Translate this page into:
Expression of galectin-3 in gastric adenocarcinoma
Reprint requests: Dr Daniel A. Ribeiro, Department of Biosciences, Federal University of São Paulo– UNIFESP Av. Ana Costa, 95, Vila Mathias, Santos - SP, 11060-001, Brazil e-mail: daribeiro@unifesp.br, daribeiro@pesquisador.cnpq.br
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Galectin-3 a member of the galectin family is an endogenous β-galactoside binding lectin. It has been found to be associated with cell adhesion, recognition, proliferation, differentiation, immunomodulation, angiogenesis, apoptosis and can be a reliable marker for cancer aggressiveness. The aim of this study was to verify protein expression in gastric adenocarcinoma tissues and correlate the results with the clinical aspects in the study population.
Methods:
Galectin-3 expression was examined by immunohistochemistry in 57 samples of gastric adenocarcinomas tissues. Galectin-3 protein expression was observed in the cytoplasm and the nucleus of examined tissues.
Results:
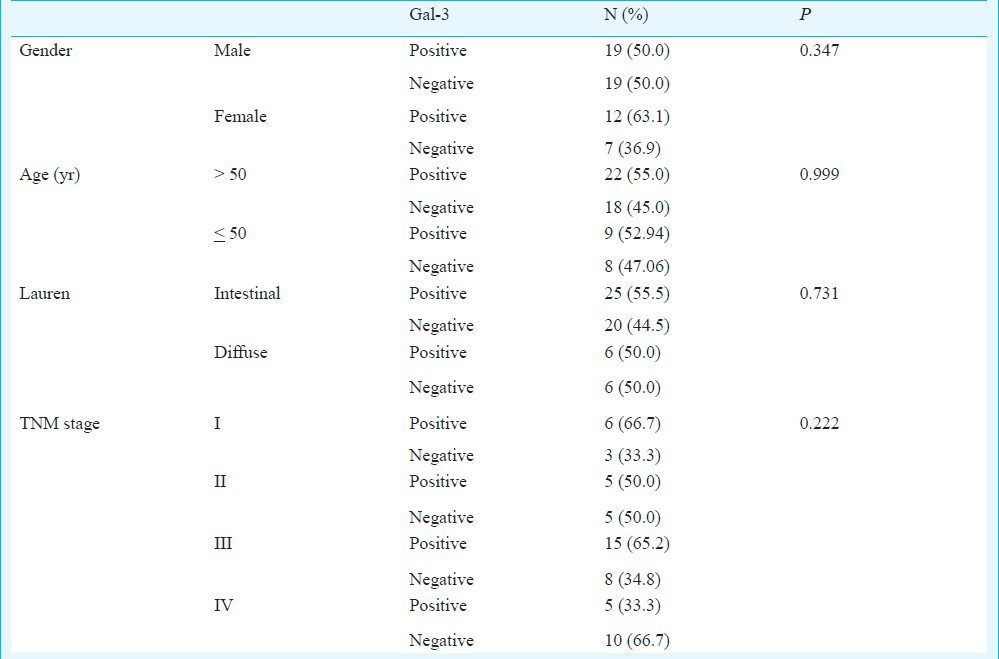
Thirty one (54.4%) samples had strong or moderate staining and 26 (45.6%) tumours had negative or weak staining. The galectin-3 did not show association with the sex (P=0.347), age (P=0.999), Lauren's classification (P=0.731) and TNM stage (P=0.222). Regarding the TNM stage, 66.7 per cent of stage I tumours had strong or moderate staining; with tumours stage IV this percentage was 33.3 per cent.
Interpretation & conclusion:
Our results suggest that gal-3 is not a reliable biomarker for prognosis of the gastric adenocarcinoma by immunohistochemistry. Further studies need to be done on a large sample of tumour tissues in different clinical staging.
