Translate this page into:
Feasibility study of a novel intraosseous device in adult human cadavers
Reprint requests: Dr Balram Bhargava, Professor of Cardiology, Executive Director, Stanford-India Biodesign Program, Old OT Block, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110 029, India e-mail: balrambhargava@yahoo.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Intraosseous (IO) access is an alternative to difficult intravenous (iv) access during emergency clinical situations. Existing IO solutions are expensive, require power supply and trained manpower; limiting their use in resource constrained settings. To address these limitations, a novel IO device has been developed. The objectives of this study were to evaluate functionality and safety of this device in adult human cadavers.
Methods:
The ability of the IO device to penetrate the proximal and/or distal tibia was evaluated in three adult cadavers. Subjective parameters of loss of resistance, stable needle hold, easy needle withdrawal and any damage to the device were evaluated during the study. The insertion time was the objective parameter measured. Four sets of radiographs per insertion confirmed the position of the needle and identified complications.
Results:
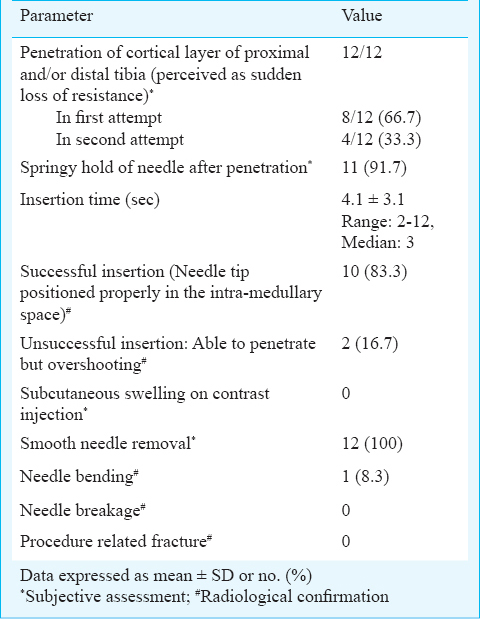
A single physician performed 12 IO access procedures using the same device. Penetration of proximal and/or distal tibia was achieved in all instances. It was successful in the first attempt in eight (66.7%) and during second attempt in the remaining. The mean time to insertion was 4.1 ± 3.1 sec. Appropriate insertion of needle in the intra-medullary space of bone was confirmed with radiological examination in 10 (83.3%) insertions. In two occasions after penetrating the cortical layer of bone, the device overshot the intra-medullary space, as detected by radiological examination. Device got bent during insertion in one instance. There was no evidence of needle breakage or bone fracture. The needle could be withdrawn effortlessly in all instances.
Interpretation & conclusions:
The novel IO device could successfully penetrate the adult cadaver bones in most cases. Further studies are needed to confirm these results on a large sample.
Keywords
Difficult intravenous access
emergency medicine
intraosseous access
intraosseous device
resuscitation
vascular access
Rapid vascular access is critical for resuscitation of patients during clinical emergency. Though peripheral intravenous (iv) access is preferred, it is difficult to gain venous access in many cases. This is frequently witnessed in patients with cardiac arrest, trauma, profound blood loss, severe dehydration and burn injury, especially in the pre-hospital settings. Failed peripheral iv access in such emergency conditions has been reported in 10-40 per cent cases1. The alternate procedure of inserting central venous catheter (CVC) in jugular or subclavian veins requires high technical skills2.
Intraosseous (IO) access is an alternative to such difficult iv access situations. IO access allows infusion of fluids directly into the intra-medullary venous channels which drain into systemic circulation. Clinical studies have shown that IO access is safe, simple, cost-effective and associated with low rate of complications345678. Success rate has been shown to be significantly higher and insertion time significantly shorter with IO access as compared to CVC insertion15.
The traditional IO needles are available in India and are used in paediatric patients. However, these devices are placed manually, hence not ideal for piercing the thick cortical bone of adult patients. There are only a few IO devices available in the developed countries which are suitable for both paediatric and adult patients. All these devices are expensive and are not easily available in India.
Due to the technical shortcomings of the existing IO devices, high cost, and lack of easy availability in India, a novel IO device under Stanford-India Biodesign (SIB) programme has been developed for emergency vascular access. This pilot study was designed to test the functionality and safety of this device. The objectives were to assess the (i) ability of the device to penetrate the cortical layers of proximal and/or distal tibia in adult human cadavers, and (ii) ability to use this device safely without any device or cadaver-related complications.
