Translate this page into:
Evaluation of real-time reverse-transcription loop-mediated isothermal amplification assay for clinical diagnosis of West Nile virus in patients
For correspondence: Dr Jyoti S. Kumar, Division of Virology, Defence Research & Development Establishment, Jhansi Road, Gwalior 474 002, Madhya Pradesh, India e-mail: jyotishukla2@drde.drdo.in
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
West Nile virus (WNV) is a mosquito-borne flavivirus. The disease can be diagnosed by isolation followed by fluorescent antibody tests, enzyme-linked immunosorbent assay and polymerase chain reaction (PCR) assay. These diagnostic methods are laborious and time-consuming. The present study was aimed to evaluate the real-time reverse-transcription loop-mediated isothermal amplification (RT-LAMP) method for rapid, early and accurate diagnosis of WNV.
Methods:
A one-step single tube accelerated quantitative RT-LAMP assay was evaluated by targeting the Env gene of WNV. The gene amplification was accomplished by incubating the reaction mixture at 63°C for 60 min in both real time turbidimeter as well as routine laboratory water bath/dry heating bath. To rule out contamination issues, proper negative controls, including no template, no primer; and no enzyme, were always kept alongside each run. The RT-LAMP assay was evaluated on 105 clinical samples from individuals having ocular infection.
Results:
Of the 105 samples tested, 27 were positive for WNV by RT-LAMP assay. The comparative evaluation with conventional RT-PCR revealed 100 per cent accordance with sensitivity and specificity of 100 and 95 per cent, respectively. The specificity of this assay was confirmed with serum samples obtained from patients with dengue and chikungunya.
Interpretation & conclusions:
The RT-LAMP test seemed to be a sensitive and specific method for rapid detection of WNV infection and would be useful for rapid screening of a large number of clinical samples in endemic areas during outbreaks.
Keywords
Arbovirus
detection assay
envelope gene
reverse transcription loop
mediated isothermal amplification test and clinical diagnosis
West Nile virus
West Nile virus (WNV) is an arthropod-borne virus of public health importance. The mortality rate following neuroinvasive infection is approximately 10 per cent and long-term neurological sequel is common (50%)12. Diagnosis of WNV is done through the detection of virus-specific antibody immunoglobulin M (IgM) and neutralizing antibodies. Specimens collected within eight days following onset of illness may not test positive for WNV-specific IgM, and testing should be repeated. A positive test for WNV-specific IgG in the absence of a positive WN IgM is indicative of a previous flavivirus infection and is not by itself evidence of an acute WNV infection. However, confirmation of the virus is based on virus-specific neutralizing antibody titre by plaque reduction neutralization (PRNT) assay3456. Both virus isolation and PRNT assays are time-consuming and tedious, requiring more than a week for completion.
Reverse-transcription polymerase chain reaction (RT-PCR) has been used to detect flavivirus genome in rapid and specific tests7. Two-step RT-PCR assay requires agarose gel analysis for the detection of amplicons after PCR cycling. Hence, the assay is labour intensive and has a very high risk of contamination. In addition to conventional RT-PCR, more rapid and sensitive real-time PCR-based assays, such as SYBR Green and TaqMan-based real-time RT-PCR, and nucleic acid sequence-based amplification (NASBA) have been used to develop rapid diagnostic tests for several pathogenic viruses with single-stranded RNA genomes, e.g. WNV, foot-and-mouth disease virus, severe acute respiratory syndrome (SARS)-associated coronavirus, human bocavirus and also parasites such as Trypanosoma brucei89. However, all of these advanced nucleic acid amplification methods require a high-precision instrument for amplification and skilled person to perform assay89.
The RT loop-mediated isothermal amplification (RT-LAMP) assay has emerged as a powerful gene amplification tool for rapid identification of microbial infections and is increasingly being used for rapid detection and typing of emerging viruses, viz. chikungunya, SARS, dengue (DEN) and Japanese encephalitis210111213. One-step, single-tube real-time accelerated RT-LAMP assay was developed by targeting the immunodominant envelope (E1) gene for rapid and real-time detection of WNV14. In the present study, the applicability of this method for clinical diagnosis of WNV infection was validated by evaluation with acute-phase serum samples collected from patients suspected to have WNV infection.
