Translate this page into:
Fas cell surface death receptor/Fas ligand genetic variants in gastric cancer patients: A case-control study
For correspondence: Dr Ali Bidmeshkipour, Department of Biology, Faculty of Sciences, Razi University, Kermanshah, Iran e-mail: a.bidmeshkipour@gmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Various studies have suggested a correlation between Fas cell surface death receptor/Fas ligand (FAS/FASL) variants and multiple types of cancers. The present study aimed to investigate the association between FAS-670A/G and FASL-844C/T and the synergistic effects of both variants on the risk of gastric cancer (GC) in the Kurdish population of west of Iran.
Methods:
This study was conducted by polymerase chain reaction-restriction fragment length polymorphism technique using MvaI and BsrDI restriction enzymes in 98 GC patients and 103 healthy control individuals.
Results:
According to the obtained results, a significant association (P=0.008) of FASL polymorphism among GC patients and the control group was detected. Furthermore, no significant differences were found in the FAS polymorphism frequencies between GC patients and the control group. Codominant and dominant models in FASL polymorphism showed significant protective effects against GC [odds ratio (OR)=0.307, 95% confidence interval (CI) (0.134-0.705), P=0.005; OR=0.205, 95% CI (0.058-0.718), P=0.013 and OR=0.295, 95% CI (0.129-0.673), P=0.004 for models of codominant CC vs. CT, codominant CC vs. TT and dominant, respectively]. Furthermore, the presence of both FAS-670G and FASL-844T alleles represented a significant protective effect against GC occurrence [OR=0.420, 95% CI (0.181-0.975), P=0.043].
Interpretation & conclusions:
So far, we believe this is the first study, the results of which suggest that FASL gene variation and its synergistic effects with FAS gene could be associated with the risk of GC in the Kurdish population in the west of Iran.
Keywords
FAS-670A/G
FASL-844C/T
gastric cancer
synergistic effect
variant
Gastric cancer (GC) is one of the most common malignancies and the second most common cause of cancer-related deaths in human societies1. GC, is affected by various environmental and genetic factors. According to the genetic background, lifestyle, diet, alcohol consumption, smoking and pathogenic infections, the prevalence of this type of cancer varies between populations2-6. Single nucleotide polymorphisms (SNPs) have an essential role in pathogenesis of GC. At the outset, various human diseases, such as cancers, are associated with apoptosis dysregulation. Apoptosis is one of the most important regulatory mechanisms in cellular homoeostasis, regulation of cell density and removing undesirable multicellular organisms7. Programmed cell death occurs via two pathways: intrinsic or mitochondrial dependent and extrinsic or receptor mediated8. Interaction between Fas cell surface death receptor (FAS) and Fas ligand (FASL) is considered a crucial factor particularly in the in extrinsic pathways9,10.
According to previous reports, association between genetic polymorphisms is involved in apoptosis and cancer incidence11,12. FAS (Apo-1, CD95) and its normal ligand (FASL, CD95L) have a vital role in initiating the apoptotic signalling pathway13. FAS and FASL genes are found on 10q24.1 and 1q23 chromosomes, respectively14. The genes responsible for FAS and FASL encoding represented several functional polymorphisms affecting FAS and FASL expression levels. FAS-670A/G and FASL-844 C/T polymorphisms are the functional SNPs in the promoter region of these two genes15. FAS-670A/G polymorphism is caused by two events of A to G substitution at the position of -670 in the promoter region of FAS gene and disorder in binding site of STAT-1 (signal transducer and activator of transcription-1)16. These alterations can lead to a reduction in the promoter activity and as a result, a decreased expression level of the FAS gene. In addition, FASL-844C/T polymorphism at FASL gene promoter could affect the expression rate of this gene by T to C substitution17. FASL-844C/T variant occurs in a putative binding motif of a transcription factor (including CAAT/enhancer-binding protein β) which leads to alteration of its binding site. This polymorphism can lead to a higher basal expression level in FASL-844C allele carriers than FASL-844T17. Some studies have demonstrated a significant association between FAS-670A/G and FASL-844C/T polymorphisms with GC18,19. The present study was designed to investigate the association between these two functional polymorphisms in the promoter regions of FAS and FASL genes and their synergistic effects on GC risk in the Kurdish population in the west of Iran.
