Translate this page into:
Extended-spectrum β-lactamase & carbapenemase-producing Gram-negative bacilli in neonates from a tertiary care centre in Dibrugarh, Assam, India
*For correspondence: jmahanta@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
The choice of first-line antibiotic for the initiation of treatment in cases of neonatal sepsis/meningitis is a challenge to a clinician. It is further complicated when it is suspected to be caused by drug-resistant bacteria. β-lactam antibiotics are used widely worldwide against infections caused by Gram-negative bacteria. Resistance is known to be due to various mechanisms among which production of extended-spectrum β-lactamases (ESBLs) and metallo-β-lactamases (MBLs) is reported123456789101112.
Presence of ESBLs and MBLs has been reported among bacterial isolates from the neonates34567. New Delhi metallo-β-lactamase (NDM-1)-producing Enterobacteriaceae has also been isolated from neonates with sepsis in India347. The present study reports the presence of blaSHV, blaTEM, blaCTX-MU, blaOXA23-like, blaOXA51-like, blaOXA58-like and blaNDM1 among the Enterobacteriaceae (Klebsiella and Escherichia coli) as well as non-fermenters (Acinetobacter baumannii and Pseudomonas spp. ) obtained from the cerebrospinal fluid (CSF) of neonates from a tertiary care centre in Dibrugarh, Assam, India.
Consecutive admissions in the neonatal ward of Paediatrics department, Assam Medical College and Hospital, Dibrugarh, with symptoms of suspected meningitis were screened for bacterial aetiology from January 2013 to January 2015. All microbiological procedures were carried out in the Bacteriology division of Microbiology Laboratory, Regional Medical Research Centre, Dibrugarh. Lumbar puncture for CSF collection (volume up to 1 ml) was done at the neonatology unit. CSF analysis was done when there was clinical or proven sepsis and in whom blood culture grew microorganism. Ethical clearance for the study was obtained from the ethics committees of both the institutions (AMC/EC/8094 and RMRC/Dib/IEC(human)/2012-13/329). Written informed consent was obtained from parents/ guardians of all neonates.
A total of 67 (22.1%) of the 303 CSF samples tested were positive for pathogens. Gram-negative organisms were predominant (n=32, 48%). The most frequent Gram-negative bacteria isolated were Acinetobacter baumannii (n=12), Klebsiella spp. (n=8) and Pseudomonas spp. (n=6). Less frequently isolated were Neisseria meningitidis (n=2), Escherichia coli (n=1), Sneathia (n=1), Cronobacter sakazakii (n=1) and Roseomonas cervicalis (n=1). In the present study, isolates of A. baumannii (n=12), Klebsiella (n=8), Pseudomonas spp. (n=6) and E. coli (n=1) were characterized. Three isolates of A. baumannii, two of Klebsiella and one isolate of Pseudomonas detected by the direct CSF polymerase chain reaction (PCR)13 were included for genotypic characterization. Isolates resistant to at least one of the following: cefotaxime, ceftriaxone, ceftazidime, or cefepime were screened for ESBL as per the Clinical Laboratory Standard Institute (CLSI) guidelines14.
Screening for carbapenemase production was done by disc diffusion using ertapenem (10 μg) disc as per the CLSI14. A zone size of 19-21 mm was considered positive for carbapenemase production. Those showing carbapenemase production by screening test were tested by modified Hodge test (MHT)14. For quality control, Klebsiella pneumoniae ATCC BAA 1705 and BAA 1706 were taken as positive and negative controls. Carbapenem-resistant isolates were also tested for MBL production by phenotypic disc confirmatory test (PDCT)15.
Genotypic analysis for blaNDM-1, blaSHV, blaTEM, blaOXA23-like, blaOXA51-like, blaOXA58-like and blaCTX-MU was performed for isolates found to be positive in ESBL and MBL. Established primers were used on the genomic DNA for amplification of the different genes including blaSHV, blaTEM16, blaCTX-MU17, blaOXA23-like, blaOXA51-like, blaOXA58-like18 and blaNDM119. Bacterial extraction was done with the Wizard Genomic DNA Purification Kit (Promega, USA). PCR on each isolate was carried out using Veriti 96-Well Thermal Cycler (Applied Biosystems, USA). Clinical isolates which were confirmed for the presence of different genes, namely blaSHV, blaTEM, blaCTX-MU, blaOXA23-like, blaOXA51-like and blaNDM-1, by sequencing were taken as positive control.
