Translate this page into:
Evaluation of various culture techniques for identification of hookworm species from stool samples of children
For correspondence: Dr Onila Nongmaithem, Department of Microbiology, Sikkim Manipal Institute of Medical Sciences, Tadong, Gangtok 737 102, Sikkim, India e-mail: nongmaithem2008@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Different coproculture techniques have been developed for culturing the hookworm (HW) larvae for morphological identification in the resource-limited settings. The objective of this study was to compare the performances of Harada-Mori culture (HMC), agar plate culture (APC) and modified APC (MAPC) of HW positive stool specimens for identification of HW species in East Sikkim.
Methods:
This prospective study was done in East Sikkim from May 2015 to May 2016. Stool and blood samples were collected from paediatric patients with gastrointestinal symptoms. The HW positive stool specimens by microscopy were subjected to HMC, APC and MAPC techniques to harvest HW larvae. Stoll's dilution egg count for determining egg intensity and blood parameters were performed in all the 12 HW-positive patients.
Results:
Twelve of the 180 samples were found positive for HW by microscopy and predominance of Necator americanus (75%) over Ancylostoma duodenale (25%) was observed. Blood parameters results showed high pack cell volume (PCV) values in 78.6 per cent, anaemia in 75 per cent and high eosinophil count in most patients. Stoll's dilution egg count showed moderate infection in 66.6 per cent, light and heavy infections in 16.7 per cent each.
Interpretation & conclusions:
Our results showed that APC yielded 100 per cent results and was easier to perform in the laboratory compared to MAPC and HMC techniques.
Keywords
Ancylostoma duodenale
coproculture
hookworm
Necator americanus
Hookworm (HW) infection is one of the important causes of iron deficiency anaemia in children worldwide1. Human infection is primarily caused by two HW species namely Ancylostoma duodenale (835 million) and Necator americanus (135 million), which belong to the family ancylostomatidae2. A. duodenale is mainly distributed in Middle East, North Africa, India, Australia and Europe whereas N. americanus is more common in the Western Hemisphere, Sub-Saharan, Eastern Asia and South East Asia3. In India, N. americanus is predominant in south India and A. duodenale in north India4. Approximately, 400 million children worldwide are infected with intestinal parasites and compromised by anaemia which gives negative effects on growth, iron status, irritability and cognitive impairment to increase susceptibility to other infection and acute complications4. In schoolgoing children, the infection leads to attention deficits, learning disabilities, school absenteeism and higher dropout rates5. Different species of HW differ in their morphology, pathogenesis, life cycle and clinical features. Therefore, specific identification and differentiation of HW species is important at community level as well as in school children for monitoring the efficacy of mass and effective treatment and severity of illness. For treatment purposes, the drugs of choice is similar; however, the severity of anaemia differs depending on the different species and worm load in the intestine67. The clinical manifestations also depend on the egg intensity and also positive correlation exists between the infecting HW species and the rate of anaemia89.
A direct microscopy examination of stool is a simple and rapid test for the diagnosis of HW infection; however, microscopy alone cannot differentiate between the HW species and other similar species like strongyloid nematodes such as Trichostrongylus spp. and Oesophagostomum spp. because all the eggs are morphologically similar10. Various coproculture techniques are employed for morphological characterization of A. duodenale and N. americanus and strongyloides. The aim of this study was to compare various culture techniques used for identification of HW species, namely, Harada-Mori culture (HMC), agar plate culture (APC) and modified APC (MAPC) techniques.
Material & Methods
This prospective study was carried out in the department of Microbiology of Sikkim Manipal Institutue of Medical Sciences, Gangtok, India, during May 2015 to May 2016. Children up to 15 yr of age who presented with gastrointestinal symptoms to Sir Thodup Namgyal Memorial Hospital and Central Referral Hospital (STNM and CRH) located in East Sikkim, were investigated for intestinal parasitic infection. All stool specimens were collected before radiological studies using barium or the administration of bismuth, mineral oils and anti-diarrhoeal medication that could interfere with the detection and identification of intestinal parasites. Children without gastrointestinal symptoms were excluded. Written informed consent was obtained from parents/guardians and/or children. This study was approved by the Sikkim Manipal University Ethics Committee.