Material & Methods
This observational pilot study was performed at the Anatomy Department of All India Institute of Medical Sciences (AIIMS), New Delhi, India in November 2010. The study protocol was approved by the Institute's Ethics Committee.
Three human cadavers were prepared as per standard protocol. The bone structure of the cadavers was not changed. The proximal and distal ends of tibia were chosen as the IO insertion sites. Proximal tibial insertion was done approximately 1-3 cm below the tibial tuberosity while distal tibial insertion was done around 1-3 cm above the medial malleolus. All the insertions were performed by a single operator so as to avoid inter-operator variation in skill and subjective assessment of penetration. A single independent monitor was responsible for device inspection.
Device description & study procedure: The device consisted of a needle-trocar assembly attached to a lead screw, which in turn was attached to the handle of the device (Fig. 1). The needle was positioned using a ‘guide’ which was gripped on the target site with the non-dominant hand. This needle-trocar assembly was rotated by pushing the handle downward with the dominant hand. This downward movement rotated the lead screw which in turn rotated the needle-trocar assembly, making it penetrate the bone. Once the needle-trocar assembly pierced through the cortical layer of the bone (perceived as give-away feel by the operator), downward push to handle was stopped. Further, the ‘guide’ was removed and the trocar was pulled out of the needle-trocar assembly resulting in positioning the distal end of the hollow needle inside the bone.
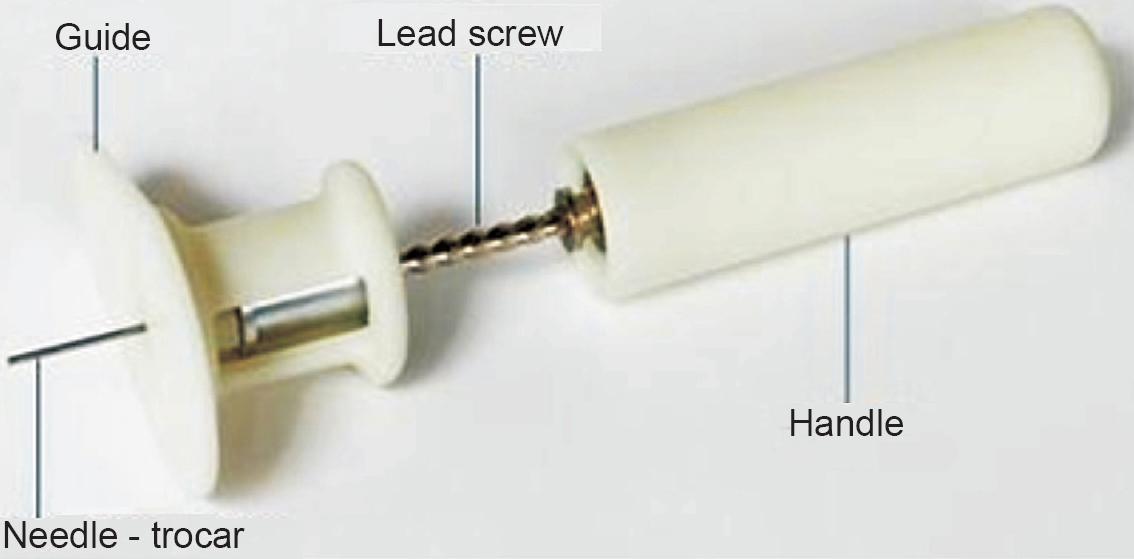
- Intraosseous (IO) device and its various parts.
A single device was used for all the procedures to prevent any variation due to device configuration. The device was inspected by the monitor before and after every insertion using a magnifying glass. The device was used only after it was certified by the monitor. The procedure of every insertion was video recorded.
