Translate this page into:
Experience with non-cremophor-based paclitaxel-gemcitabine regimen in advanced pancreatic cancer: Results from a single tertiary cancer centre
For correspondence: Dr Anant Ramaswamy, Department of Medical Oncology, Tata Memorial Hospital, Dr. E. Borges Road, Parel, Mumbai 400 012, Maharashtra, India e-mail: anantr13@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Gemcitabine combined with non-cremophor-based paclitaxel is one of the standards of care in advanced inoperable pancreatic cancer. This study was undertaken to retrospectively evaluate real world non-trial outcomes with this combination.
Methods:
Patients with histologically proven advanced inoperable pancreatic adenocarcinoma (PDAC), treated with non-cremophor-based paclitaxel-gemcitabine combination (PG) (gemcitabine-nanoxel or gemcitabine-abraxane) between January 2012 and June 2015, were retrospectively analyzed. Response assessment was done every 8-12 wk with computed tomography scan and responses were measured as per the Response Evaluation Criteria in Solid Tumours 1.1 criteria where feasible. Toxicity was recorded as per the Common Terminology Criteria for Adverse Events (CTCAE) v4 criteria. Progression-free survival (PFS) and overall survival (OS) were calculated using the Kaplan-Meier method.
Results:
A total of 78 patients with PDAC were treated with the combination. Of these, 83.3 per cent of patients had metastatic disease. The median number of chemotherapy cycles administered was three. The objective response rate for the whole group was 30.8 per cent. Grade III/IV toxicities were seen in 35.9 per cent of patients. Median PFS was 5.6 months and median OS was 11.6 months.
Interpretation & conclusions:
Non-cremophor-based paclitaxel in combination with gemcitabine appeared efficacious for advanced pancreatic cancers in routine clinical practice. Within the confines of a single-centre retrospective analysis, gemcitabine-nanoxel and gemcitabine-abraxane appeared to have similar efficacy and toxicity in advanced pancreatic cancers.
Keywords
Advanced pancreatic cancer
non-cremophor-based paclitaxel-gemcitabine
PDAC
unresectable cancer
Advanced pancreatic cancer remains a major clinical problem with a very high mortality to incidence ratio and accounts for roughly seven per cent of all cancer-related deaths worldwide, although it does not figure amongst the top ten most common cancers in India as per population-based registries1234. Although research in the area has improved our understanding of pancreatic cancers, this has not yet translated into improvement in outcomes5. Conventionally, locally advanced and metastatic pancreatic cancers have been associated with poor prognosis with median survival of about 8-14 and 4-8 months, respectively5678. Various chemotherapeutic agents and regimens have been evaluated, and a few have been shown to improve survival. Gemcitabine-based combination chemotherapy regimens and FOLFIRINOX (5 Fluorouracil-Irinotecan-Oxaliplatin-Leucovorin) appear to be the most active regimens91011. Results of the phase III Metastatic Pancreatic Adenocarcinoma Clinical Trial (MPACT) study have established gemcitabine in combination with albumin-bound paclitaxel as one of the standard first-line regimens for metastatic pancreatic cancer based on an overall survival benefit compared to gemcitabine monotherapy12.
Cremophor free paclitaxel has the advantage of lesser pre-medications and fewer allergic infusion reactions compared to conventional paclitaxel13. Abraxane is also proposed to have some unique molecular and biological characteristics that contribute to its anti-tumour mechanisms141516. Nanoxel is a polymer and surfactant bound paclitaxel that also avoids the infusion reactions associated with paclitaxel17. A cost-efficacy analysis by Ranade et al18 showed a three-week cycle with nanoxel cost-effective when compared with cremophor-based paclitaxel using a complex economic model that took all costs associated with administration and adverse events with the two drugs into consideration19. Other nanotechnology-based non-cremophor paclitaxel formulations have been used in advanced pancreatic cancers with reasonable efficacy1920. The primary objective of this study was to evaluate the survival of patients with unresectable/metastatic pancreatic cancer treated with non-cremophor-based paclitaxel-doublets (abraxane-gemcitabine and nanoxel-gemcitabine) in the routine clinical practice while the secondary objective was to assess adverse events and toxicity profile.
