Translate this page into:
Evaluation of HER2/neu oncoprotein in serum & tissue samples of women with breast cancer
Reprint requests: Dr Shailaja Shukla, Professor of Pathology, Lady Hardinge Medical College New Delhi 110 001, India e-mail: shailajashukla@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The proto-oncogene HER2/neu has been extensively studied in breast cancer patients. Serum levels of HER2/neu by ELISA in breast cancer patients were compared with tissue HER2/neu expression and with other clinicopathological parameters with the aim to investigate whether the serum assay could replace the established tests (IHC/FISH) for HER-2 status.
Methods:
Blood and Tru-cut biopsy samples were collected for determining HER2/neu status in 64 breast cancer patients. The tissue specimens were processed routinely and immunohistochemistry (IHC) for HER2/ER/PR (oestrogen/progesterone receptors) performed. Fluorescence in-situ hybridization (FISH) was performed on all HER2/neu 2 positive cases. Sixty age matched healthy females and females with benign breast disease were taken as controls for ELISA.
Results:
Of the 64 breast cancer cases, 25 (39.1%) had elevated serum HER2/neu levels accompanied with increased tissue expression of HER2/neu receptors. On IHC, HER2/neu score was 3+ in 24 (37.5%) cases, 2+ in three (4.6%), 1+ in 18 (28.1%); while 19 cases (29.7%) showed no HER2/neu expression. Of the three 2+ cases on IHC, two showed amplification on FISH. Twenty one (32.8%) patients were ER positive and 17 (26.6%) were PR positive. There was a significant correlation (P<0.001) of serum HER2 concentration with tumour size, lymph node involvement, stage of disease and histological grade. Serum HER2/neu levels showed a negative correlation with ER status (P=0.047) but no correlation with PR status.
Interpretation & conclusions:
The results suggest that elevated serum HER2 level was associated with a clinicopathological aggressive phenotype of breast carcinoma and was related to tissue HER2 overexpression. Therefore, serum HER2 may be useful for monitoring the course of the disease and response to treatment.
Keywords
Breast cancer
ELISA
extracellular domain (ECD)
FISH
HER2/neu
immunohistochemistry
Breast cancer is the most common malignant tumour of women in urban India accounting for 27 per cent of all cancers in women1. According to Globocan 2012, 144,937 women were newly diagnosed with breast cancer in India in year 2012 and 70,218 women died of it.
An intensively studied proto-oncogene in human breast cancer is HER2/neu also known as c-erbB-2. It is a human epidermal growth factor receptor (member of epithelial HER1, HER3 and HER4 family). It is located on chromosome 17q21 and encodes a 185 kDa transmembrane glycoprotein receptor with tyrosine kinase cytoplasmic domain and a growth factor binding extracellular domain (ECD). The ECD of the receptor protein (also called as p105) can be cleaved from the cell surface by matrix metalloproteinases and then released into the blood2. Amplification and/or overexpression of the HER2/neu oncogene can be detected in 10-30 per cent of primary breast cancers34. The shed HER-2 ECD can be detected in the serum of approximately 15-40 per cent of breast cancer patients256. The ECD of the HER2 receptor which is shed into the blood of patients with breast cancer, can be measured in the serum by different techniques including enzyme linked immunosorbent assay (ELISA). Fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC) identify amplification and over-expression of the HER2/neu gene and its product, the receptor, but each technique requires a high quality tissue sample, which may not always be available. On the other hand, estimation of ECD of the HER2 receptor shed in blood by ELISA is simple, easy to perform and cost-effective. Therefore, an association between serum and tissue HER2 levels was sought as serum HER2 estimations are advocated for follow up of breast cancer patients. The present study was undertaken with the aim to explore the clinical utility of serum HER2/neu estimation by ELISA in breast cancer patients and evaluate whether it could be used in place of FISH/IHC for diagnostic purpose.
Material & Methods
Study design: This was a hospital-based observational cross-sectional study conducted in the department of Pathology in collaboration with the department of Surgery, Lady Hardinge Medical College and Smt. Sucheta Kriplani Hospital, New Delhi, India, from November 2011 to March 2013. Study population included all women who presented in Surgery outpatient department with breast lump having a clinical suspicion of breast cancer. Sixty age matched healthy females and females with histopathologically or cytologically proven benign breast disease (BB) were taken as controls. Written informed consent was obtained from all cases and controls. The study protocol was approved by the institutional ethics committee.