Keywords
Biomarker
galectin-3
gastric adenocarcinoma
immunoexpression
prognosis
Gastric cancer is among the most common types of malignancy in some parts of the world, such as in Japan and in parts of China, South America, and Scandinavia, where it is associated with ingestion of foods that may contain relatively high amounts of nitrosamines1. Among all cancers in Sao Paulo City, Brazil, gastric cancer ranks 5th in the frequency of new cases per year and 2th in cancer related deaths among men and 4th among women2.
Galectins are a family of non-integrin β-galactoside-binding lectins with related amino acid sequences which are found in many animals3. Galectin-3 (Gal-3), formerly known as CBP35, Mac-2, εBP, is predominantly localized in the cytoplasm456. It can translocate to the nucleus from the cytoplasm via nonclassical secretion pathway after it is synthesized on the cytoplasmic ribosomes7. This protein is expressed by human neutrophils, macrophages and mast cells, and Langerhans cells, through which it could also be involved in inflammatory processes8910. Gal-3 has been known to be associated with the various biological processes including cell adhesion, recognition, proliferation, differentiation, immunomodulation, angiogenesis and apoptosis11 and is not considered as a reliable marker for cancer aggressiveness and metastasis12.
The expression of gal-3 is inversely associated with progression of human breast cancer13. Cytoplasmic gal-3 immunoexpression seems to be a prognostic factor in colorectal cancer because a higher risk of recurrence has been observed in tumours with a high score of galectin-3. The expression of gal-3 is associated with tumour progression and metastatic potential in gastric cancers14. Further, reduced gal-3 expression has been suggested as an indicator of unfavourable prognosis in gastric cancer15.
In this study, we evaluated the immunoreactivity of gal-3 in gastric cancer tissues and its clinical relevance. We also sought to determine whether the expression of gal-3 was associated with the progression of gastric cancer in this population.
Material & Methods
Fifty seven gastric adenocarcinomas tissues in paraffin blocks, previously classified as either intestinal or diffuse type were retrieved from the files of Department of Pathology, Federal University of Sao Paulo UNIFESP, EPM ranging from 2000 to 2010. Ethical approval for this study was granted by the local Ethics Committee.
Immunohistochemistry: Conventional 4 μm thick sections were obtained and mounted on the slides pretreated with 3-minopropyl-triethoxysilane (Sigma, USA). Sections were deparaffinized in three changes of xylene and hydrated in a graded series of ethanol finishing in distillated water. For antigen retrieval, the slides were placed in 0.01M sodium citrate buffer (pH 6.0) in a steamer for 30 min. Endogenous peroxidase was quenched by 10 per cent hydrogen peroxide for 20 min. The slides were then washed in distillated water and phosphate buffer saline (PBS) and incubated in milk powder solution for 20 min to block non specific binding of primary antibody. Sections were incubated with mouse monoclonal NCL-GAL-3 (Novocastra, UK) overnight at 4°C. Subsequently, slides were washed with PBS and incubated with biotinylated secondary antibody for 30 min, washed with PBS, and incubated with streptavidin –horse radish peroxidase (HRP) conjugate for 30 min (LSAB-Dako, Denmark). The reactions were visualized by incubation with 3, 3’-diaminobenzidine tetrahydrocloride (Sigma) for 5 min. Slides were briefly counterstained in Harris's hematoxylin and dehydrated and cover slipped with Entellan (Sigma). Negative and positive controls were made to run simultaneously. Positive control was represented by colon adenocarcinoma tissue. Negative controls were made by eliminating the primary antibody.
The galectin-3 expression was classified by the presence of dark-brown precipitate in the cytoplasm and/or nucleus. The expression was scored as follows: 1 (negative or weak) for no staining or less than 50 per cent of tumour cells and 2 (moderate or strong) when ≥50 per cent of the tumour cells were stained as described by others researchers16. The expression of galectin-3 was evaluated by two trained researchers independently and they were double blinded to the clinical data and stage. A consensus was sought for differences in opinion. Representative areas of gastric adenocarcinomas were digitalized by digital camera (Axioskop Plus, Zeiss, Germany) under ×400 magnification.