Material & Methods
Study subjects: Ninety eight GC patients and 103 healthy controls with a mean age of 53±6.43 and 47±7.43 yr, respectively, were included in the present study. All patients and healthy individuals had a Kurdish ethnic background, and were recruited among the patients referred to the Imam Reza Hospital, Kermanshah University of Medical Sciences, Kermanshah, Iran, from March 2018 to April 2019. All patients were selected among the population referred to the hospital by an oncologist for endoscopic procedure (due to the dyspeptic symptoms) and whole cancer cases of gastric adenocarcinoma (for post-operative histopathological diagnosis). Individuals with other ethnic backgrounds, incomplete clinical data and medical history of other disorders (such as diabetes and cardiovascular diseases) were excluded from the study. The control group constituted patients visiting Imam Reza Hospital during the same period, with the same ethnic background, gender and age, with no history of cancer. The study was approved by the Ethics Committee of Kermanshah University of Medical Sciences, Kermanshah, Iran. All participants were informed about the study’s objectives, and a written informed consent was also obtained.
DNA extraction and genotyping: Genomic DNA was extracted from the peripheral blood (3 ml) using the standard phenol-chloroform extraction method. Purified genomic DNA was evaluated with a NanoDrop spectrophotometer (wavelengths of 260 and 280 nm; Thermo Fisher Scientific, Wilmington, DE 19810, USA) and electrophoresis on one per cent agarose gel. FAS and FASL polymorphisms were analyzed using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method (Figs 1 and 2). PCR was carried out for polymorphism amplification using the previously published primers for FAS-670A/G variant20 and FASL-844C/T variant21. PCR and RFLP techniques were conducted according to the conditions mentioned in a previous study22.
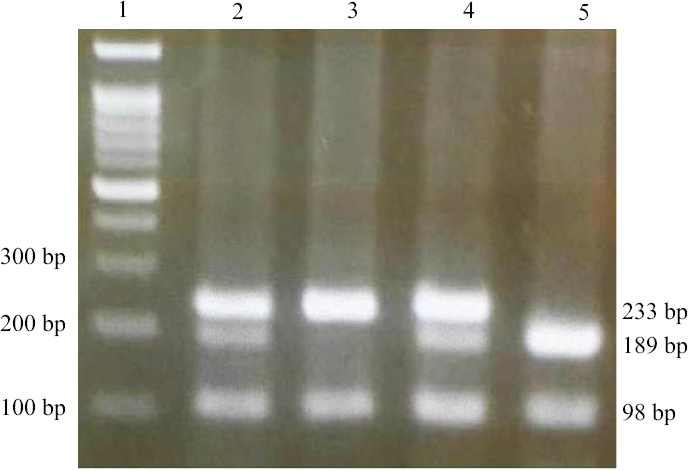
- A pattern of agarose gel electrophoresis for FAS-670A/G variant determined by PCR-RFLP method, the PCR products were digested by MvaI enzyme. Lane 1: marker (50-bp DNA ladder); lanes 2 and 4: AG genotype (233, 189, 98 and 44 bp); lane 3: GG genotype (233 and 98 bp); lane 5: AA genotype (189, 98 and 44 bp). PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism.
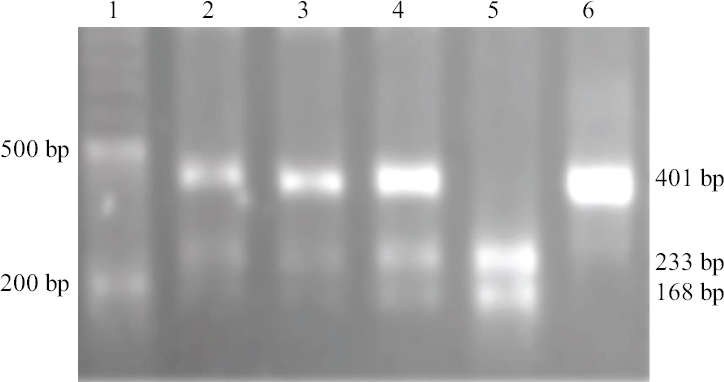
- A pattern of agarose gel electrophoresis for FASL-844C/T variant determined by PCR-RFLP method. PCR products were digested by BsrDI enzyme. Lane 1: marker (50-bp DNA ladder); lanes 2, 3 and 4: CT genotype (401, 233 and 168 bp); lane 5: CC genotype (233 and 168 bp); lane 6: TT genotype (401 bp).
