Translate this page into:
Expression profiling & functional characterization of candidate miRNAs in serum exosomes among Indians with & without HIV-tuberculosis coinfection
For correspondence: Dr Ajay Vir Singh, Department of Microbiology and Molecular Biology, ICMR-National JALMA Institute for Leprosy and Other Mycobacterial Diseases, Agra 282 001, Uttar Pradesh, India e-mail: avsjalma@gmail.com, ajayvir.s@icmr.gov.in
-
Received: ,
Abstract
Background & objectives
Despite the evidence of population differences in miRNA expression, limited information is available about the expression profile of miRNAs in Indian tuberculosis (TB) patients. The present study aimed to investigate the expression profile of candidate serum exosomal microRNAs in Indian patients with and without HIV-TB coinfection.
Methods
The pool samples of serum exosomes of study participants (HIV-TB coinfection, extra-pulmonary TB, HIV mono-infection, pulmonary TB) and healthy humans were processed for the isolation of total RNA followed by miRNA analysis using miRCURY LNA human focus PCR panel by real-time PCR. The significantly altered miRNAs were identified using differential expression analysis. The target genes prediction and potential functional analysis of exclusively differentially expressed miRNAs were performed using bioinformatics tools.
Results
The expression profile of 57, 58, 49 and 11 miRNAs was significantly altered in exosome samples of HIV–TB coinfected, extra-pulmonary TB, HIV mono-infected and pulmonary TB patients compared to healthy controls, respectively. The set of three (hsa-let-7i-5p, hsa-miR-24-3p, hsa-miR-92a-3p), three (hsa-miR-20a-5p, hsa-let-7e-5p, hsa-miR-26a-5p) and four (hsa-miR-21-5p, hsa-miR-19a-3p, hsa-miR-19b-3p, hsa-miR-146a-5p) miRNAs were exclusively significantly differentially expressed in study participants with HIV-TB coinfection, extra-pulmonary TB and pulmonary TB, respectively. Most of the target genes of exclusively differentially expressed miRNAs were enriched in pathways in cancer, MAPK signalling pathway and Ras signalling pathway.
Interpretation & conclusions
The present study demonstrates a distinct expression profile of miRNAs in serum exosomes of the study participants and identified crucial miRNAs which may have a significant impact on the biomarker analysis and pathogenesis of TB in Indian patients.
Keywords
MicroRNAs
serum exosomes
tuberculosis - human immunodeficiency virus (HIV)
biomarkers - real-time PCR
Tuberculosis (TB) is the second deadliest infectious disease (after COVID-19) caused by Mycobacterium tuberculosis (M. tb). It mainly affects the lungs (pulmonary TB) but can affect other organs causing extra-pulmonary TB (EP-TB) in about 20-30 per cent of active TB cases worldwide1. In 2022, about 10.6 million people fell ill and 1.3 million people died from TB worldwide2. People with HIV have a higher risk (16%) of experiencing TB disease and the combination of HIV-TB killed 167,000 people globally in 20222. TB is curable and preventable. Early diagnosis paired with appropriate treatment is important for the effective management of disease. The current diagnostic measures for early and accurate diagnosis of TB offer suboptimal diagnosis and necessitate the requirement to develop newer biomarker(s) for early diagnosis of TB, especially in EP-TB and HIV-positive patients3.
In recent years, exosomes (30-100 nm sized extracellular vesicles of endocytic origin) have been suggested as rich sources of biomarkers for various pathological conditions, including tumours and infectious diseases4,5. The functional and diagnostic potential of exosomal miRNAs has been demonstrated in several lung diseases, including TB6,7. However, several studies have reported a significant variation in the expression profile of clinically useful miRNAs among the human population8,9. Therefore, information about the expression profile of exosomal miRNAs in patients from different races and geographical regions is crucial to better understand the global pattern of miRNAs expression in TB disease conditions and identification of potential biomarkers for rapid diagnostic development. Despite having the highest number of TB patients in the world, exosomal miRNAs expression profiles of TB patients have not yet been described in India. Therefore, the present study aimed to investigate the expression profile of candidate microRNAs in serum exosomes of Indian patients with HIV-TB coinfection, EP-TB, HIV mono-infection and pulmonary TB, as well as from healthy humans.