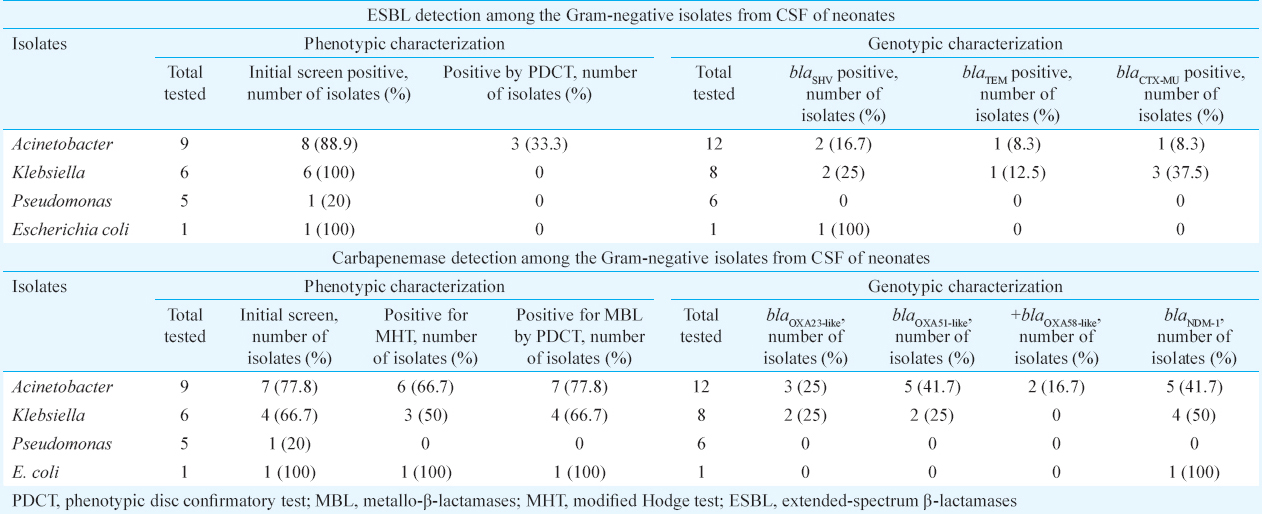
ESBL was detected in eight (88.9%) isolates of Acinetobacter, one (20%) of Pseudomonas, six (100%) of the Klebsiella and the lone isolate of E. coli, by initial phenotypic screen test. Of these, only three (33%) of the Acinetobacter were confirmed as ESBL producers by PDCT. None of the isolates of E. coli, Klebsiella and Pseudomonas were positive for the confirmatory test. This may be because the PDCT does not detect all ESBLs. Some organisms with ESBLs contain other beta-lactamases resulting in a false-negative test. These β-lactamases include AmpC and inhibitor-resistant TEM. Hyperproduction of TEM and/or SHV β-lactamases in the organism with ESBL may also cause false-negative PDCT20.
Carbapenemase production was detected in seven (77.8%) isolates of Acinetobacter, one (20%) of Pseudomonas, four (66.7%) of Klebsiella and the lone isolate of E. coli by initial screen and confirmed in the E. coli, six (66.7%) of Acinetobacter and three (50%) of the Klebsiella isolates by the MHT. The remaining isolates could be resistant to the carbapenem by a different mechanism other than carbapenemase production-like expression of an extended-spectrum cephalosporin-like Amp C. MBL was detected among seven (77.8%) Acinetobacter, the lone E. coli and four (66.7%) per cent Klebsiella isolates by PDCT.