Material & Methods
Cell culture and virus: The WNV (Eg101 strain) used in this study was obtained from the Institute of Tropical Medicine, Nagasaki, and was propagated by regular passaging in Aedes albopictus clone C6/36 cells15. The virus was titrated by plaque assay in Vero cells in accordance with the standard protocol in Eagle's minimum essential medium (Sigma, USA) supplemented with 10 per cent tryptose phosphate broth (TPB)16. TPB (DIFCO, USA), 10 per cent foetal bovine serum (Sigma, USA), 3 per cent L-glutamine and gentamicin (80 mg/l) (Sigma, USA) were purchased.
This study was conducted in the division of Virology at Defence Research and Development Establishment, Gwalior, India.
Clinical samples
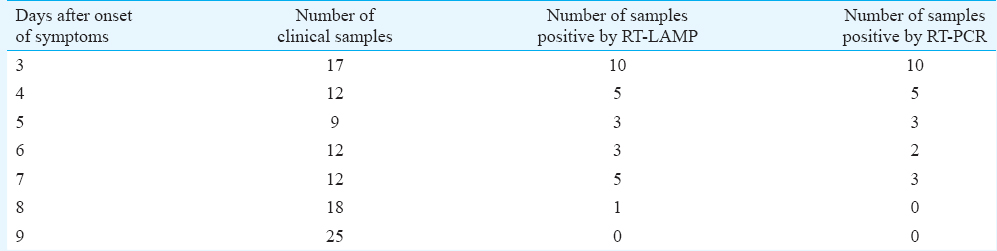
The serum and plasma samples (n=170) used in this study were collected from the department of Microbiology, Aravind Eye Hospital, Madurai, India, from patients with ocular symptoms suspected to have WNV infections during December 2012. The acute-phase serum samples collected between days 1 and 9 after the onset of symptoms were used for evaluation (Table I). In addition, a panel of positive serum samples of DEN (n=35) and chikungunya (n=25) along with healthy volunteers (n=20) was also included as negative control. The correlation, sensitivity, specificity, positive predictive value and negative predictive values were calculated17. The study protocol was approved by the ethics committee of Aravind Eye Hospital, Madurai, India.
RNA extraction: The genomic viral RNA was extracted from 140 μl of infected culture supernatant and patient serum/plasma samples using QIAamp Viral RNA Mini Kit (Qiagen, Germany), according to the manufacturer's protocols. The RNA was eluted from QIAspin columns in a final volume of 100 μl of elution buffer and stored at −70°C until used.
Reverse-transcription polymerase chain reaction (RT-PCR): To compare the sensitivity and specificity of the RT-LAMP assay, one-step RT-PCR was performed targeting the envelope gene of WNV [WN-env Forward: TGG ATT TGG TTC TCG AAG G (genome position 1028-1046) and WN-env Reverse: GGT CAG CAC GTT TGT CAT T (genome position 1228-1210)] (Table II). The amplification was carried out in a 50 μl total reaction volume using Promega AccessQuick one-step RT-PCR kit (Promega, USA) with 50 pmol of forward and reverse primers and 2 μl of RNA according to the manufacturer's protocol. The thermal profile of RT-PCR reaction was 48°C for 45 min, 95°C for 5 min, followed by 35 cycles of 94°C for one min, 62°C for one min, 72°C for one min and final extension cycle at 72°C for 10 minutes.