Selection of cases: Sixty four consecutive cases of breast carcinoma diagnosed on fine needle aspiration cytology (FNAC) and subsequently confirmed on histopathological examination were included in the study. Only newly diagnosed cases of breast cancer including both primary breast cancer (PBC) and metastatic breast cancer (MBC) were included. Patients already on treatment were excluded. Clinicopathologic features such as age, menopausal status, tumour size, lymph node involvement, site of metastases, histological type, grade, oestrogen, progesterone and HER2/neu receptor status were noted. All patients were subjected to a complete diagnostic workup including imaging modalities like mammography, chest radiography, ultrasonography and computed tomography (CT) especially to detect metastases. Lymph node involvement and liver metastases were confirmed by FNAC. Blood samples (3 ml) were obtained from all the patients at the time of diagnosis before any treatment was started. Tru-cut biopsy was performed on all cases and the tissue specimens were fixed in 10 per cent buffered formalin and embedded in paraffin. Sixty age matched controls (for ELISA) including 30 healthy females, 20 women having fibroadenoma and 10 with fibrocystic disease were also included.
Serum HER2 assays: Blood sample (3 ml) was taken by venipuncture in plain vacutainer tubes under sterile conditions from both patients and controls. Sample was allowed to clot and was centrifuged at 1000g for 10 min. Serum was separated and stored in aliquots at -70°C. The sHER2 human ELISA kit (BioVendor- Laboratorní Medicína a.s., Czech Repubic) was used for the quantitative estimation of human soluble HER2 protein (soluble p97-115 HER2; the soluble circulating fragment of p185 HER2) in serum. The cut-off value used in this study was 30.5 ng/ml as per manufacturer's recommendation.
Immunohistochemistry (IHC): IHC staining of specimens was carried out on formalin fixed paraffin embedded breast cancer tissue using the HER2 antibody targeting a peptide on C-terminus of the 185 kDa HER2 protein (clone BV5, Diagnostic Biosystems, India), oestrogen receptor (ER) antibody (clone ID5, Diagnostic Biosystems, India) and progesterone receptor (PR) antibody (clone 10A9, Diagnostic Biosystems, India) in 1:50 dilution each. College of American Pathologists (CAP) and the American Society of Clinical Oncology (ASCO) recommended guidelines for grading HER2/neu status was used7. Quick's ER/PR scoring system was used for grading oestrogen and progestrone receptors status8.
FISH
All 2+ cases on IHC were subjected to FISH analysis using the PathVysion HER2 DNA Probe Kit (Abbott Laboratories, USA) according to the manufacturer's recommendations. The results were reported as the ratio between the average copy number of the HER2/neu gene and that of the chromosome 17 centromere, analysing 100 neoplastic nuclei. Specimens with a signal ratio of <2 with an average HER2 copy number < 4.0 signals/cell were considered as non-amplified. A signal ratio of ≥ 2.0; with an average HER2 copy number ≥ 4.0 signals/cell or even < 4.0 signals/cell and specimens with a signal ratio of < 2.0; with an average HER2 copy number ≥ 6.0 signals/cell were reported as amplified7.
Statistical analysis: Normally distributed continuous variables were compared using the unpaired t test. Categorical variables were analysed using either the Chi square test or Fisher's exact test. One-way analysis of variance (ANOVA) and Pearson correlation was used for ascertaining significance of continuous variables among different groups.
Results
The patients’ age ranged from 25 to 76 yr with a mean age of 46.1 ± 12.87 yr. Maximum number of cases (n=30, 46.9%) were between 36 to 50 yr of age. The clinicopathologic characteristics of the 64 preoperative breast cancer patients on the basis of serum HER2 ECD concentration are summarised in Table I. The age of women in control group ranged from 24 to 70 yr with a mean age of 42.4 ± 11.26 yr. Among the cases, 36 (56.2%) women were premenopausal. Among the controls, 35 women (58.3%) were premenopausal. Majority of cases (59/64, 92.18%) were invasive ductal carcinoma, NOS (not otherwise specified). The other five cases included one each of invasive lobular carcinoma, neuroendocrine carcinoma, metaplastic carcinoma (squamous cell carcinoma) and two cases of mucinous carcinoma.