Statistical analysis: SPSS statistical software version 21.0 (IBM Corp., Armonk, NY, USA) was used for data analysis. Genotype and allele frequencies of polymorphisms in both patient and healthy groups were analyzed using Pearson’s Chi squared test. Odds ratios (ORs) were computed to determine the risk of GC with 95 per cent confidence intervals (CIs) for FAS-670A/G and FASL-844C/T polymorphisms in patient and control groups using logistic regression models. In this test, the GC and controls were the dependent index (Y variable), and also, different genotypes or alleles were independent variables (X variables). P<0.05 was considered as significant and the data were shown as the mean±standard deviation.
Results
Association of Fas cell surface death receptor (FAS) and Fas ligand (FASL) variants with gastric cancer (GC) risk: The frequency of G and C alleles was found to be higher in patients as compared to the healthy controls. In FAS-670A/G polymorphisms, AA and GG genotype frequency was lower in patients than healthy controls. However, the frequency of AG genotypes was higher in patients compared to the control group (P=0.13). In addition, the results of FASL-844C/T polymorphisms showed that the frequency of CT and TT genotypes was significantly lower in patients than the control group, while the frequency of CC genotypes was significantly higher in patients compared to the healthy controls (P=0.008). Analysis models of dominant and codominant in FASL polymorphism had a significant protective effect against GC incidence (P<0.05) (Table I). Moreover, the OR of CT, TT and CT+TT genotypes suggested that these genotypes may have protective effects against GC risk (P<0.05). The OR of FASL-844C/T genotypes with respect to CC genotype in the patient group were 0.307 (0.134-0.705, P=0.005), 0.205 (0.058-0.718, P=0.013, and 0.295 (0.129-0.673, P=0.004 for CT, TT and CT+TT genotypes, respectively.
| Genotype/allele | Patient group (n=98) | Control group (n=103) |
|---|---|---|
| FAS-670A/G (genotypes) | ||
| AA (%) | 22 (22.4) | 31 (30.1) |
| AG (%) | 63 (64.3) | 52 (50.5) |
| GG (%) | 13 (13.3) | 20 (19.4) |
| χ2, df, P | 3.943, 2, 0.13 | |
| FAS-670A/G (alleles) | ||
| A (%) | 107 (54.6) | 114 (55.4) |
| G (%) | 89 (45.4) | 92 (44.6) |
| χ2, df, P | 0.000, 1, 1.00 | |
| FASL-844 C/T (genotypes) | ||
| CC (%) | 24 (24.5) | 9 (8.7) |
| CT (%) | 68 (69.4) | 83 (80.6) |
| TT (%) | 6 (6.1) | 11 (10.7) |
| χ2, df, P | 9.660, 2, 0.008 | |
| Dominant (CC vs. CT+TT) | 24 (24.5%) vs. 74 (75.5%) | 9 (8.7%) vs. 94 (91.3%) |
| χ2, df, P | 9.080, 1, 0.003 | |
| Co dominant CC vs. CT | 24 (26.1%) vs. 68 (73.9%) | 9 (9.8%) vs. 83 (90.2%) |
| χ2, df, P | 8.308, 1, 0.004 | |
| Co dominant CC vs. TT | 24 (80.0%) vs. 6 (20.0%) | 9 (45.0%) vs. 11 (55.0%) |
| χ2, df, P | 6.551, 1, 0.010 | |
| FASL-844 C/T (alleles) | ||
| C (%) | 116 (59) | 101 (49) |
| T (%) | 80 (41) | 105 (51) |
| χ2, df, P | 2.013, 1, 0.156 | |
df, degree of freedom
Association of combined FAS and FASL genotypes with GC risk: The synergistic effects of FAS-670G and FASL-844T alleles on the risk of GC incidence were also analyzed. Based on our results, the presence of both FAS-670G and FASL-844T alleles in individuals suggested a significant protective effect against GC occurrence [OR=0.420, 95% CI (0.181-0.975), P=0.043]. In fact, a considerable interaction between FAS-670G and FASL-844T alleles in GC was observed (Table II).