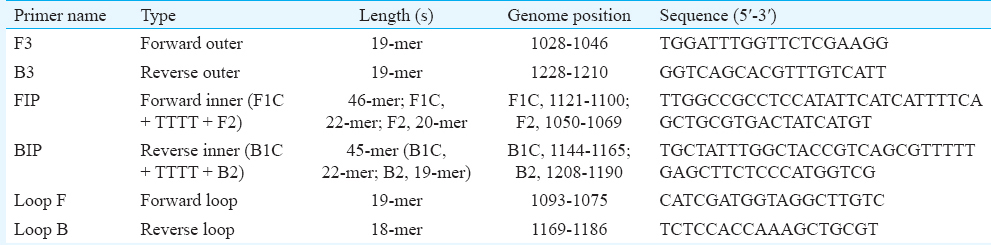
Reverse-transcription loop-mediated isothermal amplification (RT-LAMP): The RT-LAMP reaction was carried out in 25 μl reaction volume using the Loopamp RNA Amplification Kit (Eiken Chemical Co Ltd., Japan) containing 50 pmol each of the primers, forward inner primer (FIP) and backward inner primer (BIP), 5 pmol each of the outer primers F3 and B3, 25 pmol each of loop primers forward loop primer and backward loop primer and 2 μl of RNA template. The sequences of the selected primers were compared to an alignment of the E gene sequences of 14 strains of WNV. The details of the oligonucleotide primers used for amplification of the E gene of WNV are given in Table II. The real-time monitoring of RT-LAMP assay was accomplished by incubating the reaction mixture at 63°C for 60 min in Loopamp Real-time Turbidimeter (LA-200, Teramecs, Japan). Positive and negative controls were included in each experiment. The real-time monitoring of RT-LAMP amplification of WNV template was observed through spectrophotometric analysis by recording optical density (OD) at 400 nm at every six seconds with the help of Loopamp Real-Time Turbidimeter (LA-200, Teramecs, Japan). The cut-off value for positivity by real-time RT-LAMP assay was determined by taking into account the time of positivity (Tp; in min) at which the turbidity increased above the threshold value fixed at 0.1, which was two times more than average turbidity value of the negative controls of several replicates. Therefore, a sample having Tp values of ≤45 min and turbidity above the threshold value of ≥0.1 was considered positive. To facilitate the field application of RT-LAMP assay, the observance for amplification was also performed through colour change by addition of 1 μl of SYBR Green I dye to the tube. In case of positive amplification, the original orange colour of the dye will change into green that can be seen under natural light as well as under ultraviolet (UV) light (302 nm) with the help of a hand-held UV torch lamp. In case there is no amplification, the original orange colour of the dye will be retained.
Results
Sensitivity of RT-LAMP: The sensitivity of the RT-LAMP assay for the detection of WNV RNA was determined by testing serial 10-fold dilutions of virus compared with that of conventional RT-PCR. The RT-LAMP assay had a detection limit of 0.1 plaque-forming unit (pfu) of virus (Fig. 1). The comparative sensitivities of this assay and RT-PCR revealed that the RT-LAMP assay was 10-fold more sensitive than the RT-PCR assay, which had a detection limit of one pfu of virus, as indicated by the presence of a 201 bp amplicon (Fig. 1).
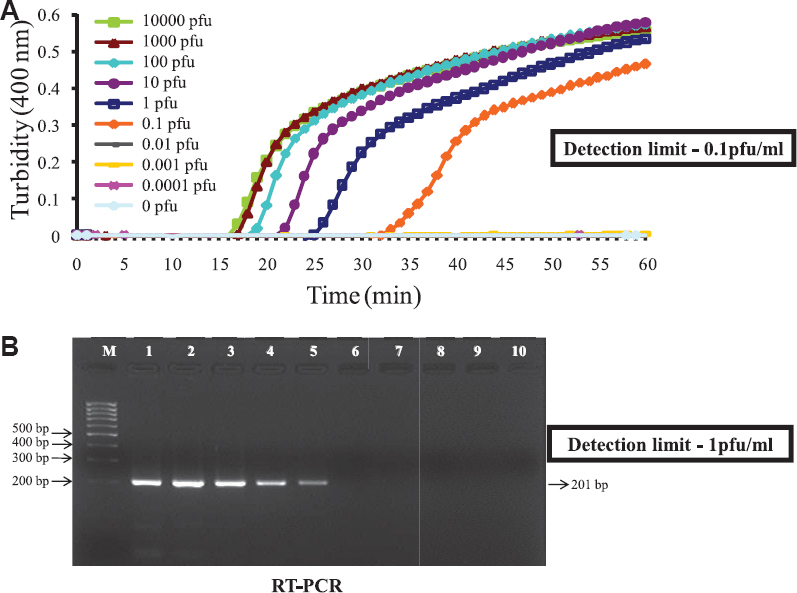
- Comparison between sensitivity (detection limit) of reverse-transcription loop-mediated isothermal amplification (RT-LAMP) and conventional RT-PCR. (A) Real-time kinetics of West Nile virus reverse-transcription loop-mediated isothermal amplification (RT-LAMP) amplification of the E gene showing the amplification curve with serial 10 fold dilutions of the virus ranging from 104 to 0.0001 plaque-forming unit (pfu). The x axis depicts the time of positivity, and the y axis shows the turbidity value in terms of the optical density at 400 nm. (B) RT-PCR performed on the same serial dilution as used for RT-LAMP. The detection limit of the RT-LAMP assay was 10-fold higher than conventional RT-PCR. M, 1 kb marker; lane1-9, 104 - 0.0001 pfu of virus; lane 10, no virus.