For objective evidence, four sets of radiographs were taken during each IO insertion. Each set of imaging consisted of an antero-posterior and lateral view of the target site. The first set was taken prior to inserting IO device to rule out any pre-procedure bone fracture or bone aberration. Second set of radiographs was taken after IO needle insertion with the needle in situ and the trocar removed. This was to confirm the exact position of the needle and to verify the needle shape after insertion (straight, bent or broken). The third set of radiographs was taken after injecting 5-10 ml of contrast (76% Trazograf: meglumine diatrizoate and sodium diatrizoate) through the IO needle. This was to confirm the position of needle inside the bone (Fig. 2). Any extravasation of the contrast resulting in soft tissue swelling was observed and recorded. The fourth set of radiographs was taken after the needle removal. This set was taken to visualize any metal pieces left inside the cadaver bone due to needle breakage. Fracture and bone aberration related to IO procedure were checked at the site of insertion in all the radiographs.
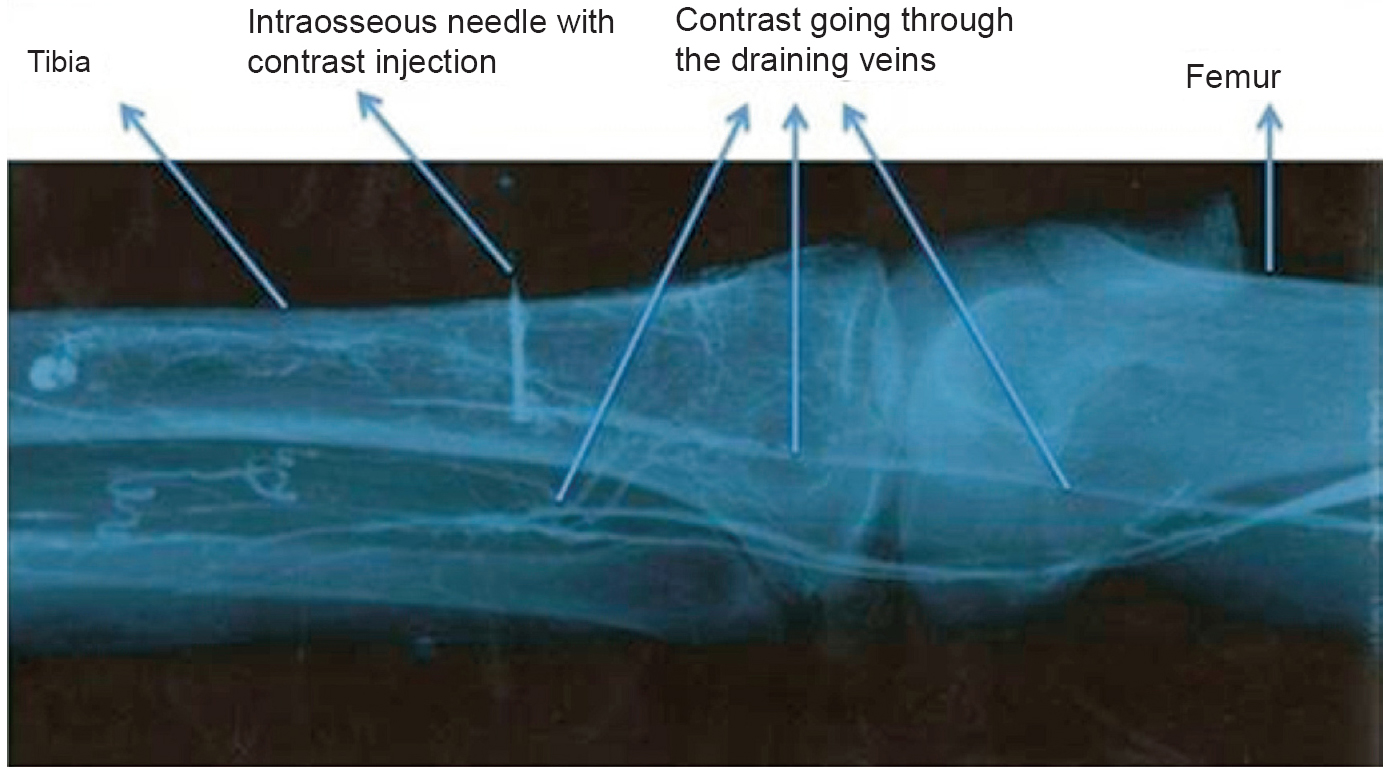
- Radiograph after contrast injection of a successful intraosseous device insertion. The needle was placed in the intra-medullary space of proximal tibia. The draining veins of the bone getting opacified in this radiograph.