Collection & processing of sample: The children/guardians were given a labelled, leak-proof container with a plastic scoop (HiMedia Laboratories Pvt. Ltd., Mumbai) to collect the stool sample and processed within four hours of collection. The samples were examined microscopically directly using saline and iodine wet mount preparation and following formol-ether concentration technique11. Positive samples for HW eggs were further subjected to three coproculture methods to obtain L3 larvae for morphological speciation. Based on the morphology of L3 larvae, the different species of HW were identified4. Stoll's dilution egg count method [number of eggs per gram (epg) of stool] to identify the worm burden and intensity of the infection was conducted in all the positive specimens. Blood samples (3-5 ml) were collected from the patients and blood parameters (Hb, Hct, MCH, MCHC and eosinophil count) were performed using standard proceedings.
Stool culture: About 0.5-1 g of fresh stool samples positive for HW eggs were cultured to the rhabditiform larvae at 25-28°C by the HMC technique6. The tube was kept for 7-10 days and checked daily. For APC, approximately 2 g of sample was used and incubated at 26-33°C for two days11. For MAPC, a canal of 1 cm wide was made and 2 g (approximately) of sample was smeared on the agar and incubated at 26-33°C for 2-3 days as described by Khanna et al12. The observation was made in concordance with the recommendations made earlier1314. Quantitative HW egg counts were obtained by Stoll's dilution egg count11 and expressed as 100-500 epg.
The sensitivity and specificity of these culture methods were determined using microscopy as standard technique.
Statistical analysis: The association between the two categorical variables was analyzed by Chi-square test or Fisher's exact test as appropriate. Different variables were summarized using frequency tables.
Results
Of the 180 patients (97 males, 83 females) screened intestinal parasites were found positive in 58 (32.2%) HW ova were observed in 12 (6.6%) (Fig. 1). Other predominant intestinal parasites found were Giardia cyst in 14 (7.7%), Entamoeba histolytica in 10 (5.5%), Taenia eggs in nine (5%), Ascaris lumbricoides in six (3.3%), Trichuris thrichiura in three, and Enterobius vermicularis and Hymenolepis nana in two each. Of the 12 samples, N. americanus and A. duodenale were identified in 10 (75%) and two (25%) samples, respectively by coproculture methods. Mixed infection was not seen. The comparison of three culture techniques is shown in Table I. Significant difference (P<0.001) was noted between HMC and APC methods. The sensitivity of HMC was 30.7 per cent, specificity 100 per cent; APC showed sensitivity of 92.3 per cent, specificity 100 per cent and MAPC showed 69.3 per cent sensitivity and specificity 100 per cent. Filariform larvae of N. americanus varied from 500 to 620 μm while A. duodenale varied from 690 to 750 μm. The highest infection rate was seen in 6-10 yr followed by 11-15 yr and least by 0-5 yr, and the infection was more common in males compared to females.
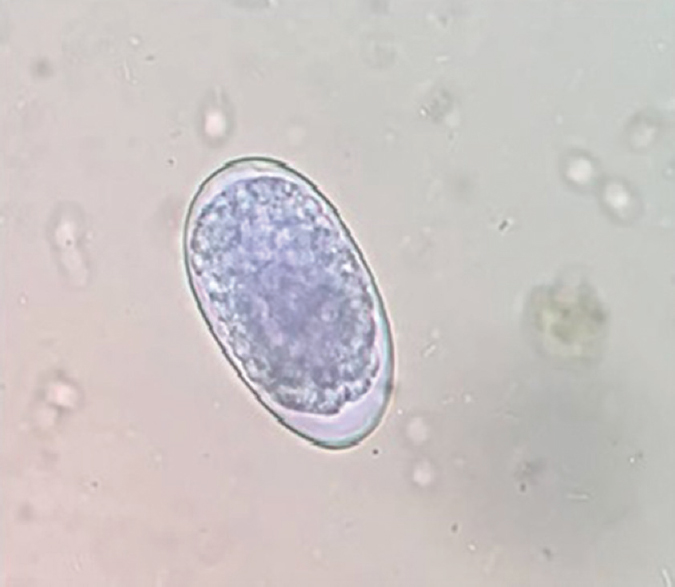
- Hookworm eggs in saline mount (×40).