Evaluation of RT-LAMP assay with clinical samples: The real-time kinetics of the RT-LAMP was studied at 63°C by monitoring turbidity. The results indicated that the time required for initiation of amplification was 17 minutes. It was also observed that there was continuous amplification of the target sequence in a step-wise gradient manner, as observed by increased turbidity compared to that of the negative control having no template, where the turbidity was fixed at around 0.01 below the threshold value. The applicability of RT-LAMP assay for clinical diagnosis of WNV was evaluated on 105 acute-phase serum samples from patients with ocular infection. The results were compared with conventional RT-PCR. A panel of 35 DEN- and 25 chikungunya-positive serum samples along with 10 negative acute-phase serum samples and 20 negative serum samples were used for comparative evaluation. Of the 105 samples, 27 were positive by RT-LAMP assay. The comparative OD values with different categories of sample panel are depicted in Fig. 2. All positive samples revealed a higher OD value of 0.2-0.6 as compared to 0.05 in the case of negative samples. The RT-LAMP assay demonstrated higher sensitivity compared to conventional RT-PCR by picking up four additional positive cases which were missed by RT-PCR. No cross-reactivity was observed with DEN- and chikungunya-positive samples along with healthy serum samples, thus establishing the specificity of RT-LAMP assay.
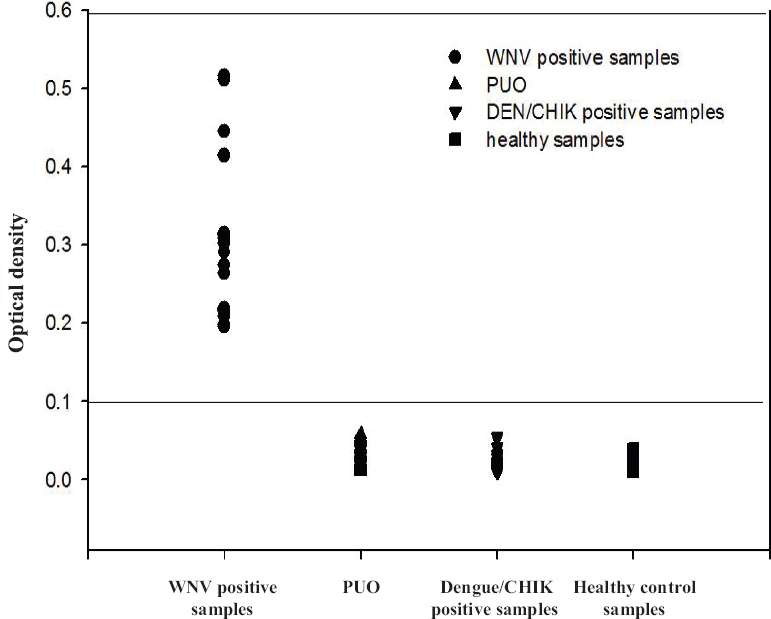
- The optical density profile of different patient samples including healthy controls as obtained through the West Nile-specific loop-mediated isothermal amplification (RT-LAMP) assay. PUO, pyrexia of unknown origin; DEN, dengue; CHIK, chikungunya.