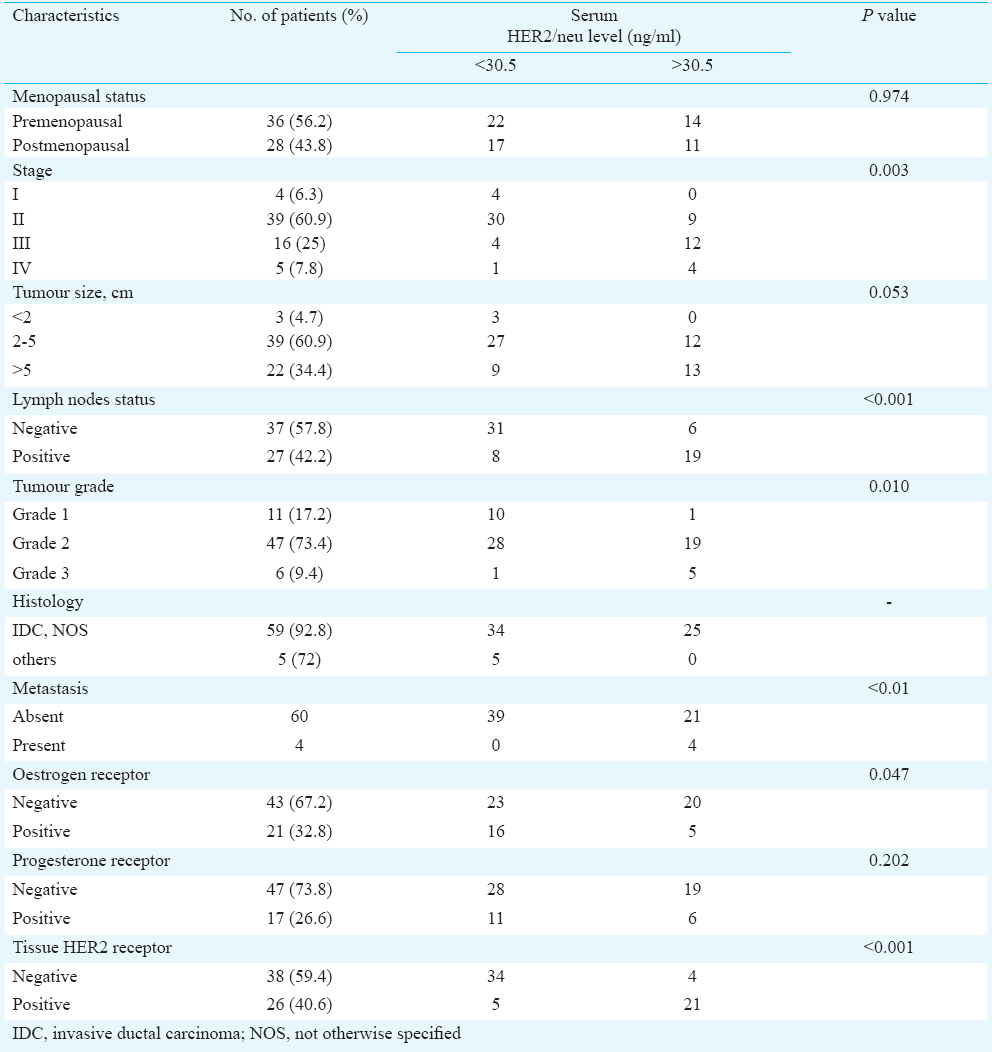
Serum HER2/neu ECD: Mean serum HER2/neu levels in cases and controls were 38.22 ± 33.39 and 9.76 ± 4.64 ng/ml, respectively. Although the mean level of HER2 ECD in controls with BBD (n=30) was slightly higher (14.64±5.28 ng/ml) than in the healthy control (n=30) group (7.3±3.1 ng/ml); it was well below the cut-off value of 30.5 ng/ml in all the controls. Twenty five (39.1%) breast cancer patients had elevated serum HER2/neu levels (33.2 -166.6 ng/ml). In breast cancer cases, the mean serum HER2 concentration in tissue HER2 positive cases (n=26) was 65.38 ± 37.92 ng/ml and in tissue HER2 negative cases (n=38) was 19.64 ± 7.34 ng/ml.
Significant correlation of serum HER2/neu concentration with tumour size was found, the levels increasing with the increase in size of tumour (P<0.001). The mean serum HER2/neu levels in patients having lymph node involvement (n=27, 59.24 ± 41.89 ng/ml) were significantly higher (P<0.001) than in those without lymph node involvement (n=37, 22.88 ± 10.50 ng/ml).
