomicin is by inhibition of RNA synthesis by interfering with the formation of DNA-RNA polymerase complex before the initiation of transcription67.
Clinical trials
Several clinical trials have been done to compare the efficacy and safety of fidaxomicin with those of vancomycin in treating CDI.
-
Phase I trial - In a double blind, randomized, dose-escalation, placebo-controlled Phase I trial, fidaxomicin was given orally to 16 healthy subjects as a single dose (Phase IA) and to 24 healthy adults as multiple doses (Phase IB) for 10 days period64. Analysis of plasma, urine and faeces showed that the plasma concentrations were mostly below the lower limit of quantification (i.e. 5 ng/ml) with either dosage strategy, except for four subjects who received 450 mg daily, and had plasma concentration of the drug ranging 6.13-6.70 ng/ml. In the urine <1 per cent of the drug was found to be excreted. Faecal concentration of OPT-1118 was greater than fidaxomicin in Phase IA, and higher in Phase IB and was directly proportional to the administered fidaxomicin dose.
No clinically adverse changes were observed in the laboratory reports inclusive of electrocardiogram and vital signs. Five mild adverse effects viz. headache, rhinorrhoea, open wound in left upper leg, elevated serum amylase and elevated serum lipase concentration were reported in Phase IA. In Phase IIB, there were eight adverse events such as weakness, difficulty in swallowing, pharyngitis, conjunctivitis, eosinophilia, and upper respiratory tract infection, though none was found to be related to fidaxomicin treatment.
-
Phase II trial - Further analysis of fidaxomicin was done in Phase II study to determine the effective dose for treatment of mild to moderate CDI68. The study included 48 subjects who received either 50 or 100 or 200 mg oral fidaxomicin every 12 hours for 10 days. Plasma concentration detected were 14.3 per cent in 100 mg/day dose, 56.3 per cent in 200 mg/day dose and 81.3 per cent in 400 mg/day dose. The majority of the subjects (93.5%) had plasma concentrations of fidaxomicin <20 ng/ml, though level OPT-1118 was higher (>20 ng/ml) in 39 per cent subjects. The mean faecal concentration of fidaxomicin per gram of faeces on day 10 was 256 µg in 100 mg/day dose, 442 µg in 200 mg/day dose, and 1433 in 400 mg/day dose. The faecal concentrations of OPT-1118 were largely similar to that of fidaxomicin68.
-
Phase III trial - two Phase III multi-centered, randomized, double blind vancomycin-controlled clinical trials were carried out with 1164 subjects, age ranging from 18-94 yr26. The subjects included in the study had diarrhoea and a positive C. difficile toxin assay. Exclusion criteria involved patients with megacolon, ileus, inflammatory bowel conditions, and those on anti-diarrhoeal or anti-C. difficile medications. In one study, 629 patients were enrolled initially, but PP patients were 268 in fidaxomicin group and 280 in vancomycin group. In the second study26, initially 535 patients were enrolled, with PP subjects 217 in the fidaxomicin group and 234 in the vancomycin group. Adequate representation of subjects with mild, moderate and severe CDI ranging from 22-39 per cent was done. Fidaxomicin was given 200 mg every 12 hours or vancomycin 125 mg every six hours for 10 days. At the end of treatment a 30 days follow up was done.
The clinical cure of CDI which meant cure at the end of therapy without any recurrence of CDI, was determined as <3 unformed bowel movements for two consecutive days or marked reduction in the number of unformed bowel movements at the end of therapy. These patients did not require CDI therapy within two days of completion of study medication. In one study, it was 88.2 per cent for fidaxomicin versus 85.8 per cent for vancomycin, and in the other study it was 87.8 versus 86.8 per cent, respectively. The sustained cure was defined as clinical cure with no recurrence during the 30 days of follow up.
The common adverse events noted in the treatment group were nausea, vomiting, hypotension, headache, abdominal pain, diarrhoea and pyrexia in <5 per cent of subjects. In fidaxomicin arm 5.9 per cent subjects and in vancomycin arm 6.9 per cent subjects discontinued treatment due to adverse effects like vomiting, respiratory failure, pneumonia, megacolon, colitis, dehydration and sepsis. The gastrointestinal bleeding events and the overall death rate were similar in both the arms. These clinical trials concluded that fidaxomicin was non-inferior to vancomycin for treatment of CDI and that there was a significant reduction of recurrences and sustained cure rate.