Material & Methods
This study was carried out at the ICMR-National JALMA Institute for Leprosy and Other Mycobacterial Diseases (ICMR-NJIL & OMD), Agra from 2020-2022 after obtaining the approval by the Human Ethics Committee of the NJIL & OMD, Agra, Uttar Pradesh, India.
Study population, isolation of exosomal RNA and cDNA synthesis
Written informed consent was obtained from all 30 study participants (HIV-pulmonary TB coinfected patients: 6; EP-TB: 6; HIV mono-infected patients: 6; pulmonary TB: 6 and healthy humans: 6) before inclusion in this investigation. After careful evaluation of clinical examination, medical history, medication history and laboratory tests, the confirmed cases (age 18 yr or above) with and without HIV-TB coinfection, as well as healthy individuals, were included in this study. The inclusion of patients with diabetes, consumption of drugs (antiretroviral therapy and/or TB treatment) and pregnant women was not permitted to participate in this study. The study participants included males (n=13; 43.33%) and females (n=17; 56.66%). The median age of the male and female participants was 35 and 31 yr, respectively. A majority of the study participants were non-smokers (n=27, 90%), non-alcoholic (n=22, 73.33%) and without a history of cough (n=20, 66.66%) and weight loss (n=17, 56.66%). The blood samples were collected from the study participants before initiation of any treatment. The serum samples were obtained from the blood samples of the study participants and processed for isolation, followed by the characterization of exosomes as per the method described previously10. Briefly, the serum samples of the study participants were centrifuged at 3000 g for 15 min to remove cells and cell debris. The supernatant of the serum samples (250 µl) was transferred to a fresh sterilise Eppendorf tube and 63 µl ExoQuick solution was added. They were mixed well by inverting and flicking the tubes. After mixing, the samples were incubated at 4°C for 30 min and then centrifuged at 1500 g for 30 min at 4°C. Following centrifugation, the supernatant was aspirated and the exosome pellets were centrifuged at 1500 g for 5 min to remove residual ExoQuick solution. After centrifugation, the traces of fluid were removed by aspiration and the exosome pellet was eluted with phosphate-buffered saline (Gibco, USA). The eluted serum-derived exosome samples were labelled with a unique identification number and immediately used or stored at -80°C until further use. The isolated exosomes were characterized using labelled antibodies specific to CD63 and CD9 exosomes surface marker by 12% SDS-PAGE and western blotting and lysed using RIPA buffer as per the methods described by Wu et al11. The purified and characterized serum exosomes were pooled (6 samples of similar groups in one sample) and processed for the isolation of total RNA using SeraMir™ Exosome RNA amplification kit (System Biosciences, USA) as per manufacturer’s instructions. The RNA concentration was determined using Nanodrop (Implen, UK) and 78-256 ng/µl was obtained. The cDNA was obtained using miRCURY LNA RT Kit (Qiagen, Hilden, Germany) as per the manufacturer’s instructions.
miRNA quantification by quantitative reverse transcription polymerase chain reaction (qRT-PCR)
About 10 µl of cDNA sample was further diluted with 290 µl of RNase-free water (Qiagen, Hilden, Germany) to quantify the expression level of miRNAs in different study groups using miRCURY LNA SYBR Green PCR Kit (Qiagen, Hilden, Germany) using manufacturer’s instruction. A total of 10 µl of the reaction mixture (2X miRCURY SYBR Green Master mix: 5 µl, re-suspended PCR Primer mix: 1 µl, diluted cDNA template: 3 µl and RNase-free water: 2µl) was prepared. The quantification of miRNAs was performed using a PCR-based assay on 96-well plates (miRCURY LNA miRNA Focus PCR Panel, Qiagen) in the CFX96 Real-Time PCR Detection System (Bio-Rad, USA) using the cycling conditions (PCR initial heat activation at 95°C for 2 min followed by 40 cycles of de-naturation at 95°C for 10 s, combined annealing/extension at 56°C for 1 min and melting curve analysis at 60-95°C described by the manufacturer (Qiagen, Hilden, Germany). The studied miRNAs (n=84) were the set of most abundantly expressed and well-characterised human miRNAs in the miRBase database (http://www.miRBase.org). The primers for the amplification of targeted miRNAs (Supplementary Table I) were pre-designed and immobilized in the wells of the 96-well plates. The CT values of the miRNAs were exported to an excel file. The table of CT values was uploaded to the data analysis web tool at http://www.qiagen.com/geneglobe. The samples were assigned to a control group and test groups. CT values were normalised based on the geNorm (Pre-Defined Reference miRNAs only) method. The data analysis report was exported from the Qiagen web portal at GeneGlobe. The endogenous reference miRNA chosen for this analysis was miR16-5p. The selection of reference miRNA (miR16-5p) was based on the previous research literature analysis, which demonstrated that miR16-5p was widely used as an endogenous reference gene in qRT-PCR-based miRNA profiling experiments involving clinical human samples including exosomes from TB patients12,13. The data analysis web portal calculates fold change/regulation using delta-delta CT method, in which delta CT is calculated between miRNA of interest and an average of reference miRNAs, followed by delta-delta CT calculations (delta CT (Test Group)-delta CT (Control Group)). Fold Change is then calculated using 2^ (-delta-delta CT) formula. The data analysis report was exported from the QIAGEN web portal at GeneGlobe. Statistical calculations were performed using Mann-Whitney U and Kruskal-Wallis test using GraphPad Prism9 Software (https://graphad.com/guides/prism/9/userguide/citing_graphad_prism.htm). The P-value <0.05 was considered statistically significant. More than 2-fold change was considered up-regulated and less than 0.5-fold change was considered down-regulated.
Target gene prediction, functional and pathways analysis
To better understand the functions of differentially expressed miRNAs potential target genes were predicted by the TargetScan Human online software (https://www.targetscan.org/vert_80/) using cumulative weighted context++ score online as >-0.5 as described by Agarwal et al14. Functional annotations of the targeted proteins were analyzed by Gene Ontology (GO) analysis and pathway enrichment analysis using the online Database for Annotation, Visualization and Integrated Discovery (DAVID) web tool (https://david.ncifcrf.gov/). The biological functions of these target genes were further investigated using KEGG pathway analysis.
Results
Expression pattern of candidate miRNAs in serum exosomes of study participants
The present study reported distinct expression profiles of miRNAs in serum exosomes derived from different study groups (Fig. 1). We found significantly altered expression of 57, 58, 49 and 11 miRNAs in exosome samples from study participants with HIV-TB coinfection (group 1), EP-TB (group 2), HIV (group 3) and pulmonary TB (group 4) compared to healthy controls, respectively (Fig. 1 and Supplementary Table II). The Venn diagram analyses (Fig. 1) revealed unique and commonly expressed miRNAs in different study groups and depicted that the sets of three (hsa-let-7i-5p, hsa-miR-24-3p, hsa-miR-92a-3p), three (hsa-miR-20a-5p, hsa-let-7e-5p, hsa-miR-26a-5p) and four (hsa-miR-21-5p, hsa-miR-19a-3p, hsa-miR-19b-3p, hsa-miR-146a-5p) miRNAs were exclusively significantly differentially expressed in serum exosomes of study participants with HIV-TB coinfection, EP-TB and pulmonary TB, respectively (Fig. 1). None of the miRNAs was found to be exclusively expressed in serum exosome samples of HIV patients. The details of significantly unique and commonly up- and down-regulated miRNAs in different study groups are summarized in Figures 2 and 3, respectively. Among the up-regulated miRNAs, the expression of five (hsa-let-7i-5p, hsa-miR-24-3p, hsa-miR-23b-3p, hsa-miR-92a-3p, hsa-let-7b-5p) and five (hsa-miR-21-5p, hsa-miR-15a-5p, hsa-miR-19a-3p, hsa-miR-19b-3p, hsa-miR-142-3p) miRNAs was exclusively up-regulated in patients with HIV-TB coinfection and pulmonary TB, respectively (Fig. 2 and 3). In down-regulated miRNAs, the expression profile of two (hsa-miR-142-3p, hsa-miR-15a-5p), three (hsa-miR-20a-5p, hsa-let-7e-5p, hsa-miR-26a-5p) and three (hsa-miR-146a-5p, hsa-miR-23b-3p, hsa-let-7b-5p) miRNAs were exclusively down-regulated in study participants with HIV-TB coinfection, EP-TB and pulmonary TB, respectively (Fig. 2 and 3). The details of significantly up- and down-regulated miRNAs in different study groups compared to healthy controls are summarized in Figure 2 and 3.