Functionality evaluation: The ability of the device to penetrate was evaluated using subjective perception of sudden loss of resistance (give-away feel) by the operator after the needle penetrated the hard cortical layer to enter intra-medullary space. Penetration was also judged by stable and springy hold of the device after removing the trocar. The position of the tip of IO needle visualised by radiographs of the target site provided objective confirmation of successful penetration.
‘Successful’ insertion was defined as needle tip positioned in the intra-medullary space without any evidence of device or cadaver-related complications. Insertion was considered ‘failed’ if either it did not meet the radiological evidence of penetration or there was evidence of device or cadaver-related complications. ‘Overshooting’ of needle was defined as the needle tip ending beyond the medullary space as per radiological examination. Any leakage of the infused contrast into the subcutaneous tissue or soft tissue (subcutaneous) swelling at the local site was considered as ‘extravasation’. ‘Insertion time’ was calculated as per video-analysis of each successful insertion and was defined as the time elapsed between the placement of device at target site and the perception of sudden loss of resistance by the operator.
Safety evaluation: The insertion procedure was considered safe, if there was absence of (i) damage (bending and/or breakage) to the tip or body of the IO needle, as observed by visual inspection and confirmed by radiological examination, (ii) remains of broken metallic fragments of the needle or trocar at the target site as seen on radiographs, and (iii) fracture or bony aberration of the target site, as per radiological examination.
Data collection: Perception of sudden loss of resistance, springy hold of the needle after penetration and the ease of needle removal (smooth/ difficult) were noted for each insertion. Additionally, procedural success, insertion time and any device or cadaver-related complication were recorded.
Statistical analysis: All relevant study data were entered into an electronic database and evaluated using Microsoft Excel-2007 (Microsoft Corporation, Washington, USA) software. The results are presented as absolute values, percentages, and mean ± standard deviation, wherever applicable.
Results
The IO device was used for 12 consecutive insertions on three cadavers. Two anatomical locations, proximal tibia and distal tibia were used on each leg of a cadaver. This resulted in four insertions per cadaver.
In eight (66.7%) insertions, penetration (perceived as sudden of loss of resistance by the operator) was achieved in the first attempt. In the remaining four (33.3%), the operator failed to perceive the sudden loss of resistance in the first attempt. However, in these four cases after removal and placement of device at a different anatomical point of the same target site, the operator could perceive sudden loss of resistance. Stable and springy hold of the device after penetration of the needle and removal of the trocar was observed in 11 (91.7%) insertions. The mean insertion time was 4.1 ± 3.1 seconds (range: 2-12, median: 3 sec).
Overall, the procedure was successful in 10 (83.3%) insertions. The needle tip as confirmed by radiological examination was positioned properly in the intra-medullary space in these insertions. Overshooting of the needle was observed in two instances of failed insertions (Table). There was no incidence of extravasation after contrast injection. Needle bending occurred in one (8.3%) insertion which was also confirmed by radiological examination. The bent needle was repaired and thoroughly inspected and certified before being used for next insertion. No broken fragments of the needle or trocar were observed in any radiograph. There was no incidence of fracture or bone aberration related to the IO device insertion. Needle removal was smooth and effortless in all instances (Table).
Discussion
The IO infusion allows direct access to venous channels in highly vascular intra-medullary space of the long bones. These channels do not collapse even in the presence of shock as these are supported by bone matrix. IO access was initially recommended for children aged six years or younger910. However, now there is sufficient evidence that this procedure is also safe in older children and adults1112. The available guidelines recommend IO access as the first alternative to iv access in patients with cardiac arrest213. The antero-medial aspect of the tibia is preferably used for IO infusion as it lies just under the skin and can be easily located.
Compared to paediatric patients, adults have thicker bony cortex and smaller intra-medullary space1415. Traditional manual IO devices require high thrust force to pierce this thick cortex, making IO insertion difficult in adult population. In a study by Brenner et al16, traditional manual IO access has been shown to be successful in 80 per cent in the first attempt. In 13 per cent cases, IO access could not be achieved even after three attempts in adult human cadaver. In comparison, battery-driven IO device (Teleflex Inc., USA) was successful in more than 95 per cent cases in the first attempt16. Studies have revealed the superiority of battery-driven IO device over manual needles and also over other semi-automatic IO infusion devices1017. In a pre-hospital clinical setting, overall success rates of IO insertion were 50 per cent with the manual needle and 96 per cent with the battery-driven IO18. With our novel device, successful penetration could be achieved in approximately 70 per cent insertions in the first attempt, while the remaining could be accomplished in the second attempt. No instance of failure to penetrate the cortical layer of bone was observed. Needle bending was observed only in one instance with our device, even though same device was being used for all insertions. In comparison, needle bending or breakage has been seen in 15 per cent cases using traditional manual needle16. This is probably because this novel device penetrates using both rotational and axial thrust forces hence requiring less force. There was no incidence of target site fracture during this study. Per se, this complication is infrequent with available IO devices19. All insertions could be accomplished in less than 15 sec. This was comparable with the insertion time using traditional manual devices as well as automated devices16.