However, in one patient who was clinically cured C. difficile strain isolated had an elevated fidaxomicin MIC of 16 μg/ml at the time of recurrence69. This patient at baseline investigation had a C. difficile strain having a fidaxomicin MIC of 0.06 μg/ml. The patient though cured was culture positive at the end of therapy, and the strain had the same fidaxomicin MIC at the end of therapy, as at the start. The patient had a recurrence six days after the last dose of fidaxomicin, and the strain isolated at that time had an MIC of 16 μg/ml70.
Clinical efficacy
Fidaxomicin is particularly active against C. difficile (MIC90 0.03 to 0.25 µg/ml) and C. perfringens60. The drug is more potent at suppressing clostridial RNA polymerase than against other bacterial species71. It is moderately active against Staphylococcus (MIC90 2 µg/ml) and Enterococcus (MIC90 8µg/ml). However, it does not act against other gut flora like Bacteroides and other Gram-negative bacteria and yeast72. This may benefit in maintaining colonization resistance and protecting the gastrointestinal tract from colonization by C. difficile55. Fidaxomicin also has a low potential for colonization by vancomycin resistant enterococci (VRE). Nerandzic et al73 in enterococci a multicenter randomized trial of fidaxomicin versus vancomycin for CDI treatment observed that fidaxomicin was less likely than vancomycin to promote acquisition of VRE and Candida species during CDI treatment. However, selection of pre-existing subpopulations of VRE with elevated fidaxomicin MICs was common during fidaxomicin therapy.
Babakhani et al74 investigated the effect of fidaxomicin and OPT-1118 on C. difficile growth and sporulation kinetics and compared it with that of vancomycin, metronidazole and rifaximin. They found that both fidaxomicin and OPT-118, effectively inhibited sporulation by C. difficle unlike the other three comparator drugs. The authors concluded that the inhibitory effect of fidaxomicin on C. difficile sporulation may contribute to its superior performance in sustaining clinical response and reducing recurrences and may also be beneficial in decreasing shedding and transmission of the pathogen. Allen et al75 tested effects of fidaxomicin, its metabolite OPT-1118, and vancomycin on spore germination and found that none affected the initiation of spore germination but all inhibited outgrowth of vegetative cells from germinated spores.
Fidaxomicin is minimally absorbed into the system as a result of which it is well tolerated and has minimal side effects. It is a safe and effective treatment against CDI and its safety profile is comparable to oral vancomycin. The post-antibiotic effect against C. difficile due to fidaxomicin ranges from 6-10 h61 which helps to support the twice daily dosing. Fidaxomicin is required to be used only for infections that are strongly suspected or definitely known to be CDI, to avoid the development of drug resistant bacteria. It has also been suggested that fidaxomicin may be used as a prophylactic agent against the development of CDI, particularly in immune suppressed patients76. Smeltzer & Hassoun77 reported the successful use of fidaxomicin in recurrent CDI in a child. However, it is yet not recommended for the treatment of paediatric patients.
Fidaxomicin is ineffective for systemic infections as its activity is confined to the gastrointestinal tract due to minimal absorption. Prospective randomized studies comparing fidaxomicin with metronidazole in the treatment of mild or moderate CDI and with vancomycin for severe CDI need to be done to look into the exact role of fidaxomicin in clinical practice78. Though no study of fidaxomicin as yet has been carried out in patients with hepatic or renal dysfunction, but as the drug is minimally absorbed, dosage adjustment may not be required for these groups of patients.
Fidaxomicin has no significant drug-drug interactions in clinical studies. Administration of fidaxomicin one hour before digoxin, a P-glycoprotein substrate showed no clinically meaningful pharmacokinetic interaction between the two drugs79. Cytochrome P-450 enzymes are weakly inhibited by fidaxomicin and OPT-1118, as seen by co-administration of marker substrate drugs such as warfarin, omeprazole and midazolam with fidaxomicin80. Cross-resistance with any other antibiotic class has not been reported as yet. However, caution should be exercised in patients with history of infection, any allergy and those taking other medications. Caution should also be taken in children, and in women during pregnancy and breast feeding.