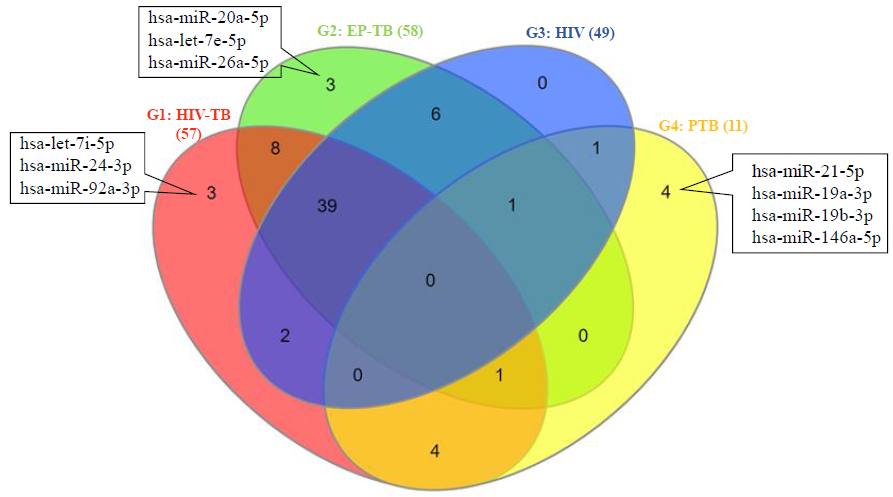
- Venn diagram showing unique and commonly significantly altered (up- and down regulated) miRNAs in different study groups. (G1: HIV-TB coinfection, G2: EP-TB individuals, G3: HIV individuals, G4: pulmonary TB individuals).

-
(A) Venn diagram showing uniquely significantly up-regulated miRNAs in different study groups (G1: HIV–TB coinfection, G2: EP-TB patients, G3: HIV individuals, G4: pulmonary TB individuals). (B) Bar diagram showing comparisons of expression profile of significantly up-regulated miRNAs in different study groups.

-
(A) Venn diagram showing uniquely significantly down-regulated miRNAs in different study groups. (B) Bar diagram showing comparisons of expression profile of significantly down-regulated miRNAs in different study groups.
Target gene prediction and functional analysis of differentially expressed miRNAs
Two hundred fifty-seven, 137 and 215 target genes were identified for the exclusively significantly differentially expressed miRNAs using cumulative weighted context++ score online >-0.5 in group 1, group 2 and group 4, respectively. Functional annotations of the targeted genes were analyzed by GO analysis and pathway (KEGG) enrichment analysis using the online DAVID web tool. The results demonstrated that a total of 177, 147 and 63 GO terms or KEGG pathways were enriched by predicted target genes of group 1, group 2, and group 4, respectively. The group-wise detail of the top 10 pathways most enriched by targeted genes of exclusively significantly differentially expressed miRNAs in different categories [biological processes (BP), cellular components (CC) and molecular functions (MF)] are depicted in Figure 4. The KEGG pathway analysis revealed that the target genes of exclusively significantly differentially expressed miRNAs of group 1 and group 2 were most enriched in pathways in cancer (Pathway: has05200) and MAPK signalling pathway (Pathway: has04010). In contrast the target genes of exclusively significantly differentially expressed miRNAs of group 4 were most enriched in Ras signalling pathway (Pathway: hsa04014, Count:6, P-value: 0.012848) followed by MAPK signalling pathway (Pathway: hsa04010, Count: 5, P value: 0.097536) and focal adhesion pathway (Pathway: hsa04510, Count: 5, P value: 0.031413) (Fig. 4).

- Top 10 GO and KEGG pathways most enriched by targeted genes of exclusively significantly differentially expressed miRNAs of (A) group 1, (B) group 2 and (C) group 4 individuals.

- Top 10 GO and KEGG pathways most enriched by targeted genes of exclusively significantly differentially expressed miRNAs of (A) group 1, (B) group 2 and (C) group 4 individuals.