Statistical analysis: The categorical variables were presented in a descriptive way in Tables containing absolute frequencies. Continuous variable of normal distribution was described using the mean. Expression of gal-3 was assessed with various clinic-pathological parameters using the χ2 test. Survival rates were calculated by the Kaplan-Meier method. The difference between the survival curves was analyzed by the log-rank test. Starting time was the day of surgical resection of gastric cancer. Differences were considered significant when the P value was less than 0.05.
Results
A total of 57 gastric adenocarcinomas were examined for galectin-3 protein expression. Among the patients, 38 were males and 19 females. Their age ranged from 27 to 89 yr, with an average age of 59 yr. The average period of follow up was 57 months. According to the Lauren's classification 45 was intestinal type and 12 was diffuse type. Nine patients belonged to clinical stage I, 10 to stage II, 23 to stage III and 15 to stage IV. The mean follow up period for all patients was 57 months (range 1-131 months). At the end of the study, 13 patients were alive without cancer, 17 alive with cancer, and 23 had died because of the tumour progression (Table 1).
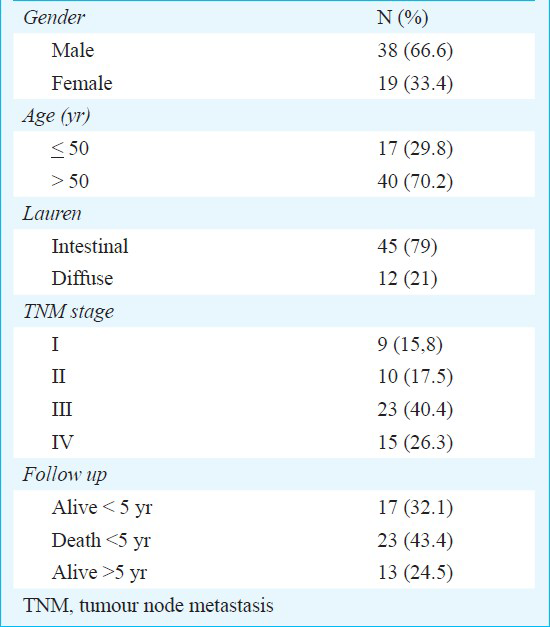
Gal-3 immunoexpression was detected in the cytoplasm and nuclei of the gastric cancer cells (Fig. 1). Thirty one (54.4%) had strong or moderate staining (score 1) and 26 (45.6%) tumours had negative or weak staining (score 2). Fig. 1 shows immunoreactivity of Galectin-3 in gastric cancer.
- Immunohistochemistry for galectin-3. A - Moderate immunoexpression. B - Strong expression. X100 magnification.
Kaplan-Meier survival curves demonstrated that both the positive gal-3 group and the negative gal-3 group had the same global survival at the end (Fig. 2). With respect to prognosis, survival curve of Kaplan-Meyer curve showed the immunoexpression of galectin-3 interfere negatively (Fig. 3).
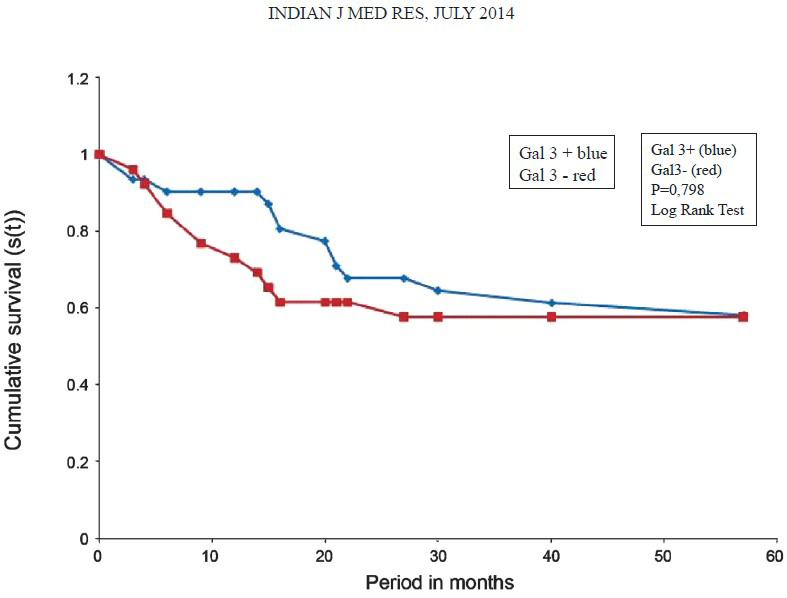
- Survival curve of Kaplan-Meyer. The prognosis became similar between the positive gal-3 group and the negative gal-3 group at the end.