Four patients presented with metastatic disease at the time of diagnosis of breast cancer; two in liver and one each in ovary and lung. Patients with metastases (n=4) had markedly elevated serum HER2/neu levels (mean 102.44 ± 50.13 ng/ml) as compared to patients without metastases (n=60, 32.78 ± 25.53 ng/ml) and the difference was significant (P<0.001). There were two cases with local recurrence of the disease during the course of the study that showed increased serum levels of HER2 at the time of recurrence as compared to those at the time of initial diagnosis with levels rising from 92.0 and 73.8 ng/ml to 116.8 and 94.6 ng/ml, respectively. One patient developed skin metastasis later during the course of the study and showed a rise in serum HER2 concentration from 28.4 ng/ml at the time of initial diagnosis to 36 ng/ml at recurrence 15 months later.
There was a significant association between mean serum levels of HER2/neu and the clinical stage of disease with the levels rising with increasing stage (P<0.001). Moreover, the mean serum HER2/neu concentration was significantly raised in tumour of higher stage as compared to those of lower clinical stage (Table II). There was a significant association between histological grade and serum HER2 levels (P<0.001). All breast cancer patients with raised serum HER2 concentration had invasive ductal carcinomas (IDC). HER2 concentration was not found to be elevated in the other histologic types of breast carcinoma in the present study; and the number of samples in each type was very small.

Tissue HER2 status: Twenty four (37.5%) cases were HER2 positive on IHC with a score of 3+; three (4.6%) were equivocal with a score of 2+ and 18 (28.1%) cases had a score of 1+ while 19 (29.7%) showed no HER2/neu immunostaining. Two of the three cases with HER2 2+ score were amplified on FISH. Elevated serum HER2/neu levels were accompanied with increased tissue expression of HER2/neu receptors and the association was significant (P<0.001).
Hormone receptor status: Of the 64 patients, 21 (32.8%) were ER positive while 17 (26.6%) were PR positive. There was a significant negative correlation (r=-0.026) between serum HER2/neu levels and ER status in breast cancer (P=0.047) (Figure). The HER2/neu levels were elevated in 60 per cent of cases where ER score was zero and not elevated on maximum ER score. There was no significant difference between the mean serum HER2 levels in PR positive and PR negative cases of breast cancer.
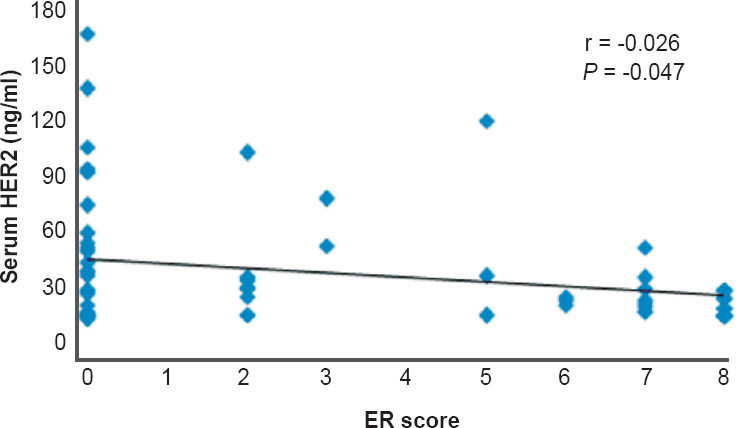
- Correlation of serum HER2 with oestrogen receptor (ER) score in breast cancer.
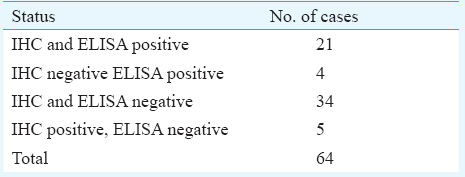
Comparison between HER2/neu in serum and tissue: Twenty one cases showed HER2 overexpression by both IHC and ELISA and 34 cases were found to be negative by both (Table III). Five cases showed overexpression of HER2/neu on IHC without increase in the serum levels while four cases were negative on IHC and positive by ELISA.