Discussion
Although many studies using various biological samples have been conducted to investigate the role of microRNAs in TB and HIV, no comprehensive study has yet been performed to identify differentially expressed miRNAs in serum exosomes of HIV patients with or without TB coinfection. This study’s primary objective was to identify differentially expressed miRNAs in serum exosomes and the potential function of these miRNAs in different study groups. Similar to the present study, the role of exosomal miRNAs has been investigated as disease-specific biomarkers and in the progression of several diseases, including infectious diseases6,7,15. In the present study, we used miRCURY LNA miRNA Focus PCR Panel to investigate miRNA levels in serum exosomes derived from study participants. These PCR panels were enabled with the Locked Nucleic Acids (LNA™) technology, which is highly sensitive to ensure accurate quantification of the miRNAs by real-time PCR16. Similar to the present study, the miRNA Focus PCR Panel have also been used for the investigation of miRNAs in several studies17,18.
In the present study, we identified that the expression profile of miRNAs in serum exosomes of individuals with HIV-TB coinfection, EP-TB, HIV individuals and pulmonary TB differs from healthy humans. The results of the present study supported previous findings on distinct expression profiles of miRNAs in several diseases as compared to healthy humans. Previously, Sabir et al19 found distinct expression pattern miRNAs in active TB and latent-TB patients as compared to healthy individuals. The present study identified that three (hsa-let-7i-5p, hsa-miR-24-3p, hsa-miR-92a-3p), three (hsa-miR-20a-5p, hsa-let-7e-5p, hsa-miR-26a-5p) and four (hsa-miR-21-5p, hsa-miR-19a-3p, hsa-miR-19b-3p, hsa-miR-146a-5p) miRNAs were exclusively significantly differentially expressed in study participants with HIV-TB coinfection, EP-TB and pulmonary TB study participants, respectively. Previously, Hu et al20 identified six exosomal miRNAs (miR-20a, miR-20b, miR-26a, miR-106a, miR-191, miR-486) which were differentially expressed in TB patients as compared to disease controls or healthy controls in China. Sun et al21 investigated the expression profile of miRNAs in macrophage-derived exosomes in the TB-infected bone microenvironment using miRNA-seq and reported that 28 miRNAs were up-regulated. In comparison, 34 miRNAs were down-regulated in individuals with spinal TB. Kathirvel et al22 reported significant up-regulation of miR-21, miR-29a, miR-31, miR-155 and down-regulation of miR-146a in children with active-TB compared to healthy subjects using SYBR green-based miScript qRT-PCR assay in Puducherry, India. Ndzi et al23 reported significant up-regulation of hsa-miR-29a-3p, hsa-miR-155-5p and hsa-miR-361-5p in active-TB patients and confirmed miR-29a-3p as a valuable candidate biomarker for the differential diagnosis and monitoring of TB in Cameroon. In Serbia, Buha et al24 investigated the association between the expression level of miRNA-146a and active pulmonary TB using RT-qPCR technique and reported that miRNA-146a has the potential to be used as a biomarker for active pulmonary TB. Nour Neamatollahi et al25 studied the expression of miR-let-7f, miR-125a and miR-125b in sputum and serum samples of pulmonary TB patients from Iran and Afghanistan. They reported overexpression of miR-125a and miR-125b in Iranian and Afghan patients. In Indonesia, Massi et al26 investigated the expression of miRNA hsa-miR-425-5p and hsa-miR-4523 in patients with pulmonary TB, latent TB infection and lymph node TB using RT-qPCR and found that the expression levels of mir-425-5p and mir-4523 could be the potential biomarkers for latent TB infection. In another study from Indonesia, Angria et al27 reported increased expression of miRNA-29a-3p in patients with active and latent pulmonary TB using qRT-PCR and highlighted the potential use of miRNA-29a-3p as a biomarker of active and latent pulmonary TB infection. Recently, Li et al28 performed a meta-analysis to evaluate the diagnostic performance of miRNA-29a as a biomarker for the diagnosis of active TB and reported that miRNA-29a had a good ability to diagnose active TB. The findings of the present study supported previous reports which suggest the diagnostic use of exosomal miRNAs in TB and highlight that the identified sets of significantly differentially expressed miRNAs (in combination with up- and down-regulated miRNAs) can be useful for biomarker analysis for early diagnosis of TB in Indian patients. Although there were methodological differences, these studies indicated that the expression profile of miRNAs may vary in TB patients in different geographical regions of the world.