Recurrences with fidaxomicin treatment
In a systematic review of 11 studies Drekonja et al81 observed that recurrent disease was less with fidaxomicin treatment (15%) compared to that with vancomycin (25%). Cornely et al82 reported from the two Phase III clinical trials of 1164 subjects enrolled, that a subgroup of 128 patients in the PP population had another recent episode of CDI diagnosis at study enrollment. When analysis of this subgroup was done, initial response to both fidaxomicin and vancomycin was similar (>90%). Recurrence occurred within 28 days in 35.5 per cent patients treated with vancomycin and 9.7 per cent patients treated with fidaxomicin. Recurrence within the first two weeks occurred in 27 per cent patients treated with vancomycin and 8 per cent patients treated with fidaxomicin. Though fidaxomicin was similar to vancomycin in achieving a clinical response at the end of therapy in patients with a first CDI recurrence, the drug was found to be superior in preventing a second recurrence within four weeks of investigation83.
Cornely et al84 investigated treatment response in 183 patients with cancer who were at increased risk for CDI and reported that fidaxomicin treatment was superior to vancomycin, resulting in higher cure and sustained response rates, shorter time to resolution of diarrhoea, and fewer recurrences. Louie et al85 investigated the effect of advancing age on the clinical outcomes of CDI treatment by regression modelling of results from the two double-blind randomized multicenter studies on the treatment of primary and first recurrent cases of CDI. Nine hundred ninety nine individuals with toxin-positive CDI were randomized to receive vancomycin (125 mg four times daily) or fidaxomicin (200 mg twice daily) for ten days. They observed that the model predicted a 17 per cent lower clinical cure, 17 per cent greater recurrence, and 13 per cent lower sustained clinical response by advancing decade than in those younger than 40. Clinical cure was similar in the fidaxomicin and vancomycin treatment groups, although fidaxomicin was associated with a more than 50 per cent lower relative risk for recurrence than vancomycin. Multivariate regression modelling showed that risk factors accounting for poorer outcomes with advancing age included infection with the BI strain type, inpatient status, renal insufficiency, leukocytosis, hypoalbuminaemia, and concomitant medication exposure. Measurable and progressive deterioration in CDI treatment outcomes occurred with advancing age in those aged 40 and older, highlighting the need for prevention and treatment strategies. Fidaxomicin treatment was associated with a 60 per cent lower risk of recurrence than vancomycin after adjusting for age, concomitant antibiotics, and C. difficile strain85.
Clutter et al86 assessed the feasibility of fidaxomicin versus vancomycin and metronidazole in 59 transplant recipients with 61 episodes of CDI. They reported that fidaxomicin was well tolerated by the patients and overall clinical cure occurred in 86 per cent of episodes, and in seven per cent of episodes, infection recurred. Clinical cures were not significantly different compared with conventional therapy (67 versus 89%, respectively). New-onset VRE colonization was not noted after fidaxomicin therapy alone. However, this occurred in 10 of 28 patients (36%) following conventional therapy, and two of three patients with subsequent bacteraemia died. Hostler and Chen87 have hypothesized that the same properties that confer reduced recurrence make fidaxomicin a promising agent for prophylaxis, particularly in high-risk patients.
Formulations available
Fidaxomicin is available in the form of 200 mg oblong tablets which are white or off-white in colour given twice daily for 10 days. Dificid was the first fidaxomicin brand approved by the US FDA. Fidaxomicin has been recently licensed by the European Medicines Agency as Dificlir. Dificlir has been accepted for use with National Health Service for Scotland, with the restriction that treatment of adults with a first CDI recurrence should be done on the advice of local microbiologists or specialists in infectious diseases. However, it has not been accepted by Scottish Medicine Consortium for first-line use in adults with severe CDI. An oral suspension of fidaxomicin is in Phase II development88.