| FAS-670 G | FASL-844 T | Control group, n (%) | Patient group, n (%) [OR (95% CI, P)] |
|---|---|---|---|
| − | − | 9 (8.7) reference group | 22 (22.4) reference group |
| − | − | 22 (21.4) | 0 (0) |
| + | − | 0 (0) | 2 (2) |
| + | + | 72 (69.9) | 74 (75.6), [OR=0.420, 95% CI (0.181-0.975, P=0.043)] |
Discussion
Numerous complexes of molecular variations are involved in the pathogenesis of GC. Previous reports suggested that the FAS/FASL system could promote the apoptotic cell death13,23,. It has also been suggested that the alteration in FAS and FASL gene expression is associated with the risk of various cancers such as GC. Furthermore, in comparison with healthy individuals, the functional variants in these genes could potentially alter the expression levels of FAS and FASL genes by affecting the promoter activity and binding sites of transcription factors16,17. The results of the present study suggest that the FASL-844C/T polymorphism is significantly related to the risk of GC (P=0.008), while the association of FAS-670A/G polymorphism was found non-significant in patients with GC in comparison with healthy controls (P=0.13). In addition, our results showed that the concomitant presence of both FAS-670G and FASL-844T alleles in individuals had a significant protective role in GC development in the Kurdish population in the west of Iran (P=0.043) (Table II).
Various investigations have so far been carried out to clarify the probable impacts of FAS-670A/G and FASL-844C/T polymorphisms on GC18,25-27. For example, Liu et al27 in China proposed the FAS/FASL polymorphisms as a risk factor for GC incidence and introduced its multifactorial interactions with MMP-2 polymorphism as an effective impact on GC development. Similar to the present study, Li et al25, in a meta-analysis investigation, demonstrated that FASL-844T>C polymorphism could be associated with the risk of developing GC. In addition, another study reported that the FAS and FASL polymorphisms are crucial factors in the development of gastric atrophy and intestinal metaplasia in Helicobacter pylori-infected patients19. In this study, it was found that the FASL-844C allele considerably increased the risk of gastric atrophy development19. In another study by Wang et al28, it was concluded that an increased level of FAS gene expression could improve the therapeutic outcomes in GC patients. However, in the study by Kupcinskas et al29, no significant association was found amongst FAS-670A/G and FASL-844C/T polymorphisms with GC in the Caucasian population. In addition, it was concluded that the FAS-670A/G genotype could reduce the risk of gastric cardiac carcinoma (GCA) in smokers. The A/G genotype among smokers were found to have a lower risk of GCA compared to A/A genotype. However, the FASL-844T/C variant was not associated with the risk of GCA development18. Thus, the previous studies and the data presented in the present investigation proposed that genetic variations in FAS/FASL system could play an essential role in the GC pathogenesis.
Some of the studies evaluated the association of FAS and FASL variants with various types of cancers (including prostate, breast, chronic myeloid leukaemia, oesophageal, head and neck and cervical cancers)11,12,21,30,31. These displayed a significant association between the alleles and genotypes of FAS and FASL with the risk of cancer. For example, Sun et al21 demonstrated that the genetic variants in FAS and FASL genes were associated with an increased risk of oesophageal squamous cell carcinoma in the Chinese population. Another study reported these to have increased the apoptosis rate of lymphocytes associated with the susceptibility of individuals to breast cancer11. Edathara et al12 suggested a considerable association between FAS-670GG, FASL-844TC and CC genotypes with the risk of chronic myeloid leukaemia in the Indian population. Various polymorphic variants have been reported in the field of GC with different results related to the sample size, techniques, heterogeneity of GC phenotype, ethnic differences and geographical variations. Phenotypic effects of gene variants are moderated by other genetic and environmental factors, which is a clear example of gene–environment interaction in specific phenotypic development. In present study, the data related to environmental risk factors (including family history, alcohol consumption, smoking, dietary and pathogenic infections) were not available for investigation of GC development and this was a limitation.
Overall, the authors believe the present study for the first time, suggests that FASL-844C/T polymorphism and its synergistic effects with FAS gene could be significantly related to the GC risk in the Kurdish population in the west of Iran. Further case-control studies among different ethnicities are however, recommended to determine the accurate biological roles of these variations in cancer development.
Acknowledgment:
Authors acknowledge Dr Farhad Shaveisi-Zadeh for effective involvement in human samples collection for oncology study and referring patients to the Imam Reza Hospital.
Financial support & sponsorship: The study was funded by the intramural grant of Kermanshah University of Medical Sciences, in Iran (vide grant no. 97278).
Conflicts of Interest: None.
References
- Competing endogenous RNA networks and gastric cancer. World J Gastroenterol. 2015;21:11680-7.
- [Google Scholar]
- Environmental and heritable factors in the causation of cancer-analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78-85.
- [Google Scholar]
- The association of cigarette smoking with gastric cancer:The multiethnic cohort study. Cancer Causes Control. 2012;23:51-8.