In the present study, the KEGG pathway analysis was performed to investigate the biological importance of the target genes of the exclusively differentially expressed miRNAs of the study groups. Similar to the present study, KEGG pathway analysis has been widely used to predict the most impacted pathways under the given conditions by grouping the targeted genes of the differentially expressed miRNAs29,30. The results of KEGG pathway analysis revealed that the target genes of exclusively differentially expressed miRNAs of group 1 (individuals with HIV-TB coinfection) and group 2 (EP-TB individuals) were mainly enriched in pathways/microRNA in cancer and MAPK signalling pathways. The findings of the present study indicated that HIV-TB coinfection and EP-TB could participate in the development of cancer. This statement can be supported by the findings of the previous studies that people co-infected with M. tuberculosis/HIV have an increased risk of developing cancer31,32. The results of the present study supported previous studies which described MAPK signalling pathways as crucial pathways in M. tuberculosis infection33-35. The present study findings are based on the analysis of a limited number of miRNAs and pooled samples, which is a major limitation. Sample pooling may decrease the level of coverage of individual samples in expression studies and not provide individual-specific information, which might be useful for biomarker discovery. Therefore, further studies with the use of individual samples and high-throughput molecular techniques (RNA-sequencing, microarray, etc.) are needed to identify more differentially expressed miRNAs to establish ‘exosome-based miRNA signature’ for early diagnosis of TB and pathophysiological significance of differentially expressed miRNAs in Indian patients.
In conclusion, the present study demonstrates a distinct expression profile of miRNAs in serum exosomes of the study participants and identified the set of three (hsa-let-7i-5p, hsa-miR-24-3p, hsa-miR-92a-3p), three (hsa-miR-20a-5p, hsa-let-7e-5p, hsa-miR-26a-5p) and four (hsa-miR-21-5p, hsa-miR-19a-3p, hsa-miR-19b-3p, hsa-miR-146a-5p) miRNAs which were exclusively significantly differentially expressed in serum exosomes of study participants with HIV-TB coinfection, EP-TB and pulmonary TB, respectively. The identified sets of miRNAs may have crucial clinical value in the direction of the search for new diagnostic markers for TB. The present study also indicates that pathways in cancer, MAPK signalling pathway and Ras signalling pathway may constitute crucial potential mechanisms in the process of development/progression of different TB disease conditions (HIV-TB coinfection, EP-TB and pulmonary TB). Further validation using signalling pathway inhibitors are warranted.
Financial support & sponsorship
This study received financial support from Indian Council of Medical Research, New Delhi (Grant number No.5/5/8/5/19/2014-ECD-I). Second author (RY) received the woman Scientist Award from the Department of Science and Technology, Government of India, New Delhi for Woman Scientist (No SR/WOS-A/LS- 129/2018).
Conflicts of Interest
None.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Extrapulmonary tuberculosis – an update on the diagnosis, treatment and drug resistance. J Respir. 2021;1:141-64.
- [Google Scholar]
- Global tuberculosis report. Available from: https://iris.who.int/bitstream/handle/10665/373828/9789240083851-eng.pdf?sequence=1, accessed on June 8, 2023.
- Diagnostic biomarkers for active tuberculosis: Progress and challenges. EMBO Mol Med. 2022;14:e14088.
- [Google Scholar]
- Clinical significance of exosomes as potential biomarkers in cancer. World J Clin Cases. 2019;7:171-90.
- [Google Scholar]
- Exosomes: Potential disease biomarkers and new therapeutic targets. Biomedicines. 2021;9:1061.
- [Google Scholar]
- The roles of exosomal miRNAs and lncRNAs in lung diseases. Signal Transduct Target Ther. 2019;4:47.
- [Google Scholar]
- Composition and clinical significance of exosomes in tuberculosis: A systematic literature review. J Clin Med. 2021;10:145.
- [Google Scholar]
- Global population-specific variation in miRNA associated with cancer risk and clinical biomarkers. BMC Med Genomics. 2014;7:53.
- [Google Scholar]
- Population variation in miRNAs and isomiRs and their impact on human immunity to infection. Genome Biol. 2020;21:187.