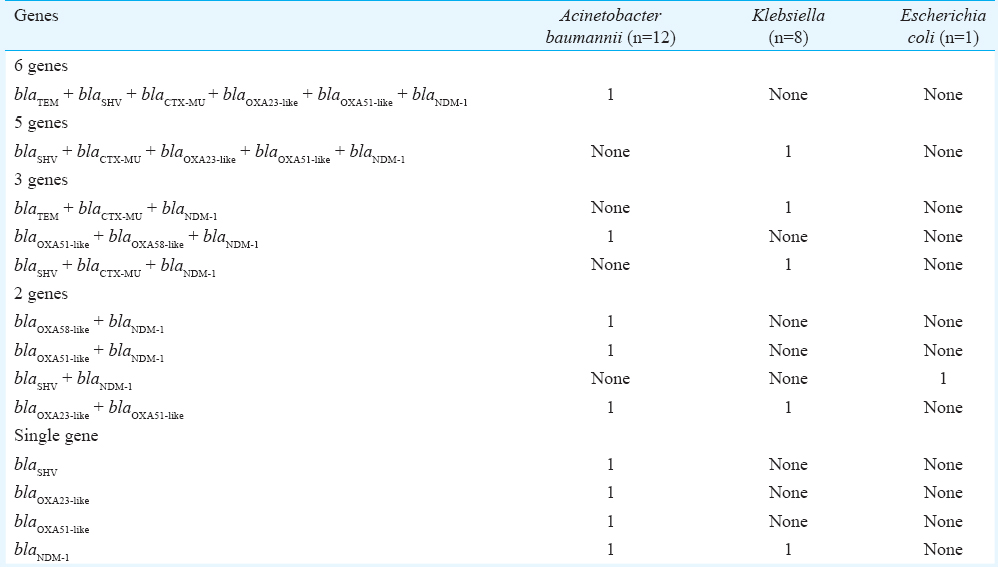
Genotypic characterization (Table I) revealed that most common resistant genes harboured by Acinetobacter were blaNDM1(41.7%) and blaOXA51-like(41.7%) followed by blaOXA23-like(25%), blaOXA58-like(16.7%), blaSHV(16.7%), blaTEM(8.3%) and blaCTX-MU(8.3%). Klebsiella harboured all the genes included except for blaOXA58-like. The E. coli isolate harboured the ESBL gene blaSHV and MBL gene blaNDM1 only. None of the six isolates of Pseudomonas harboured the β-lactamase genes tested. Table II shows the multiplicity of genes in the different isolates. The presence of single β-lactamase encoding gene was seen among 33 per cent of the A. baumannii (4/12) isolates and 12.5 per cent of Klebsiella (1/8) isolates. The presence of two genes in same host was seen in 23 per cent of A. baumannii (3/12) and 12.5 per cent of Klebsiella (1/8) isolates and intheonly E. coli isolate. Three genes were detected in 25 per cent of Klebsiella (2/8) and 8.3 per cent of the A. baumannii (1/12) isolates. One isolate each of Acinetobacter (8.3%) and Klebsiella (12.5%) showed the presence of six and five genes, respectively. Co-existence of ESBL and carbapenemase-encoding genes was seen in eight isolates.
β-lactamase-producing bacteria are a major problem worldwide, and their prevalence varies in different geographical regions. Enterobacteriaceae is the common ESBL and MBL producers known in India12345672122. Non-fermenters such as Acinetobacter and Pseudomonas are also known producers1521.
An Indian study among neonates found about 60 per cent of the Klebsiella and 75 per cent of the E. coli isolates to be ESBL producers5. In another study from West India, ESBL-producing Klebsiella and E. coli were 94.87 and 92 per cent, respectively22. Klebsiella (60%) was the most common ESBL producer followed by E. coli (30%) in a study by Vijayakanthi et al6. In a study carried out among hospitalized patients in a tertiary care centre in Assam, ESBL production was detected in 27.33 per cent isolates (41/150) and AmpC β-lactamase (both plasmid-mediated and inducible chromosomal) was detected in 32 per cent Gram-negative isolates8. In another study from a tertiary referral hospital in North-East India, the presence of blaOXA2 and other ESBL genes (blaSHV-148, blaCTX-M-15 and blaTEM-1) in different Gram-negative bacilli has been reported9. ESBL genes namely SHV, TEM and CTX-M have also been described among E. coli isolates from Assam10. There also exists report on the detection of OXA-48 β-lactamase gene in E. coli and P. aeruginosa in Assam11. NDM and ESBL producing urinary E. coli and K. pneumonia isolates have also been reported from this region12. NDM-1 was seen among the Klebsiella (50%), A. baumannii (41.7%) and the lone E. coli isolate tested in the present study. Even though the existence of NDM-1 among Gram-negative isolates has been reported from North-East region, its demonstration in isolates from neonates is extremely limited4.
Use of β-lactam antibiotics has led to the emergence of β-lactamases producers and is a great concern for the clinicians since these are multidrug-resistant causing therapeutic failure. Carbapenems are the drug of choice for life-threatening infections with such ESBL-producing organisms. This has probably led to the emergence of carbapenem resistance among the organisms creating another great challenge for treatment. The present study also found the existence carbapenem resistance among the Gram-negative isolates. Carbapenemase production was phenotypically confirmed in the lone isolate of E. coli, 77.8 per cent of A. baumannii and 66.7 per cent of the Klebsiella isolates. Emerging reports of carbapenem resistance among A. baumannii are also available371118.
Though the present study was limited due to its small sample size, it still highlighted the emergence of carbapenem resistance among common bacterial isolates from neonatal infection and the presence of carbapenemase encoding genes for OXA and NDM-1. Thus, screening for these and taking appropriate measures for control of their spread are of major importance.