- [Google Scholar]
- Alcohol consumption and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Am J Clin Nutr. 2011;94:1266-75.
- [Google Scholar]
- Contributions of the Epstein-Barr virus EBNA1 protein to gastric carcinoma. J Virol. 2012;86:60-8.
- [Google Scholar]
- Functional polymorphisms of FAS and FASL gene and risk of breast cancer –Pilot study of 134 cases. PLoS One. 2013;8:e53075.
- [Google Scholar]
- The role of intrinsic pathway in apoptosis activation and progression in Peyronie's disease. Biomed Res Int. 2014;2014:616149.
- [Google Scholar]
- The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233-43.
- [Google Scholar]
- Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992;267:10709-15.
- [Google Scholar]
- Functional polymorphisms in FAS and FASL contribute to increased apoptosis of tumor infiltration lymphocytes and risk of breast cancer. Carcinogenesis. 2007;28:1067-73.
- [Google Scholar]
- Association of promoter polymorphisms of Fas-FasL genes with development of Chronic myeloid leukemia. Tumour Biol. 2016;37:5475-84.
- [Google Scholar]
- Polymorphisms in cell death pathway genes are associated with altered sperm apoptosis and poor semen quality. Hum Reprod. 2009;24:2439-46.
- [Google Scholar]
- FAS rs2234767 and rs1800682 polymorphisms jointly contributed to risk of colorectal cancer by affecting SP1/STAT1 complex recruitment to chromatin. Sci Rep. 2016;6:1-8.
- [Google Scholar]
- Functional FAS promoter polymorphisms are associated with increased risk of acute myeloid leukemia. Cancer Res. 2003;63:4327-30.
- [Google Scholar]
- A novel polymorphic CAAT/enhancer-binding protein beta element in the FasL gene promoter alters Fas ligand expression:A candidate background gene in African American systemic lupus erythematosus patients. J Immunol. 2003;170:132-8.
- [Google Scholar]
- Polymorphisms in promoter region of FAS and FASL gene and risk of cardia gastric adenocarcinoma. J Gastroenterol Hepatol. 2010;25:555-61.
- [Google Scholar]
- Polymorphisms of death pathway genes FAS and FASL and risk of premalignant gastric lesions. Anticancer Res. 2008;28:97-103.
- [Google Scholar]
- Identification and characterization of polymorphisms in the promoter region of the human Apo-1/Fas (CD95) gene. Mol Immunol. 1997;34:577-82.
- [Google Scholar]
- Polymorphisms of death pathway genes FAS and FASL in esophageal squamous-cell carcinoma. J Natl Cancer Inst. 2004;96:1030-6.
- [Google Scholar]
- Association analysis of FAS-670A/G and FASL-844C/T polymorphisms with risk of generalized aggressive periodontitis disease. Biomed Rep. 2018;8:391-5.
- [Google Scholar]
- Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol Cell Biol. 1999;77:312-7.
- [Google Scholar]
- Prognostic significance of Fas and Fas ligand system-associated apoptosis in gastric cancer. Ann Surg Oncol. 2000;7:750-7.
- [Google Scholar]
- Association between the FAS/FASL polymorphisms and gastric cancer risk:A meta-analysis. Asian Pac J Cancer Prev. 2012;13:945-51.
- [Google Scholar]
- Functional polymorphisms in FAS/FASL system contribute to the risk of occurrence but not progression of gastric cardiac adenocarcinoma. Hepatogastroenterology. 2012;59:141-6.
- [Google Scholar]
- Association of candidate genetic variations with gastric cardia adenocarcinoma in Chinese population:A multiple interaction analysis. Carcinogenesis. 2011;32:336-42.
- [Google Scholar]
- Lack of association between gene polymorphisms of angiotensin converting enzyme, Nod-like receptor 1, Toll-like receptor 4, FAS/FASL and the presence of Helicobacter pylori-induced premalignant gastric lesions and gastric cancer in Caucasians. BMC Med Genet. 2011;12:112.
- [Google Scholar]
- Polymorphisms of FAS and FAS ligand genes involved in the death pathway and risk and progression of squamous cell carcinoma of the head and neck. Clin Cancer Res. 2006;12:5596-602.
- [Google Scholar]
- FASL–844C polymorphism is associated with increased activation-induced T cell death and risk of cervical cancer. J Exp Med. 2005;202:967-74.
- [Google Scholar]