Material & Methods
All patients with histologically proven locally advanced or metastatic pancreatic cancers diagnosed at the department of Medical Oncology at Tata Memorial Hospital, Mumbai, India, between January 2012 and June 2015 and treated with either gemcitabine-abraxane (GA) or gemcitabine-nanoxel (GN) were included in this retrospective analysis. The study was approved by the Institutional Ethics Committee (IEC No IEC/0216/1644/001). Baseline clinical and demographic variables were recorded. The decision to use either abraxane or nanoxel-based therapy was taken by the primary treating physician based on the in-house availability of the drug, discussion of the cost-benefit ratio of the regimens and patient preference. From January 2014 onwards the patients were offered nanoxel as an alternative non-cremophor paclitaxel. Patients not able to afford abraxane were also offered nanoxel.
Treatment details: Patients received a 30 min intravenous infusion of abraxane or nanoxel at a dose of 125 mg/m2 followed by an infusion of gemcitabine at a dose of 1000 mg/m2, on days 1, 8 and 15 every four weeks. The dose was reduced to 75 per cent in cycle 1 in patients with serum albumin <3.0 g/dl and in subsequent cycles in case of a grade III/IV toxicity in the previous cycle and restarted once the toxicity had settled to grade I or completely recovered as per our institutional practice. Therapy was withheld in the event of any life-threatening toxicity, deterioration in patient's performance status or disease progression. Response assessment was done every 8-12 wk or as and when felt clinically relevant with a contrast-enhanced computed tomography (CECT) scan of the thorax, abdomen and pelvis. Response was assessed using Response Evaluation Criteria in Solid Tumours (RECIST) v1.1 by treating clinician or with the help of radiologists associated with gastrointestinal disease management group21. In case of non-measurable lesions, the response was not quantified. Complete response, partial response, stable disease and progressive disease were defined as per RECIST 1.1. Clinical benefit (CB) was defined as lack of disease progression at two consecutive response assessments (16-24 wk after starting treatment). Toxicity was documented using the Common Terminology Criteria for Adverse Events (CTCAE) criteria v4.0322. Subsequent therapy on progression was based on patient's performance status and was at the treating physician's discretion.
Statistical analysis: The data were retrieved from a prospectively maintained database. Overall survival (OS) was calculated from the date of starting chemotherapy to date of death from any cause or date of the last follow up. In patients with locally advanced pancreatic cancer, once the decision for inoperability was taken, then the date of start of chemotherapy was retrospectively used for calculation of OS. Progression-free survival (PFS) was calculated from the date of start of GA or GN until the date of documented radiologic or clinical progression, death or loss to follow up. Survival functions were calculated using the Kaplan-Meier method. The median PFS and OS of the two treatment groups, GA and GN, were compared using the log-rank test.
Results
Seventy eight patients of locally advanced or metastatic pancreatic cancer were treated with GA or GN between January 2012 and June 2015. Median age at diagnosis of metastatic disease or inoperable disease was 60 yr (range: 24-84 yr) and 62.8 per cent of patients were males. Majority of the patients had metastatic disease at presentation (83.3%) and were treatment naïve (68%). The baseline characteristics are summarized in Table I.
GA combination chemotherapy was used in 57 (73.1%) patients, whereas 21 patients (26.9%) received the GN combination. The median number of cycles of chemotherapy received for the whole group was three. Eighteen patients (23.1%) received cycle 1 at a reduced dose of 75 per cent because of low albumin (<3.0 g/dl). Dose reduction in subsequent cycles was done in 14 patients (17.9%) due to grade III/IV toxicity. Delay in chemotherapy due to toxicity was seen in 18 patients (23.1%). The CB rate was 48.7 per cent [95% confidence interval (CI) –37.2-60.3] and overall response rate was 30.7 per cent (95% CI –21.8-42.3).