The novel device used in this study is intended to be for single-use and disposable, and does not require any power supply.
The present study had certain limitations. The IO device was evaluated in adult human cadavers. These results cannot be extrapolated to paediatric patients. Owing to the human cadaver, the occurrence of other live patient-related complications such as hematoma, infection, fat embolism could not be assessed. However, such complications have been reported to be rare with IO insertion14151619. In the present study, all 12 insertions were done using a single device by a single operator. The device needs to be tested by multiple operators using each device only once. It is plausible that with single use, better results may be observed.
In conclusion, the newly-developed IO device could successfully penetrate the adult cadaver bones in most cases. The device needs further testing in pre-clinical studies using large sample size and comparing with the commercially available IO devices.
Acknowledgment
The authors acknowledge the Department of Biotechnology, Ministry of Science and Technology, Government of India, for providing funds under the SIB Program, and Johnson & Johnson's Corporate Office of Science and Technology, USA, for providing additional grant-in-aid to develop this device. The authors also acknowledge the services of Biotech Consortium India Limited, New Delhi, for managing the funds and intellectual property; and LUNAR (San Francisco, California) for assisting in prototype refinement.
Conflicts of Interest: None.
References
- Comparison of intraosseous versus central venous vascular access in adults under resuscitation in the emergency department with inaccessible peripheral veins. Resuscitation. 2012;83:40-5.
- [Google Scholar]
- Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122(18 Suppl 3):S729-67.
- [Google Scholar]
- Five-year experience in prehospital intraosseous infusions in children and adults. Ann Emerg Med. 1993;22:1119-24.
- [Google Scholar]
- Intraosseous vascular access is safe, effective and costs less than central venous catheters for patients in the hospital setting. J Vasc Access. 2013;14:216-24.
- [Google Scholar]
- EZ-IO(®) intraosseous device implementation in a pre-hospital emergency service: A prospective study and review of the literature. Resuscitation. 2013;84:440-5.
- [Google Scholar]
- Intraosseous versus intravenous vascular access during out-of-hospital cardiac arrest: a randomized controlled trial. Ann Emerg Med. 2011;58:509-16.
- [Google Scholar]
- “PALS for life!” A required trauma-oriented pediatric advanced life support course for pediatric and emergency medicine house staff. Ann Emerg Med. 1984;13:1044-7.
- [Google Scholar]
- Current advances in intraosseous infusion - a systematic review. Resuscitation. 2012;83:20-6.
- [Google Scholar]
- The role of intraosseous vascular access in the out-of-hospital environment (resource document to NAEMSP position statement) Prehosp Emerg Care. 2007;11:63-6.
- [Google Scholar]
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2005;112((24 Suppl)):IV 1-203.
- [Google Scholar]
- Intraosseous devices for intravascular access in adult trauma patients. Crit Care Nurse. 2011;31:76-89.
- [Google Scholar]
- The use of a powered device for intraosseous drug and fluid administration in a national EMS: a 4-yr experience. J Trauma. 2008;64:650-4.
- [Google Scholar]
- Comparison of two intraosseous infusion systems for adult emergency medical use. Resuscitation. 2008;78:314-9.
- [Google Scholar]
- Intraosseous access in the prehospital setting: literature review. Prehosp Disaster Med. 2012;27:468-72.
- [Google Scholar]
- Emergency intraosseous access in a helicopter emergency medical service: a retrospective study. Scand J Trauma Resusc Emerg Med. 2010;18:52.
- [Google Scholar]
- Intraosseous drug administration in children and adults during cardiopulmonary resuscitation. Ann Pharmacother. 2007;41:1679-86.
- [Google Scholar]