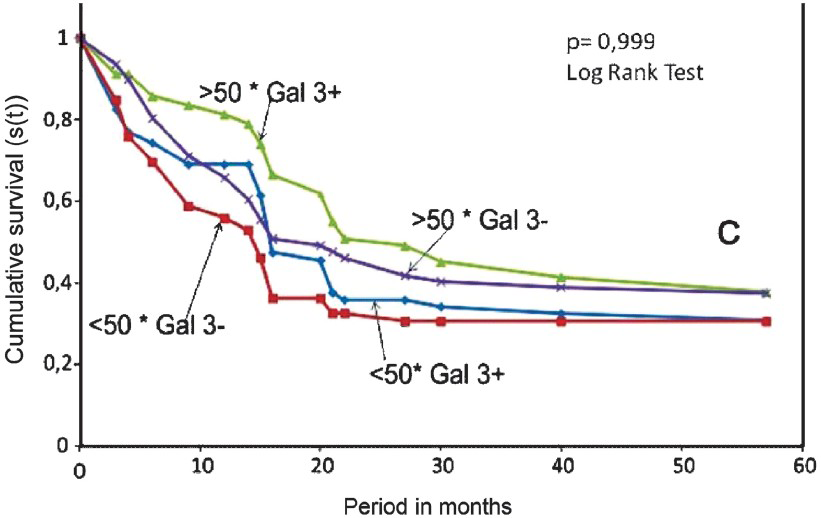
- Survival curve of Kaplan-Meier. Considering the age the prognosis was worse in the negative gal-3 group than the positive galectin-3 group.
Lauren's classification pointed out worse prognosis in diffuse tumours when compared to intestinal group (Fig. 4). Regarding the TNM stage, 66.7 per cent of stage I tumours had strong or moderate staining (Fig. 5). When we analyzed tumours stage IV this percentage was 33.3 per cent (Table II).
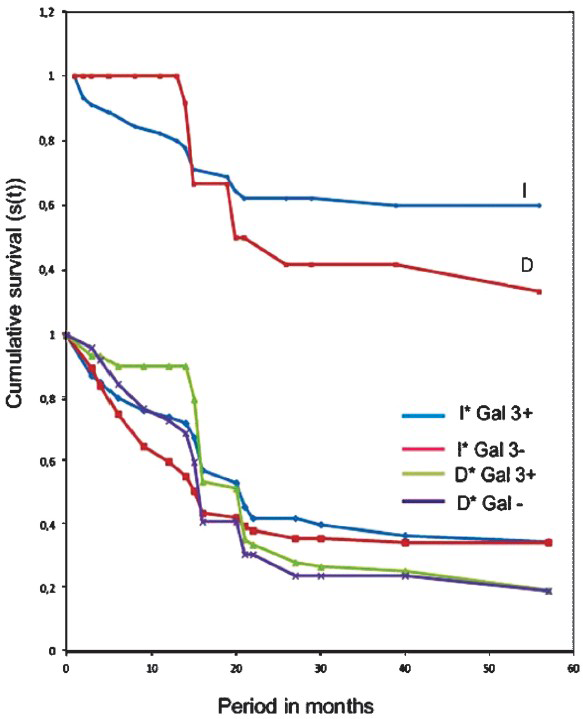
- Survival curve of Kaplan-Meier. Considering the Lauren's classification the prognosis was worse in diffuse (D) tumours than in intestinal (I) tumours group.