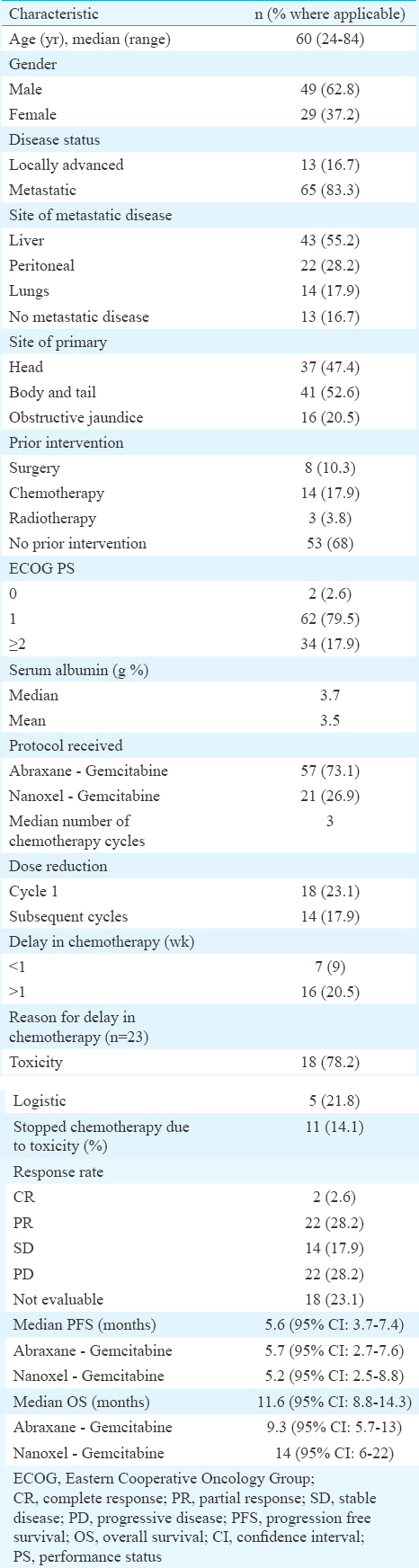
Toxicity profile: Grade III/IV toxicity was seen in 28 patients (35.9%). There was no significant difference in any grade III/IV toxicity between the GA and GN regimens. The rate of grade III/IV neutropenia and thrombocytopenia was higher in the GN group, but this did not reach statistical significance. Chemotherapy was withheld in 11 patients (14.1%) of whom nine had received GA chemotherapy. One patient died due to chemotherapy-related neutropenic sepsis. The toxicity details are shown in Table II.
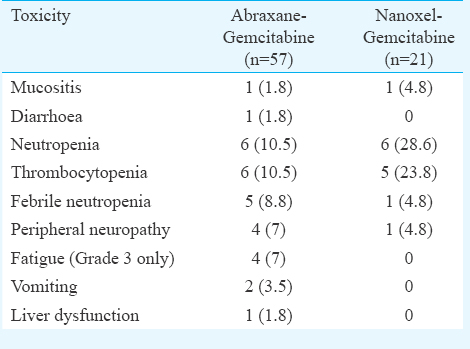
Treatment outcomes: At a median follow up for all patients of 11.4 months (range: 2-23 months), the median overall survival was 11.6 months (95% CI –8.8-14.3) and two-year estimated OS was 12 per cent. There was no significant difference in the median overall survival between the chemotherapeutic regimens [median OS - GA vs. GN –9.3 months (95% CI –5.7-13) vs. 14 months (95% CI –6.0-22); P=0.255] (Fig. 1).