- [Google Scholar]
- Higher abundance of vitronectin (s-protein) in serum-derived exosomes of pulmonary and extra-pulmonary tuberculosis patients as compared to HIV-tuberculosis dual-infected patients and healthy humans. Curr Proteomics. 2023;20:75-9.
- [Google Scholar]
- Exosomes: improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst. 2015;140:6631-42.
- [Google Scholar]
- Reference genes for qPCR-based miRNA expression profiling in 14 human tissues. Med Princ Pract. 2022;31:322-32.
- [Google Scholar]
- A dual marker for monitoring MDR-TB treatment: host-derived miRNAs and M. tuberculosis-Derived RNA sequences in serum. Front Immunol. 2021;12:760468.
- [Google Scholar]
- Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:e05005.
- [Google Scholar]
- MicroRNA quantitation from a single cell by PCR using SYBR® Green detection and LNA-based primers. Nat Methods. 2008;5:iii-iv.
- [Google Scholar]
- Exosome microRNAs in amyotrophic lateral sclerosis: A pilot study. Biomolecules. 2021;11:1220.
- [Google Scholar]
- Increased expression of six-large extracellular vesicle-derived miRNAs signature for nonvalvular atrial fibrillation. J Transl Med. 2022;20:4.
- [Google Scholar]
- miRNAs in tuberculosis: new avenues for diagnosis and host-directed therapy. Front Microbiol. 2018;9:602.
- [Google Scholar]
- Integrating exosomal microRNAs and electronic health data improved tuberculosis diagnosis. Ebio M. 2019;40:564-73.
- [Google Scholar]
- Differential expression analysis of miRNAs in macrophage-derived exosomes in the tuberculosis-infected bone microenvironment. Front Microbiol. 2023;14:1236012.
- [Google Scholar]
- Expression levels of candidate circulating microRNAs in pediatric tuberculosis. Pathog Glob Health. 2020;114:262-70.
- [Google Scholar]
- MicroRNA hsa-miR-29a-3p is a plasma biomarker for the differential diagnosis and monitoring of tuberculosis. Tuberculosis (Edinb). 2019;114:69-76.
- [Google Scholar]
- Association between active pulmonary tuberculosis and miRNA-146a: a preliminary study from Serbia. J Infect Dev Ctries. 2022;16:1317-22.
- [Google Scholar]
- Evaluation of miR-let-7f, miR-125a, and miR-125b expression levels in sputum and serum samples of Iranians and Afghans with pulmonary tuberculosis. Iran J Microbiol. 2023;15:665-73.
- [Google Scholar]
- microRNA hsa-miR-425-5p and hsa-miR-4523 expressions as biomarkers of active pulmonary tuberculosis, latent tuberculosis infection, and lymph node tuberculosis. Noncoding RNA Res. 2023;8:527-33.
- [Google Scholar]
- Expression of miRNA-29a-3p and IFN-γ as biomarkers in active and latent pulmonary tuberculosis. Ann Med Surg (Lond). 2022;83:104786.
- [Google Scholar]
- Diagnostic performance of microRNA-29a in active pulmonary tuberculosis: a systematic review and meta-analysis. Clinics (Sao Paulo). 2023;78:100290.
- [Google Scholar]
- Binformatics analysis of microRNA expression between patients with and without latent tuberculosis infections. Exp Ther Med. 2019;17:3977-88.
- [Google Scholar]
- miRNA expression profiles and potential as biomarkers in nontuberculous mycobacterial pulmonary disease. Sci Rep. 2020;10:3178.
- [Google Scholar]
- Cancers attributable to infections among adults with HIV in the United States. AIDS. 2015;29:2173-81.
- [Google Scholar]
- Cancer risk in tuberculosis patients in a high endemic area. BMC Cancer. 2021;21:679.
- [Google Scholar]
- Macrophage signaling upon mycobacterial infection: the MAP kinases lead the way. Cell Microbiol. 2003;5:133-42.
- [Google Scholar]
- Mitogen-activated protein kinases mediate Mycobacterium tuberculosis-induced CD44 surface expression in monocytes. J Biosci. 2012;37:41-54.
- [Google Scholar]
- microRNAs associated with the pathogenesis and their role in regulating various signaling pathways during Mycobacterium tuberculosis infection. Front Cell Infect Microbiol. 2022;12:1009901.
- [Google Scholar]