Cost utility analysis and economic impact
The use of fidaxomicin has not yet become popular due to cost constraints compared to vancomycin and metronidazole89. The price for fidaxomicin is $135 (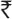 8,265) per 200 mg tablet compared to $31.81 (
8,265) per 200 mg tablet compared to $31.81 (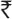 1,947) per 125 mg vancomycin capsule and $0.72 (
1,947) per 125 mg vancomycin capsule and $0.72 (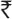 44) per 500 mg of metronidazole. Some hospitals add intravenous vancomycin, thereby increasing the price. The cost of treatment with vancomycin is approximately $139 (
44) per 500 mg of metronidazole. Some hospitals add intravenous vancomycin, thereby increasing the price. The cost of treatment with vancomycin is approximately $139 (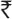 8,510) per day and that with fidaxomicin is about $296 (
8,510) per day and that with fidaxomicin is about $296 (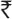 18,121) per day. Treatment of recurrent CDI increases the cost of therapy three-folds that of the primary infection90.
18,121) per day. Treatment of recurrent CDI increases the cost of therapy three-folds that of the primary infection90.
A cost utility analysis comparing fidaxomicin with oral vancomycin for the treatment of CDI91 reported that the drug remained cost-effective under all fluctuates of both fidaxomicin and oral vancomycin costs. Fidaxomicin was also cost-effective in patients receiving concomitant antimicrobials, in patients with mild to moderate CDI, and when compared with oral metronidazole in patients with mild to moderate disease. However, oral vancomycin was the drug of choice for CDI caused by NAP1/B1/027. The cost of fidaxomicin for refractory cases would be justified due to the lower recurrence rate by the drug. Bartsch et al92 developed a decision analytic simulation model to determine the economic value of fidaxomicin for CDI treatment from the third-party payer perspective. They looked at CDI treatment in cases where no fidaxomicin was given, those who received only fidaxomicin and those receiving fidaxomicin based on strain typing results. They concluded that in regards to the current cost and NAP1/BI/027 accounting for approximately 50 per cent of isolates, using fidaxomicin as a first-line treatment for CDI was not cost-effective, even though typing and treatment with fidaxomicin based on strain could be more promising depending on the costs of fidaxomicin. However, studies that define the risk stratification strategy are not available. Patients to be treated by fidaxomicin must be carefully selected, as for recurrent CDI, treatment with the same drugs used during the first CDI episode would be required93.
Fidaxomicin may have an economic impact on hospital budgets, as the drug may be used with increasing frequency for patients who do not respond to oral vancomycin in the management of recurrent CDI which may require hospitalization. The cost anticipated from subsequent hospitalization and retreatment can justify the use of fidaxomicin. Thus, it seems reasonable to use fidaxomicin as it reduces CDI recurrences. However, multiple factors would probably influence the use of fidaxomicin, such as willingness of health providers to prescribe a new medication, safety data accumulation when fidaxomicin becomes more common, patterns of incidence locally and CDI recurrence and costs.
Conclusion
Fidaxomicin is particularly active against C. difficile and its bactericidal properties make it an ideal alternative for CDI treatment. The cost factor and the institution capability of doing strain typing will ultimately decide the place of this new antibiotic in CDI treatment. Due to increase in frequency and severity of CDI, further research on the role of fidaxomicin for CDI treatment remains a priority. Moreover, clinical trials comparing fidaxomicin with metronidazole for mild to moderate CDI are required. The efficacy and safety data of fidaxomicin compared to vancomycin for patients with multiple recurrences and severe CDI are also needed. It is yet not known whether fidaxomicin can be efficiently used as a salvage therapy for recurrent CDI. In conclusion, the use of fidaxomicin for treatment of CDI should be considered taking into account the potential benefits of the drug, along with the medical requirements of the patient, the risks of treatment and the high cost of fidaxomicin compared to other treatment regimens.
References
- Preliminary investigation of environmental prevalence of Clostridium difficile affecting inpatients in a north Indian hospital. Indian J Med Microbiol. 2012;30:89-92.
- [Google Scholar]
- Emergence of Clostridium difficile infection due to a new hypervirulent strain, polymerase chain reaction ribotype 078. Clin Infect Dis. 2008;47:1162-70.
- [Google Scholar]
- Clostridium difficile associated infection, diarrhea and colitis. World J Gastroenterol. 2009;15:1554-80.
- [Google Scholar]
- Thinking beyond the colon-small bowel involvement in Clostridium difficile infection. Gut Pathog. 2009;19:7.
- [Google Scholar]
- Bacteremia due to Clostridium difficile--review of the literature. Int J Infect Dis. 2009;13:e305-9.
- [Google Scholar]
- Bacteremia due to Clostridium difficile: Case report and review of the literature. Clin Med Case Reports. 2009;2:5-9.