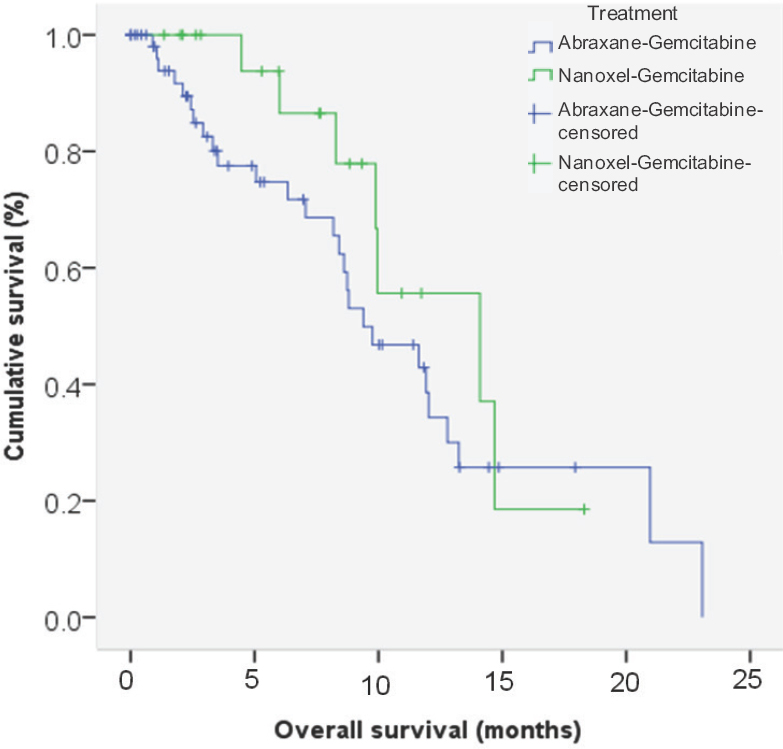
- Kaplan-Meier curve for overall survival for abraxane-gemcitabine and nanoxel-gemcitabine.
The median PFS for the whole group was 5.6 months (95% CI –3.7-7.4) with a one year PFS of 11.2 per cent. There was no significant difference in PFS between the two regimens [median PFS - GA vs. GN –5.7 months (95% CI –2.7-7.6) vs. 5.2 months (95% CI –2.5-8.8); P=0.84] (Fig. 2).
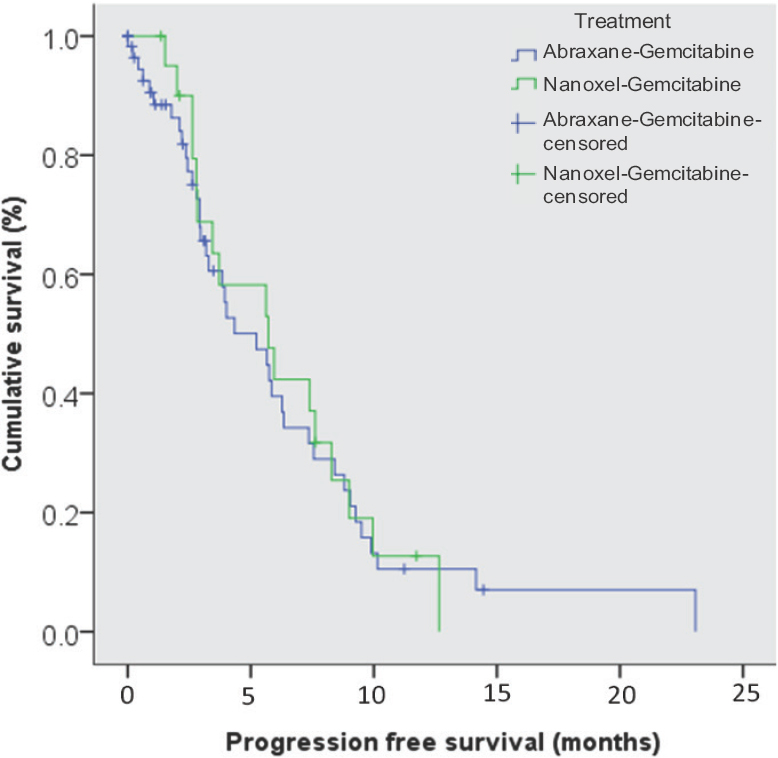
- Kaplan-Meier curve for progression free survival for abraxane-gemcitabine and nanoxel-gemcitabine.
Discussion
Limited efficacies of chemotherapeutic agents and dismal outcomes have plagued the treatment of advanced pancreatic cancers over the years. With current evidence supporting the use of gemcitabine-based combination with albumin-bound paclitaxel and FOLFIRINOX, it is important to choose an appropriate regimen for patients, taking into account age, comorbidity status, Eastern Cooperative Oncology Group Performance Status (ECOG PS) amongst other variables. The reduced infusional toxicity with non-cremophor-based paclitaxel leading to the lesser need of premedication with dexamethasone along with the preclinical evidence of improved efficacy with a more linear dose-response curve compared to traditional paclitaxel (Taxol) and improved survival have made these agents an important component in the therapeutic armamentarium against advanced pancreatic cancer1214151623.