| Characteristics | HMC | APC | Total |
|---|---|---|---|
| Positive | 4 | 12 | 16 |
| Negative | 8 | 0 | 8 |
| Total*** | 12 | 12 | 24 |
| HMC | MAP | ||
| Positive | 4 | 9 | 13 |
| Negative | 8 | 3 | 11 |
| Total | 12 | 12 | 24 |
| APC | MAP | ||
| Positive | 12 | 9 | 21 |
| Negative | 0 | 3 | 3 |
| Total | 12 | 12 | 24 |
***P<0.001 (Fisher exact two-tailed test); HMC, Harada-Mori culture; APC, agar plate culture; MAP, modified agar plate
Stoll's dilution egg count showed moderate infection (eggs=600-1000 per g) in 8/12 (66.6%), light (100-500 epg) and heavy infections (>1000 epg) in 4/12 (33.3%) each. Complete blood count (CBC) results showed lower haemoglobin in 19 (76%) females, higher PCV (Hct) in 16 (64%) females and low MCH and MCHC in 29 (87.8%) and 28 (84.8%) males, respectively. Higher eosinophil count was seen more in males 27 (81.8%) (Table II). Diarrhoea accompanied with dehydration, weakness, fever and bloating were the common symptoms at presentation 119 (66%), other symptoms included diarrhoea alone 27 (15%), loss of weight and appetite 14 (8%), abdominal pain 13 (7%), dysentery 6 (3%) and constipation (1%).
| Parameters | Normal range | Males (n=33) | Females (n=25) |
|---|---|---|---|
| Haemoglobin (g/dl) | >11 | 13 (39.4) | 6 (24) |
| <11 | 20 (60.6) | 19 (76) | |
| Hct (%) | <36 | 16 (48.5) | 9 (36) |
| <54 | 17 (51.5) | 16 (64) | |
| MCH (pg) | <27 | 29 (87.8) | 25 (100) |
| >32 | 4 (12.2) | - | |
| MCHC (g/dl) | <31 | 28 (84.8) | 23 (92) |
| >35 | 5 (15.2) | 2 (8) | |
| Eosinophil count | <0.04 | 6 (18.2) | 4 (16) |
| >0.40 | 27 (81.8) | 21 (84) |
Values in parentheses are percentage. Hct, haematocrit; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration
Discussion
In this study, three stool culture techniques, namely HMC, APC, and MAPC were evaluated for the isolation and identification of HW larvae from the stool samples. The study showed APC as the most effective technique.
Twelve samples yielded larvae for morphological identification by coproculture technique, of which 10 samples were identified as N. americanus and two as A. duodenale (Figs 2 and 3). In other studies done by Shahid et al15 found 10.15 per cent HW, of these 11.53 were A. duodenale and 88.47 per cent were N. americanus. Parija et al16 reported, 53.6 per cent A. duodenale, 43.7 per cent N. americanus and 2.7 per cent mixed infection of both. Adenusi and Ogunyomi17 found 6.1 per cent A. duodenale, 68.2 per cent N. americanus and 25.7 per cent mixed infection of both. All these studies were based on HMC techniques only.