- [Google Scholar]
- Prevalence of PCR ribotypes among Clostridium difficile isolates from pigs, calves, and other species. J Clin Microbiol. 2007;45:1963-4.
- [Google Scholar]
- Decrease of hypervirulent Clostridium difficile PCR ribotype 027 in the Netherlands. Euro Surveill. 2009;14:19402.
- [Google Scholar]
- Changing epidemiology of Clostridium difficile infection following the introduction of a national ribotyping-based surveillance scheme in England. Clin Infect Dis. 2012;55:1056-63.
- [Google Scholar]
- Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis. 2011;53:42-8.
- [Google Scholar]
- Epidemiology and molecular characterization of Clostridium difficile strains from patients with diarrhea: low disease incidence and evidence of limited cross-infection in a Swedish teaching hospital. J Clin Microbiol. 2003;41:4031-7.
- [Google Scholar]
- Clostridium difficile colitis after kidney and kidney-pancreas transplantation. Clin Transplant. 1999;13:318-23.
- [Google Scholar]
- Clostridium difficile-associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol. 2008;198:635.
- [Google Scholar]
- Epidemiological features of Clostridium difficile-associated disease among inpatients at children's hospitals in the United States, 2001-2006. Pediatrics. 2008;122:1266-70.
- [Google Scholar]
- Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989-95.
- [Google Scholar]
- Increase in Clostridium difficile-related mortality rates: United States, 1999-2004. Emerg Infect Dis. 2007;13:1417-9.
- [Google Scholar]
- Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare associated infection due to methicillin resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32:387-90.
- [Google Scholar]
- Fidaxomicin (Dificid), a novel oral macrocyclic antibacterial agent for the treatment of Clostridium difficile-associated diarrhea in adults. Pharm Ther. 2012;37:278-81.
- [Google Scholar]
- Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34:346-53.
- [Google Scholar]
- The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28:1219-27.
- [Google Scholar]
- Clostridium difficile infection: clinical spectrum and approach to management. Indian J Gastroenterol. 2011;30:245-54.
- [Google Scholar]
- A new macrocyclic antibiotic, fidaxomicin (OPT-80), causes less alteration to the bowel microbiota of Clostridium difficile-infected patients than does vancomycin. Microbiology. 2010;156:3354-9.
- [Google Scholar]
- Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422-31.
- [Google Scholar]
- Clinical spectrum and pathogenesis of Clostridium difficile associated diseases. Indian J Med Res. 2010;131:487-99.
- [Google Scholar]
- The burden of Clostridium difficile in surgical patients in the United States. Surg. Infect (Larchmt). 2007;8:557-66.
- [Google Scholar]
- A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442-9.
- [Google Scholar]
- Clostridium difficile: an emerging epidemic in nursing homes. Geriatr Nurs. 2007;28:161-4.
- [Google Scholar]
- Risk factors for Clostridium difficile ribotype 027 infection in Germany: preliminary results of a retrospective case-control study. 18th European Congress of Clin. Microbial & Infectious Diseases, Barcelona, Spain, Abst 2008:1480.
- [Google Scholar]
- Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis. 2005;5:549-57.
- [Google Scholar]
- Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhoea: A cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41:254-60.
- [Google Scholar]
- An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433-41.
- [Google Scholar]
- Update of Clostridium difficile infection due to PCR ribotype 027 in Europe. Euro Surveill. 2008;31:13. pii=18942
- [Google Scholar]
- Toxinotype V Clostridium difficile in humans and food animals. Emerg Infect Dis. 2008;14:1039-45.
- [Google Scholar]
- Prevalence of Clostridium difficile in pseudomembranous and antibiotic associated colitis in North India. J Diarrhoeal Dis Res. 1986;4:157-60.
- [Google Scholar]
- Clostridium difficile and its cytotoxin in hospitalized children with acute diarrhea. Indian Pediatr. 1991;28:1129-32.
- [Google Scholar]
- Prevalence of Clostridium difficile in hospitalised patients with acute diarrhoea in Calcutta. J Diarrhoeal Dis Res. 1991;9:16-9.
- [Google Scholar]
- Clinical manifestation of Clostridium difficile enteritis in Calcutta. J Assoc Physicians India. 1991;39:683-4.