Our study looked at two non-cremophor-based paclitaxel preparations which were used in combination with gemcitabine for locally advanced and metastatic pancreatic cancers. The primary aim of the study was to examine the usage and performance of these agents in routine clinical practice. A retrospective analysis has suggested that a modified two weekly abraxane/gemcitabine schedule appears to retain its survival benefit along with lesser toxicity and is more cost effective24.
In our study, 73.1 per cent of the patients opted for abraxane-based combination therapy. This was expected as the efficacy and overall survival advantage of abraxane-gemcitabine combination has been proven in phase III randomized controlled trial12. Nanoxel has not been compared in a clinical trial with abraxane. Although a preclinical study conducted in athymic nude mice comparing abraxane and nanoxel along with a third cremophor free paclitaxel formulation found superior anti-tumour activity with abraxane, at equitoxic doses; the interpretability of this study was limited by the small numbers in each group (n=10 per group)25. Other non-cremophor paclitaxel formulations have shown efficacy in phase II studies in advanced pancreatic cancers further supporting the role of nanoxel as a potential option in advanced pancreatic cancers1920.
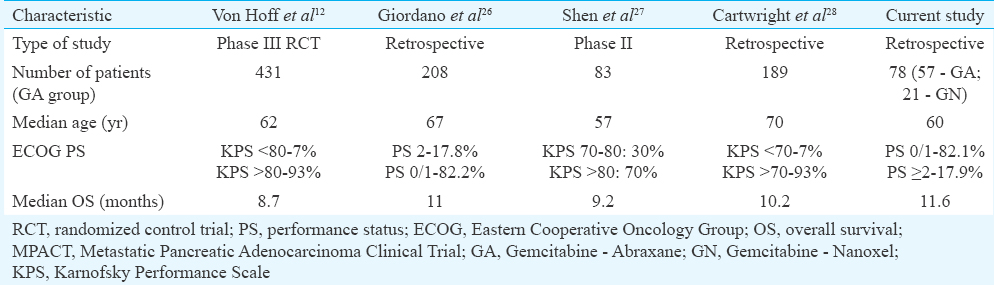
The overall survival of our patients compares well with published data from other studies using gemcitabine-non cremophor paclitaxel in advanced pancreatic cancers (Table III)12262728. The survival of the patients on abraxane and nanoxel was not significantly different in our study. This suggested that nanoxel might have comparable activity to abraxane, but the small numbers undermined the strength of this finding. This study was not powered to statistically compare the abraxane and nanoxel group and should only be considered as preliminary data to suggest the feasibility of using gemcitabine-nanoxel in pancreatic cancers. With regard to toxicity, both drugs were comparable with no significant difference in rates of grade III/IV adverse events. Compared with the toxicity data from the MPACT trial12, the rates of grade III/IV neutropenia were much lower in our study. This could be due to the difference in the dose modification protocols used in our study. However, the febrile neutropenia rate was higher in our study. Grade III/IV peripheral neuropathy also appeared to be lower in our patients even though the median number of chemotherapy cycles were similar to that delivered in the MPACT trial.
Amongst the other chemotherapy regimens used in advanced pancreatic cancers, FOLFIRINOX has shown improvement in survival and quality of life, but its applicability is limited to only the fit patients of the lot11. This was reflected in a previous report from our centre where only 6.9 per cent of treatment-naïve metastatic pancreatic cancers received FOLFIRINOX as their first-line treatment29. Barring the minuscule but significant survival benefit observed with gemcitabine/erlotinib combination in a single phase III randomized study, other combination chemotherapies like gemcitabine-capecitabine have failed to show improvement in survival over gemcitabine alone30. Thus, gemcitabine in combination with abraxane remains an important and a standard first-line regimen for metastatic pancreatic cancer.
Limitations of this study included the small numbers and its retrospective nature. The study was not powered to show the actual efficacy of a nanoxel-based chemotherapeutic regimen; it only suggested feasibility. However, it provided some evidence of the two regimens being relatively comparable in their toxicity and efficacy.