- Survival curve of Kaplan-Meier. Considering stages (I+II) and (III+IV) positive gal-3 group had best prognosis compared with the negative gal-3 group.
Discussion
In the present study we investigated the potential clinical relevance of gal-3 expression in gastric adenocarcinoma. Several attempts are reported exploring gal-3 expression as a prognostic biomarker and a diagnostic indicator in gastric cancer. The mechanisms underlying the role of gal-3 in carcinogenesis are not clearly defined, but these probably involve the regulation of intracellular signal pathways17. Gal-3 gene contains a responsive element to the tumour suppressor p53 and it is downregulated by p5318. Also, Gal-3 was reported to exhibit an anti-apoptotic activity, due to its considerable similarity to Bcl2, thereby promoting survival of malignant cells17.
A positive correlation between the expression of gal-3 and tumour progression has been observed in various tumour types, including colon, thyroid, and lung cancer19. In addition to its expression levels, gal-3 localization pattern seems to play a role in cancer progressions20.
Many researchers had evaluated the staining pattern of gal-3 in other malignant tissues. Some have detected gal-3 in the cytoplasm and nucleus of normal cells and predominantly in the cytoplasm of cancer cells in colon, prostate and tongue cancer21. Baldus et al22 reported that in gastric cancer nuclear gal-3 reactivity was significantly stronger in diffuse-type tumours than it was in intestinal-type tumours. These results suggest that the cellular localization of gal-3 may play an important role in malignant transformation. In this study, gal-3 was found in the cytoplasm and in the nucleus of tumour cells.
Yoshii et al23, detected gal-3 immunoreactivity in cytoplasm of thyroid papillary carcinoma cells and it could also be detected in cell nucleus, cell surface or outside cell. Another study reported the staining of light yellow, dark yellow or brown yellow at a low power field and diffuse or granular staining at a high power field under microscopy in the well, moderately and poorly differentiated gastric cancerous tissues24.
Nakahara et al25 have shown that gal-3 is mainly localized in the cytoplasm, but can also be detected in the nucleus, suggesting that gal-3 is a protein shuttling between the nucleus and cytoplasm and this different localization of gal-3 in the cell seems to be significant for its anti-apoptotic activity. Honjo et al26 analyzed gal-3 expression in 77 tongue carcinoma specimens (including 54 squamous cell carcinoma and 23 normal mucosa specimens) and observed that the levels of nuclear expression of galectin-3 markedly decreased during the progression from normal to cancerous states, whereas cytoplasmic expression increased. No association was observed between the immunoexpression of gal-3 and sex, age, Lauren's classification and TNM stage in the present study. Regarding the clinical stage, 66.7 per cent of stage I tumours had moderate or strong staining pattern of galectin-3, unlike stage IV tumours, (33.3 %). Possibly, there was no statistical significance due to small number of tumours.
Only a few researchers have discussed the relationship between the immunoexpression of gal-3 and prognosis in gastric cancer patients. Miyazaki et al14 observed a significant stronge expression of gal-3 in poorly differentiated carcinoma which was also correlated with tumour progression. On the other hand, Lotan et al27 observed that well differentiated tubular carcinomas expressed a higher level of gal-3 than the corresponding non-neoplastic mucosa.
Okada et al15 showed that the patients with low immunoreactivity for gal-3 had a short survival and gal-3 expression was a prognostic factor. In our study, the prognosis for survival was the same for both positive and negative galectin-3 groups.
In conclusion, our results suggest that gal-3 may not be a reliable biomarker for prognosis of the gastric adenocarcinoma by immunohistochemistry. This requires further study.
Acknowledgment
The authors thank JoaquimSoares de Almeida for technical support. The first author (TSG) was a recipient of CAPES PhD fellowship, and the last author (DAR) received CNPq fellowship.