- [Google Scholar]
- Detection of Clostridium difficile toxin by an indigenously developed latex agglutination assay. Trop Gastroenterol. 1999;20:33-5.
- [Google Scholar]
- Faecal lactoferrin latex agglutination assay for Clostridium difficile associated intestinal disease. Indian J Med Microbiol. 1998;16:81-3.
- [Google Scholar]
- Etiology of diarrhea in patients undergoing allogenic bone marrow transplantation in South India. Transplantation. 2002;73:1247-51.
- [Google Scholar]
- Simultaneous assay for Clostridium difficile and fecal lactoferrin in ulcerative colitis. Trop Gastroenterol. 2003;24:13-6.
- [Google Scholar]
- Clostridium difficile toxin assay in psoriatic patients. Trop Gastroenterol. 2004;25:164-7.
- [Google Scholar]
- Diagnostic role of stool culture and toxin detection in antibiotic associated diarrhoea due to Clostridium difficile in children. Indian J Med Res. 2005;122:52-6.
- [Google Scholar]
- Changing pattern of Clostridium difficile associated diarrhoea in a tertiary care hospital: a 5 year retrospective study. Indian J Med Res. 2008;127:377-82.
- [Google Scholar]
- Clinical Practice Guidelines for Clostridium difficile Infection in Adults: 2010 Update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431-55.
- [Google Scholar]
- Clostridium difficile: controversies and approaches to management. Curr Opin Infect Dis. 2009;22:517-24.
- [Google Scholar]
- Recurrent Clostridium difficile diarrhea: characterstics of and risk factors for patients enrolled in a prospective, randomized, double blinded trial. Clin Infect Dis. 1997;24:324-33.
- [Google Scholar]
- Breaking the cycle: treatment strategies fort 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769-75.
- [Google Scholar]
- Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis. 2010;50:S16-S23.
- [Google Scholar]
- Fidaxomicin: A novel macrocyclic antibiotic approved for treatment of Clostridium difficile infection. Clin Infect Dis. 2012;54:568-74.
- [Google Scholar]
- Fidaxomicin: A novel macrocyclic antibiotic for the treatment of Clostridium difficile infection. Am J Health Syst Pharm. 2012;69:933-43.
- [Google Scholar]
- Lipiarmycin, a new antibiotic from Actinoplanes. I. Description of the producer strain and fermentation studies. J Antibiot. 1975;28:247-52.
- [Google Scholar]
- Lipiarmycin, a new antibiotic from Actinoplanes. II. Isolation, chemical, biological and biochemical characterization. J Antibiot. 1975;28:253-9.
- [Google Scholar]
- Tiacumicins, a novel complex of 18- membered macrolide antibiotics. I. Taxonomy, fermentation, and antibacterial activity. J Antibiot. 1987;40:567-74.
- [Google Scholar]
- Tiacumicins, a novel complex of 18-membered macrolides. II. Isolation and structure determination. J Antibiot. 1987;40:575-88.
- [Google Scholar]
- In vitro activities of OPT-80 and comparator drugs against intestinal bacteria. Antimicrob Agents Chemother. 2004;48:4898-902.
- [Google Scholar]
- In vitro activity of OPT-80 against Clostridium difficile. Antimicrob Agents Chemother. 2004;48:2280-2.
- [Google Scholar]
- European Medicines Agency (EMA). committee for medicinal products for human use. London, UK: EMA; 2011. p. :1-83.
- [Google Scholar]
- Safety, tolerance, and pharmacokinetic studies of OPT-80 in healthy volunteers following single and multiple oral doses. Antimicrob Agents Chemother. 2008;52:1391-5.
- [Google Scholar]
- Dificid (fidaxomicin) Tablets, prescribing information. San Diego, Calif: Optimer Pharmaceuticals; May, 2011. daccessed on December 12, 2013. Available from: www.dificid.com/files/prescribing.pdf
- [Google Scholar]
- Typing and susceptibility of bacterial isolates from the fidaxomicin (OPT-80) phase II study for Clostridium difficile infection. Anaerobe. 2009;15:234-6.
- [Google Scholar]
- Fidaxomicin is an inhibitor of the initiation of bacterial RNA synthesis. Clin Infect Dis. 2012;55:S127-31.