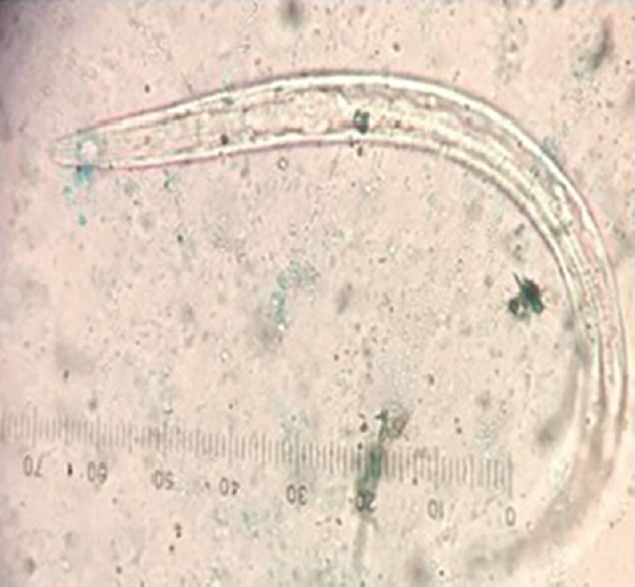
- Filariform larvae L3 of Ancylostoma duodenale and Necator americanus in agar plate culture (×40).
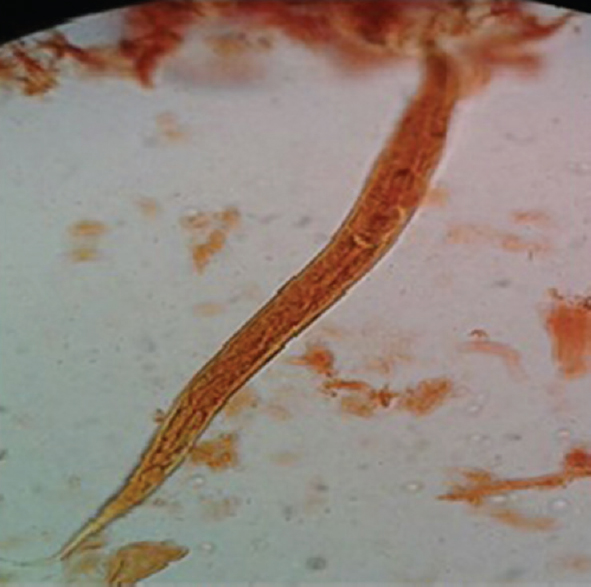
- Filariform larvae, L3 of Ancylostoma duodenale and Necator americanus in iodine stain obtained from agar plate culture.
Compared to the HMC and MAPC, APC showed higher positive rates. In terms of total time taken to hatch the filariform larva, APC and MAPC took almost similar time period of around 2-5 days, in some cases, MAPC took only two days. However, in HMC, it took 7 to 10 days. In terms of sensitivity in our study, APC showed 92 per cent compared to 69 per cent by MAPC and 30.7 per cent by HMC. Khanna et al12, have mentioned that, in case of MAPC, infective larva may be found after second or third day or even after the first day in case of heavy infections. Their study also reported more parasites by this modified method, which was a contrast with our findings.
Anaemia was observed more in females compared to males. Furthermore, the infection rate was more in 6-10 yr age group, and in males. However, in the study done in Malaysia15, HW infection was more prevalent in females (62.7%) compared to males (37.3%). Higher occurrence in male in our study might be due to close with household pets, playing with barefoot, frequent contact with polluted soil and less care about the hygiene18. Depending on the status of host iron, an HW burden of 40-160 worms per individuals is associated with haemoglobin level below 11 g/dl19. Among the HW species, A. duodenale causes more blood loss than N. americanus. It has been estimated that a single A. duodenale worm ingests about 150 μl (0.15 ml) of blood per day and N. americanus worm about 30 μl (0.03 ml)20. However, MCH and MCHC decreases may be due to malnutrition of the children, and there was increase in the eosinophil count which may be the reason of allergy or parasitic infection.
The limitation of this study was the use of the single faecal sample which might lose the chances of HW infections from the same patients. Coproculture is also time-consuming and needs well experienced person to differentiate the species. Coproculture techniques are successful only when the larvae present are viable. Even though this study used the fresh stool samples, we could not rule out the false positive or false negative results for species identification. This differential identification can be ruled out by molecular methods. Furthermore, the number of positive samples was too small to make a significant conclusion.