References
- Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci. 2010;14:249-58.
- [Google Scholar]
- Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807-10.
- [Google Scholar]
- Carbohydrate binding protein 35. Complementary DNA sequence reveals homology with proteins of the heterogeneous nuclear RNP. J biol chem. 1988;263:6009-11.
- [Google Scholar]
- The major non-integrin laminin binding protein of macrophages is identical to carbohydrate binding protein 35 (Mac-2) J biol chem. 1990;265:7097-9.
- [Google Scholar]
- Human IgEbinding protein: a soluble lectin exhibiting a highly conserved interspecies sequence and differential recognition of IgE glycoforms. Biochemistry. 1990;29:8093-100.
- [Google Scholar]
- Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim Biophys Acta. 1999;1473:172-85.
- [Google Scholar]
- Human neutrophils express immunoglobulin E (IgE)-binding proteins (Mac-2/epsilon BP) of the S-type lectin family: role in IgE-dependent activation. J exp med. 1993;177:243-8.
- [Google Scholar]
- Surface expression of functional IgE binding protein, an endogenous lectin, on mast cells and macrophages. J immunol. 1992;148:861-7.
- [Google Scholar]
- IgE-binding molecules on human Langerhans cells. Acta Derm Venereol Suppl (Stockh). 1992;176:54-7.
- [Google Scholar]
- Nuclear export of phosphorylated galectin-3 regulates its antiapoptotic activity in response to chemotherapeutic drugs. Mol cell biol. 2004;24:4395-406.
- [Google Scholar]
- Identification of a 14-kDa laminin binding protein (HLBP14) in human melanoma cells that is identical to the 14-kDa galactoside binding lectin. Arch biochem biophys. 1992;297:132-8.
- [Google Scholar]
- Increased expression of galectin-3 in primary gastric cancer and the metastatic lymph nodes. Oncol rep. 2002;9:1307-12.
- [Google Scholar]
- Reduced galectin-3 expression is an indicator of unfavorable prognosis in gastric cancer. Anticancer Res. 2006;26:1369-76.
- [Google Scholar]
- Implication of the Galectin-3 in colorectal cancer development (about 325 Tunisian patients) Bull cancer. 2010;97:E1-8.
- [Google Scholar]
- The second intron of the human galectin-3 gene has a strong promoter activity down-regulated by p53. FEBS lett. 1995;363:165-9.
- [Google Scholar]
- Involvement of galectin-3 expression in colorectal cancer progression and metastasis. Int J Oncol. 1999;15:143-8.
- [Google Scholar]
- Dual activities of galectin-3 in human prostate cancer: tumor suppression of nuclear galectin-3 vs tumor promotion of cytoplasmic galectin-3. Oncogene. 2004;23:7527-36.
- [Google Scholar]
- Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. Int J Cancer. 2000;89:361-7.
- [Google Scholar]
- Increased galectin-3 expression in gastric cancer: correlations with histopathological subtypes, galactosylated antigens and tumor cell proliferation. Tumour biol. 2000;21:258-66.
- [Google Scholar]
- Galectin-3 maintains the transformed phenotype of thyroid papillary carcinoma cells. Int J Oncol. 2001;18:787-92.
- [Google Scholar]
- Li-cadherin is inversely correlated with galectin-3 expression in gastric cancer. Dig Dis Sci. 2008;53:1811-7.
- [Google Scholar]
- Regulation of cancer-related gene expression by galectin-3 and the molecular mechanism of its nuclear import pathway. Cancer Metastasis Rv. 2007;26:605-10.
- [Google Scholar]
- Expression of cytoplasmic galectin-3 as a prognostic marker in tongue carcinoma. Clin Cancer Res. 2000;6:4635-40.
- [Google Scholar]
- Expression of a 31-kDa lactoside-binding lectin in normal human gastric mucosa and in primary and metastatic gastric carcinomas. In j cancer. 1994;56:474-80.
- [Google Scholar]