- [Google Scholar]
- Comparative susceptibilities to fidaxomicin (OPT-80) of isolates collected at baseline, recurrence, and failure from patients in two phase III trials of fidaxomicin against Clostridium difficile Infection. Antimicrob Agents Chemother. 2011;55:5194-9.
- [Google Scholar]
- Citron DM. Antimicrobial activities of fidaxomicin. Clin Infect Dis. 2012;55(Suppl 2):S143-8.
- [Google Scholar]
- Clinical outcomes, safety, and pharmacokinetics of OPT-80 in a phase 2 trial with patients with Clostridium difficile infection. Antimicrob Agents Chemother. 2009;53:223-8.
- [Google Scholar]
- Emerging therapies for Clostridium difficile infection - focus on fidaxomicin. Infect Drug Resist. 2013;6:41-53.
- [Google Scholar]
- OPT-80 eliminates Clostridium difficile and is sparing of bacteroides species during treatment of C. difficile infection. Antimicrob Agents Chemother. 2009;53:261-3.
- [Google Scholar]
- Reduced acquisition and overgrowth of vancomycin-resistant enterococci and Candida species in patients treated with fidaxomicin versus vancomycin for Clostridium difficile infection. Clin Infect Dis. 2012;55(Suppl 2):S121-6.
- [Google Scholar]
- Fidaxomicin inhibits spore production in Clostridium difficile. Clin Infect Dis. 2012;55:S162-9.
- [Google Scholar]
- Both fidaxomicin and vancomycin inhibit outgrowth of Clostridium difficile spores. Antimicrob Agents Chemother. 2013;57:664-7.
- [Google Scholar]
- Economic impact of fidaxomicin on CDI treatment in the United States. Am J Pharmacy. 2012;4:114-7.
- [Google Scholar]
- Successful use of fidaxomicin in recurrent Clostridium difficile infection in a child. J Antimicrob Chemother. 2013;68:1688-9.
- [Google Scholar]
- Fidaxomicin: the newest addition to the armamentarium against Clostridium difficile infections. Clin Ther. 2012;34:1-13.
- [Google Scholar]
- Optimer Pharmaceuticals, Inc. Dificid (fidaxomicin) tablets prescribing information. San Diego, CA; 2011 May. Available from: www.optimerpharma.com
- [Google Scholar]
- Fidaxomicin: New therapy for Clostridium difficile-associated diarrhea. Formulary. 2011;46:297-308.
- [Google Scholar]
- Comparative effectiveness of Clostridium difficile treatments: A systematic review. Ann Intern Med. 2011;155:839-47.
- [Google Scholar]
- Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis. 2012;55:S154-61.
- [Google Scholar]
- Comparative effectiveness of Clostridium difficile treatments: A Systematic Review. Ann Intern Med. 2011;155:839-47.
- [Google Scholar]
- Resolution of Clostridium difficile-associated diarrhea in patients with cancer treated with fidaxomicin or vancomycin. J Clin Oncol. 2013;31:2493-9.
- [Google Scholar]
- Effect of age on treatment outcomes in Clostridium difficile infection. J Am Geriatr Soc. 2013;61:222-30.
- [Google Scholar]
- Fidaxomicin versus conventional antimicrobial therapy in 59 recipients of solid organ and hematopoietic stem cell transplantation with Clostridium difficile-associated diarrhea. Antimicrob Agents Chemother. 2013;57:4501-5.
- [Google Scholar]
- Fidaxomicin for treatment of Clostridium difficile-associated diarrhea and its potential role for prophylaxis. Expert Opin Pharmacother. 2013;14:1529-36.
- [Google Scholar]
- Fidaxomicin: A novel macrocyclic antibiotic for the treatment of Clostridium difficile infection. Am J Health Syst Pharm. 2012;69:933-43.
- [Google Scholar]
- Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trails. Clin Infect Dis. 2012;55:S93-103.
- [Google Scholar]
- Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect. 2010;74:309-18.
- [Google Scholar]
- Cost-effectiveness analysis evaluating fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in the United States. Value Health. 2013;16:297-304.
- [Google Scholar]
- Is fidaxomicin worth the cost?. An economic analysis. Clin Infect Dis. 2013;57:555-61.
- [Google Scholar]
- Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431-55.
- [Google Scholar]