Discussion
This study showed a correlation between serum HER2 ECD concentration and tissue HER2 status as determined by IHC and FISH. Kandl et al9 did not find any correlation between the serum and tissue HER2 levels probably because of the small sample size in their study. A couple of other studies also did not find a correlation between sHER2 ECD and tissue HER2 status1011, especially in primary breast cancer without distant metastases. However, a significant association have been reported especially in MBC disease1213.
In the present study, high serum HER2 ECD levels were significantly associated with tumour size, clinical stage, nodal status, histological grade, distant metastasis, tissue HER2 status and negatively associated with oestrogen receptor status. Ludovini et al14 found significant association between serum HER2/neu concentration and most of the clinicopathological parameters mentioned above except the tumour size having taken 2 cm as the cut-off size. The only discordant parameter in the study by James et al15 was lymph node involvement. Imoto et al16 did not find any significant association between raised serum HER2 and oestrogen receptor status. Tchou et al13 reported a significant association only with tissue HER2 status and ER/PR negative status.
No significant relationship was found between other variables like age, menopausal status and progesterone receptor status like the other previous studies101718. Pallud et al19 reported a significant correlation between elevated serum HER2 and higher age. Kong et al20 Ludovini et al14, Lee et al12 and others12131420 observed significant association between serum HER2 levels and progesterone receptor status. Thriveni et al21 found a positive correlation of serum HER2 with age, clinical stage and lymph node status and negative association with hormone status. There was no association with menopausal status. They also reported that serum HER2 levels were related to tumour size, histological grade, presence of comedo type carcinoma and tissue HER2/neu expression.
In the present study, patients with distant metastases (liver, lung and ovary) had markedly elevated serum HER2/neu levels as compared to those without metastases, similar to many previous studies51112132223. In addition, serum HER2/neu levels were only moderately elevated with local recurrence but markedly with distant metastases as reported by Isola et al22. Anderson et al24 found elevated serum HER2 levels significantly more often in patients with distant metastasis than in patients with loco-regional recurrence (68 vs 19%). Thus, elevated serum HER2 levels are associated with HER-2 over-expression and amplification in breast cancer and reflect increased tumour aggressiveness and metastatic potential.
In the present study, four of the 64 cases were negative for HER2/neu overexpression on IHC but had raised serum HER2 levels while five were positive on IHC and negative on ELISA. Lack of tissue immunostaining may be related to the sensitivity of the method, tissue fixation playing an important role. The issue can be resolved to some extent by employing FISH analysis for ascertaining tissue HER2 status. However, it has been postulated that even a few HER2/neu positive cells within an essentially HER2 negative tumour can shed sufficiently detectable amount of HER2/neu protein in the serum25.
Serial estimations of HER2 ECD, showing changes from the baseline level, may be useful for monitoring HER2/neu targeted therapy. Patients with decreased levels after receiving trastuzumab (with or without adjuvant chemotherapy) were more likely to have a higher response rate and longer disease free survival25. Similarly, Tchou et al13 reported that in all patients who developed metastases, a steady rise in serum HER2 levels was noted before metastatic disease became clinically evident. Therefore, HER2 ECD has the potential of being a real-time biomarker for longitudinal assessments in breast cancer patients25.
Thus, ELISA can be useful in assessing the response to therapy; estimating the tumour burden in the body; detecting occult metastases and selecting cases for trastuzumab therapy owing to its simple and inexpensive methodology. Moreover, serial estimations of HER2/neu by ELISA can be of value in the follow up of patients, often being the first clue to the detection of occult metastases. Immunohistochemistry, being a one-time procedure lacks this distinct advantage. It is recommended that ELISA be used in conjunction with IHC. IHC cannot be excluded, as it provides a permanent record of HER2/neu overexpression. Only ELISA may be performed when tissue is not available or is unsuitable for IHC and during follow up of the patient in whom tissue HER2 status has already been established.
The present study had some limitations. First, the number of patients included was small. Second, the patients were not stratified as PBC and MBC cases although the serum levels of HER2 were higher in patients having metastases at the time of diagnosis. It is known that serum HER2 testing is useful in monitoring treatment in MBC patients111213. Third, serum HER2 levels were measured only once at the time of initial diagnosis before commencement of chemotherapy. The assay was repeated in a few cases at the time when they developed recurrence or distant metastasis. Longitudinal sequential measurements of HER2 ECD at definite time intervals are desirable. Fourth, relationship of serum HER2 levels with chemotherapy and/or trastuzumab therapy was not studied and the utility of serum HER2 assay as a prognostic tumour marker in follow up of patients was not assessed.