The field applicability of RT-LAMP assay was also evaluated on 105 clinical samples along with negative panel. Incubation was carried out at 63°C for 30 minutes. Following incubation, all positive samples showed green fluorescence, whereas negative samples retained orange colour while adding 1 μl of SYBR Green I (1:1000) nucleic acid dye. The parameter of comparative evaluation with RT-PCR indicated an accordance of 100 per cent with a sensitivity and specificity of 100 and 95 per cent, respectively.
Discussion
Outbreaks or sporadic cases of WN viral disease in human beings or equines have been documented in Africa, the Middle East, Europe, West and South Asia, Australia and North America6181920212223. Several outbreaks in different countries with various range of severity have been reported earlier. A recent outbreak of WNV was seen in the United States. More than 1100 cases and at least 44 deaths have been reported24. During the past decade, various nucleic acid amplification techniques such as RT-PCR, TaqMan real-time RT-PCR, SYBR Green real-time RT-PCR and NASBA have been developed to address the need for rapid identification of WNV with accuracy. Despite the high magnitude of amplification, these PCR-based methods are costly and require trained personnel to perform assay. In addition, these methods are often cumbersome to adapt for routine clinical use, especially in peripheral healthcare settings and private clinics. Therefore, a rapid, specific, cost-effective and sensitive test is necessary for effective surveillance of new WNV circulating strains. Based on TaqMan chemistry, two real-time RT-PCR assay systems for the detection of WNV RNA genome in serum, cerebrospinal fluid (CSF) and brain tissues samples from human, field-collected mosquitoes and avian tissue samples have been reported8.
The real-time PCR assay has many advantages over conventional RT-PCR methods, including rapidity, quantitative measurement, lower contamination rate, higher sensitivity, higher specificity and easy standardization. The development of fluorogenic PCR utilizing 5’-3’ nuclease activity of Taq DNA polymerase made it possible to eliminate post-PCR processing such as visualization in agarose7. However, all these nucleic acid amplification methods have several intrinsic disadvantages of requiring either a high precision instrument for amplification or an elaborate complicated method for the detection of amplified products. These rapid molecular tests might not be the method of choice in basic clinical settings in developing countries or in field situations because of the requirement of sophisticated instrumentation and expensive reagents. It is, therefore, critical to develop simple and rapid molecular tests to complement the existing techniques.
In this regard, RT-LAMP assay reported in this study is advantageous due to its simple operation, rapid and easy detection. The RT-LAMP assay is a simple diagnostic tool in which the reaction is carried out in a single tube by mixing of the buffer, primers, reverse transcriptase and DNA polymerase and incubating the mixture at 63°C for 30 minutes. The higher sensitivity and specificity of the RT-LAMP reaction are attributed to continuous amplification under isothermal condition employing six primers recognizing eight distinct regions of the target. Besides, the higher amplification efficiency RT-LAMP reaction yields large amount of by-product, pyrophosphate ion, leading to white precipitate of magnesium pyrophosphate in the reaction mixture. Since the increase in turbidity of the reaction mixture according to the production of precipitate correlates with the amount of DNA synthesized, real-time monitoring of the RT-LAMP reaction can be achieved by real-time measurement of turbidity13.
RT-LAMP assay seems to have advantage in terms of monitoring of amplification that can be accomplished by SYBR Green I dye-mediated naked eye visualization and by real-time monitoring using an inexpensive turbidimeter according to the situation. The RT-LAMP assay was rapid as its result appeared after one hour and sensitive than the conventional diagnostic methods. The LAMP assay requires only a regular laboratory water bath and is hence suitable as a routine diagnostic tool in private clinics and field applications where equipment such as PCR and real-time RT-PCR apparatus are not available. LAMP was found to be effective when the template was pure; however, the usefulness of LAMP methods could be limited by the presence of inhibitors in the analysis of clotted blood samples and urine without centrifuge2.