In conclusion, non-cremophor paclitaxel in combination with gemcitabine appeared to have modest efficacy in unresectable/metastatic pancreatic cancer, and the outcomes in this study were similar to previously published data. Within the confines of a single-centre retrospective analysis, gemcitabine-nanoxel and gemcitabine-abraxane appeared to have similar efficacy and toxicity in advanced pancreatic cancers. Prospective studies looking at cost-effective nanoparticle-based paclitaxel formulations represent an important area for future research.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- American Cancer Society. Cancer facts and figures 2013. Atlanta: American Cancer Society; 2013.
- European cancer mortality predictions for the year 2013. Ann Oncol. 2013;24:792-800.
- [Google Scholar]
- Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86.
- [Google Scholar]
- SEER cancer statistics review, 1975-2013. Bethesda, MD: National Cancer Institute; 2012.
- Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma:A prospective, randomized phase III study of the Gruppo Oncologia Dell'italia Meridionale. Cancer. 2002;94:902-10.
- [Google Scholar]
- Gemcitabine combined with oxaliplatin in advanced pancreatic adenocarcinoma: Final results of a GERCOR multicenter phase II study. J Clin Oncol. 2002;20:1512-8.
- [Google Scholar]
- Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403-13.
- [Google Scholar]
- Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513-8.
- [Google Scholar]
- FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-25.
- [Google Scholar]
- Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-703.
- [Google Scholar]
- 2005. U S Food and Drug Administration. Abraxis oncology: Abraxane: Prescribing information. Schaumburg, IL: Abraxis Oncology, a Division of American Pharmaceutical Partners, Inc.; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021660lbl.pdf
- Phase I and pharmacokinetic study of ABI-007, a cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8:1038-44.
- [Google Scholar]
- Designing paclitaxel drug delivery systems aimed at improved patient outcomes: Current status and challenges. ISRN Pharmacol 2012 2012:623139.
- [Google Scholar]
- Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol. 2005;23:7785-93.
- [Google Scholar]
- NANOXEL Injection Package Insert [Version Number IND/00/2006 dated December, 2006]. New Delhi: Dabur Pharma Ltd; 2006.
- Clinical and economic implications of the use of nanoparticle paclitaxel (Nanoxel) in India. Ann Oncol. 2013;24(Suppl 5):v6-12.
- [Google Scholar]
- Cationic liposomal paclitaxel plus gemcitabine or gemcitabine alone in patients with advanced pancreatic cancer: A randomized controlled phase II trial. Ann Oncol. 2012;23:1214-22.
- [Google Scholar]
- Phase II clinical trial of paclitaxel loaded polymeric micelle in patients with advanced pancreatic cancer. Cancer Invest. 2010;28:186-94.
- [Google Scholar]
- New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228-47.
- [Google Scholar]
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0; 28 May, 2009. 14, June 2010
- Nab-paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2:260-9.
- [Google Scholar]
- Modified gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer (MPC): A single institution experience. Poster presented at: ASCO 2015 Gastrointestinal Cancers Symposium. [Abstract 366] 2015
- [Google Scholar]
- Comparison of antitumor activity of three cremophor-free paclitaxel formulations, Abraxane, Nanoxel, and Genexol PM, 99th AACR Annual Meeting 2008. San Diego, CA; 12-16 April, 2008 [Abstract 5619]
- [Google Scholar]
- Nab-paclitaxel (Nab-P) and Gemcitabine (G) as first line chemotherapy (CT) in advanced pancreatic cancer (APDAC) patients (pts): An Italian “real life” study. European J Cancer. 2015;S444(Suppl) Poster 2334
- [Google Scholar]
- A phase II study of Chinese patients (pts) treated with nab-paclitaxel (nab-P) plus gemcitabine (Gem) for metastatic pancreatic cancer (MPC) J Clin Oncol. 2016;34(Suppl) Abstract 327
- [Google Scholar]
- Use of first-line chemotherapy for advanced pancreatic cancer: FOLFIRINOX versus gemcitabine-based therapy. J Clin Oncol. 2014;32(Suppl) Abstract 4132
- [Google Scholar]
- Treatment of patients with metastatic pancreatic cancer: Experience from a tertiary Indian cancer center. Indian J Cancer. 2015;52:449-52.
- [Google Scholar]
- Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-6.
- [Google Scholar]