In conclusion, baseline levels of HER2 ECD may not correlate with tissue HER2 status. Serum HER2 assay cannot replace IHC/FISH testing but may complement the tissue assay in monitoring therapy of breast cancer patients by providing information that is lacking in IHC and FISH testing, such as early diagnosis of recurrence and metastases.
Conflicts of Interest: None.
References
- GLOBOCAN 2012 cancer incidence and mortality worldwide: IARC cancerbase No.11. Lyon, France: International Agency for Research on Cancer; 2013.
- In vitro and in vivo release of soluble erbB-2 protein from human carcinoma cells. Jpn J Cancer Res. 1990;81:489-94.
- [Google Scholar]
- Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177-82.
- [Google Scholar]
- Laboratory assessment of the status of HER-2/neu protein and oncogene in breast cancer specimens : comparison of immunohistochemistry assay with fluorescence in situ hybridisation assays. J Clin Pathol. 2000;53:374-81.
- [Google Scholar]
- Tissue expression and serum levels of HER-2/neu in patients with breast cancer. Oncology. 1997;54:475-81.
- [Google Scholar]
- C-erbB-2 CEA and Ca 15.3 serum levels in the early diagnosis and recurrence of breast cancer patients. Anticancer Res. 1999;19:2551-5.
- [Google Scholar]
- Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997-4013.
- [Google Scholar]
- Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155-68.
- [Google Scholar]
- Soluble c-erbB-2 fragment in serum correlates with disease stage and predicts for shortened survival in patients with early-stage and advanced breast cancer. Br J Cancer. 1994;70:739-42.
- [Google Scholar]
- c-erb B-2 protein level in tissue and sera of breast cancer patients : a possibly useful clinical correlation. Tumori. 2006;92:311-7.
- [Google Scholar]
- Serum HER-2 concentrations for monitoring women with breast cancer in a routine Oncology setting. Clin Chem Lab Med. 2009;47:1117-23.
- [Google Scholar]
- Preoperative serum HER 2 extracellular domain levels in primary invasive breast cancer. BMC Cancer. 2014;14:929.
- [Google Scholar]
- Monitoring serum HER 2 levels in breast cancer patients. Springer Plus. 2015;4:237.
- [Google Scholar]
- Evaluation of serum HER2 extracellular domain in early breast cancer patients: correlation with clinicopathological parameters and survival. Ann Oncol. 2008;19:883-90.
- [Google Scholar]
- Clinical outcome of adjuvant endocrine treatment according to HER2/neu status in breast cancer. Indian J Med Res. 2011;133:70-5.
- [Google Scholar]
- Serum c-erbB-2 protein is a useful marker for monitoring tumor recurrence of the breast. Int J Cancer. 2007;120:357-61.
- [Google Scholar]
- Serum her-2/neu and survivin levels and their relationship to histological parameters in early-stage breast cancer. J Int Med Res. 2007;35:165-72.
- [Google Scholar]
- Impact of serum HER2 levels on survival and its correlation with clinicopathological parameters in women with breast cancer. J Breast Cancer. 2012;15:71-8.
- [Google Scholar]
- Tissue expression and serum levels of the oncoprotein HER-2/neu in 157 primary breast tumours. Anticancer Res. 2005;25:1433-40.
- [Google Scholar]
- Serum HER-2 concentration in patients with primary breast cancer. J Clin Pathol. 2006;59:373-6.
- [Google Scholar]
- Clinical utility of serum human epidermal receptor-2/neu detection in breast cancer patients. Indian J Med Res. 2007;125:137-42.
- [Google Scholar]
- Elevated erbB-2 oncoprotein levels in preoperative and follow-up serum samples define an aggressive disease course in patients with breast cancer. Cancer. 1994;73:652-8.
- [Google Scholar]
- Serum epidermal growth factor receptor and HER2 expression in primary and metastatic breast cancer patients. Breast Cancer Res. 2007;9:R75.
- [Google Scholar]
- Detection of c-erbB-2 related protein in sera from breast cancer patients. Relationship to ERBB2 gene amplification and c-erbB-2 protein overexpression in tumour. Acta Oncol. 1995;34:499-504.
- [Google Scholar]
- Challenges in the clinical utility of the serum test for HER 2 ECD. Biochim Biophys Acta. 2012;1826:199-208.
- [Google Scholar]