Acknowledgment
Authors thank Dr Lokendra Singh, Director, Defence Research and Development Establishment (DRDE), Ministry of Defence, Government of India, for support, constant inspiration and providing the necessary facilities for this study.
Financial support & sponsorship: None
Conflicts of Interest: None.
References
- The West Nile virus outbreak of 1999 in New York: The Flushing Hospital experience. Clin Infect Dis. 2000;30:413-8.
- [Google Scholar]
- Development and evaluation of a novel loop-mediated isothermal amplification method for rapid detection of severe acute respiratory syndrome coronavirus. J Clin Microbiol. 2004;42:1956-61.
- [Google Scholar]
- Detection of West Nile and Japanese encephalitis viral genome sequences in cerebrospinal fluid from acute encephalitis cases in Karachi, Pakistan. Microbiol Immunol. 1994;38:827-30.
- [Google Scholar]
- Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J Clin Microbiol. 2000;38:1827-31.
- [Google Scholar]
- Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol. 2000;38:1823-6.
- [Google Scholar]
- Origin of the West Nile virus responsible for an outbreak of encephalitis in the Northeastern United States. Science. 1999;286:2333-7.
- [Google Scholar]
- Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066-71.
- [Google Scholar]
- Nucleic acid sequence-based amplification assays for rapid detection of West Nile and St. Louis encephalitis viruses. J Clin Microbiol. 2001;39:4506-13.
- [Google Scholar]
- Detection of foot-and-mouth disease virus by nucleic acid sequence-based amplification (NASBA) Vet Microbiol. 2008;126:101-10.
- [Google Scholar]
- Development and evaluation of reverse transcription-loop-mediated isothermal amplification assay for rapid and real-time detection of Japanese encephalitis virus. J Clin Microbiol. 2006;44:4172-8.
- [Google Scholar]
- Rapid detection and differentiation of dengue virus serotypes by a real-time reverse transcription-loop-mediated isothermal amplification assay. J Clin Microbiol. 2005;43:2895-903.
- [Google Scholar]
- Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes. 2002;16:223-9.
- [Google Scholar]
- Rapid and real-time detection of Chikungunya virus by reverse transcription loop-mediated isothermal amplification assay. J Clin Microbiol. 2007;45:351-7.
- [Google Scholar]
- Real-time reverse transcription loop-mediated isothermal amplification for rapid detection of West Nile virus. J Clin Microbiol. 2004;42:257-63.
- [Google Scholar]
- Isolation of a Singh's Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol. 1978;40:531-44.
- [Google Scholar]
- Arboviruses. In: Schmidt NJ, Emmons RW, eds. Diagnostic procedures for viral, rickettsial and chlamydial infections. Washington: American Public Health Association; 1989. p. :797-856.
- [Google Scholar]
- Monoclonal antibody-based antigen capture immunoassay for detection of circulating non-structural protein NS1: Implications for early diagnosis of Japanese encephalitis virus infection. J Med Virol. 2011;83:1063-70.
- [Google Scholar]
- Outbreak and cocirculation of three different Usutu virus strains in eastern Germany. Vector Borne Zoonotic Dis. 2017;17:662-4.
- [Google Scholar]
- The changing epidemiology of Kunjin virus in Australia. Int J Environ Res Public Health. 2013;10:6255-72.
- [Google Scholar]
- West Nile fever in Israel: The reemergence of an endemic disease. J Infect. 2014;68:170-5.
- [Google Scholar]
- West Nile Virus Outbreak in Houston and Harris County, Texas, USA, 2014. Emerg Infect Dis. 2017;23:1372-6.
- [Google Scholar]
- Current epidemiology and clinical practice in arboviral infections - implications on blood supply in South-East Asia. ISBT Sci Ser. 2014;9:262-7.
- [Google Scholar]
- Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Vet Microbiol. 2013;165:61-70.
- [Google Scholar]