Financial support & sponsorship: Authors thank the Indian Council of Medical Research (ICMR), New Delhi, for the financial support received.
Conflicts of Interest: None.
References
- Extended spectrum β-lactamases among clinical isolates of Enterobacteriaceae sp.: Prevalence and susceptibility pattern at a tertiary care hospital. Sch J Appl Med Sci. 2014;2:862-4.
- [Google Scholar]
- Prevalence and molecular characterization of extended-spectrum-β-lactamase-producing Enterobacteriaceae in a pediatric patient population. Antimicrob Agents Chemother. 2012;56:4765-70.
- [Google Scholar]
- Sepsis in neonates due to imipenem-resistant Klebsiella pneumoniae producing NDM-1 in India. J Antimicrob Chemother. 2011;66:1411-3.
- [Google Scholar]
- Detection of NDM-1 in clinical isolates of Klebsiella pneumoniae from Northeast India. J Clin Diagn Res. 2012;6:794-800.
- [Google Scholar]
- Neonatal septicaemia caused by diverse clones of Klebsiella pneumoniae & Escherichia coli harbouring blaCTX-M-15. Indian J Med Res. 2013;137:791-9.
- [Google Scholar]
- Frequency and characteristics of infections caused by extended-spectrum beta-lactamase-producing organisms in neonates: A prospective cohort study. Biomed Res Int. 2013;2013:756209.
- [Google Scholar]
- Correction: A five-year experience of carbapenem resistance in Enterobacteriaceae causing neonatal septicaemia: Predominance of NDM-1. PLoS One. 2015;10:e0134079.
- [Google Scholar]
- Multi-drug resistance in clinical isolates of Gram-negative bacilli in a tertiary care hospital of Assam. Indian J Med Res. 2014;139:643-5.
- [Google Scholar]
- Genetic environment of OXA-2 beta-lactamase producing Gram-negative bacilli from a tertiary referral hospital. Indian J Med Res. 2015;141:368-9.
- [Google Scholar]
- Prevalence and identification of extended spectrum β-lactamases (ESBL) in Escherichia coli isolated from a tertiary care hospital in North-East India. Indian J Exp Biol. 2016;54:108-14.
- [Google Scholar]
- First report on the detection of OXA-48 β-lactamase gene in Escherichia coli and Pseudomonas aeruginosa co-infection isolated from a patient in a Tertiary Care Hospital in Assam. Indian J Med Microbiol. 2016;34:252-3.
- [Google Scholar]
- New Delhi metallo-β-lactamase and extended spectrum β-lactamases co-producing isolates are high in community-acquired urinary infections in Assam as detected by a novel multiplex polymerase chain reaction assay. Indian J Med Microbiol. 2016;34:173-82.
- [Google Scholar]
- Bacterial aetiology of neonatal meningitis: A study from north-east India. Indian J Med Res. 2017;145:138-43.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. In: Performance standards for antimicrobial susceptibility testing. 20th informational supplement. Wayne, PA: CLSI; 2010.
- [Google Scholar]
- Detection of metallo beta lactamase producing Pseudomonas aeruginosa in hospitalized patients. Indian J Med Res. 2005;122:148-52.
- [Google Scholar]
- Mechanisms of decreased susceptibility to cefpodoxime in Escherichia coli. Antimicrob Agents Chemother. 2002;46:3829-36.
- [Google Scholar]
- Multiple CTX-M-type extended-spectrum beta-lactamases in nosocomial isolates of Enterobacteriaceae from a hospital in Northern Italy. J Clin Microbiol. 2003;41:4264-9.
- [Google Scholar]
- Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2006;27:351-3.
- [Google Scholar]
- Detection of NDM-1-producing Klebsiella pneumoniae in Kenya. Antimicrob Agents Chemother. 2011;55:934-6.
- [Google Scholar]
- Centers for Disease Control and Prevention. Healthcareassociated Infections. Laboratory Detection of Extended Spectrum β-lactamases (ESBLs). Available from: http://www.cdc.gov/HAI/settings/lab/lab_esbl.html
- [Google Scholar]
- Multidrug resistant Gram-negative bacilli from neonatal septicaemia at a tertiary care centre in North India: A phenotypic and genotypic study. Indian J Med Microbiol. 2014;32:97-8.
- [Google Scholar]
- Blood culture isolates in neonatal sepsis and their sensitivity in Anand district of India. Indian J Pediatr. 2014;81:785-90.
- [Google Scholar]