In conclusion, the APC technique yielded better results for the isolation of larvae from the eggs in the stool and was also easier to perform in the laboratory. Chances of laboratory infection are lesser because of mishandling while making the canal with the blade in case of MAPC and takes lesser time (2-3 days) compared to HMC (7-10 days). Among the HW species, N. americanus was predominant in this study. A large number of samples need to be studied to confirm our findings.
Acknowledgment
The authors thank the hospital superintendent of Central Refferal Hospital (CRH), Tadong, and Sir Thodup Namgyal Memorial Hospital (STNM), Gangtok, for allowing collecting the samples and other related information. The authors also thank Dr Rekha for statistical analysis and Shri Dhurba Bhandari for laboratory work.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Hookworm infection and hemoglobin concentration among students in three selected primary schools. Western Ethiopia: Abobo Woreda, Gambella Region; 2012.
- One world health: Neglected tropical diseases in a flat world. PLoS Negl Trop Dis. 2009;3:e405.
- [Google Scholar]
- Soil-transmitted helminth infections: Updating the global picture. Trends Parasitol. 2003;19:547-51.
- [Google Scholar]
- Small intestinal nematodes. In: Sastry AS, Bhat SK, eds. Medical parasitology (1st ed). Puducherry: Jaypee Brothers Medical Publisher (P) Ltd; 2014.
- [Google Scholar]
- Control of Schistosomaisis and soil-transmitted helminths infections. In: Report by the Secretariat, Executive Board. 107th Session, Provisional agenda Item 3.3 (EB 107/31). Geneva: World Health Organization; 2001.
- [Google Scholar]
- Guidelines for the evaluation of soil transmitted helminthiasis and schistomiasis at the community level. (WHO/CTD/SIP/98.1). Geneva: Schistosomiasis an Intestinal Parasite Unit, Division of Control Tropical Diseases. WHO; 1998.
- Medical parasitology (2th ed). Berlin, New York: Springer-Verlag Press; 1989.
- Effect of age and initial infection intensity on the rate of reinfection with Trichuris trichiura after treatment. Parasitology. 1988;97(Pt 3):469-76.
- [Google Scholar]
- Emerging patterns of hookworm infection: Influence of aging on the intensity of necator infection in Hainan province, people's republic of China. Clin Infect Dis. 2002;35:1336-44.
- [Google Scholar]
- Textbook of medical parasitology: Protozoology and helminthology (4th ed). New Delhi: All India Publishers and Distributors; 2013.
- Diagnostic medical parasitology (4th ed). Washington, DC: ASM Press; 2001.
- Modified agar plate culture method for culture of Strongyloides stercoralis. Trop Parasitol. 2015;5:136-8.
- [Google Scholar]
- Movement of the rhabditiform larva of Strongyloides stercoralis. Lancet. 1993;342:1310.
- [Google Scholar]
- Increased sensitivity of routine laboratory detection of Strongyloides stercoralis and hookworm by agar-plate culture. Trans R Soc Trop Med Hyg. 1999;93:398-400.
- [Google Scholar]
- Identification of hookworm in stool by Harada Mori culture. Bangladesh J Med Microbiol. 2010;4:3-14.
- [Google Scholar]
- Prevalence of hookworm species in Pondicherry, India. Trop Geogr Med. 1992;44:378-80.
- [Google Scholar]
- Relative prevalence of the human hookworm species, Necator americanus and Ancylostoma duodenale in an urban community in Ogun state. Niger Afr J Biotechnol. 2003;2:470-3.
- [Google Scholar]
- Evaluation of the utility of conventional polymerase chain reaction for detection and species differentiation in human hookworm infections. Trop Parasitol. 2017;7:111-6.
- [Google Scholar]
- A new approach to morbidity risk assessment in hookworm endemic communities. Epidemiol Infect. 1992;108:469-81.
- [Google Scholar]
- Jan MS, Liu D, eds. Molecular detection of human parasitic pathogens. USA: CRC Press; 2012. p. :613.






