Translate this page into:
Evaluation of cell adhesion molecules (LFA-1 and L-selectin) in ankylosing spondylitis patients after treatment with β-D-mannuronic acid (M2000)
For correspondence: Prof Abbas Mirshafiey, Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran e-mail: mirshafiey@tums.ac.ir
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
To examine β-D-mannuronic acid (M2000) effects on L-selectin shedding and leucocyte function-associated antigen-1 (LFA-1) expression as mechanisms of action of this drug in patients with ankylosing spondylitis (AS).
Methods:
To investigate the molecular consequences of β-D-mannuronic acid on L-selectin shedding, flow cytometry method was used. Furthermore, the effect of it on LFA-1 gene expression was analyzed by using quantitative real time (qRT)-PCR technique.
Results:
The LFA-1 expression in patients with AS was higher than controls (P=0.046). The LFA-1 expression after 12 wk therapy with β-D-mannuronic acid was meaningfully decreased (P=0.01). After 12 wk treatment with β-D-mannuronic acid, the frequency of CD62L-expressing CD4+ T cells in patients with AS, was not considerably altered, compared to the patients before therapy (P=0.5). Furthermore, after 12 wk therapy with β-D-mannuronic acid, L-selectin expression levels on CD4+ T-cells in patients with AS, were not remarkably changed, compared to the expression levels of these in patients before treatment (P=0.2).
Interpretation & conclusions:
The results of this study for the first time showed that β-D-mannuronic acid can affect events of adhesion cascade in patients with AS. Moreover, β-D-mannuronic acid presented as an acceptable benefit to AS patients and could aid in the process of disease management.
Keywords
Ankylosing spondylitis
leucocyte function-associated antigen-1
L-selectin
M2000
nonsteroidal anti-inflammatory drugs
β-D-mannuronic acid
Cell adhesion molecules (CAMs) are crucial transmembrane proteins that control interactions between cells and their extracellular matrices. According to their molecular structures, they are categorized into four major classes: selectins, integrins, immunoglobulin-like proteins (proteins of the immunoglobulin superfamily) and cadherins1. They play pivotal roles in the control of basic vital processes such as cell proliferation, differentiation, survival and migration2.
Leucocyte recruitment is a complex and multistep process in which each step is tightly orchestrated by the sequential expression and activation of specific CAMs on the surface of leucocytes and endothelial cells3. It can be divided into four steps, including tethering/rolling, firm adhesion, intraluminal crawling and transmigration4,5. The first step is initiated by members of the selectin family of CAMs such as L-selectin that causes the rolling of leucocytes along the endothelium. During rolling, leucocytes are stimulated by chemokines that results into changes in the expression level and activation state of specific integrins such as leucocyte function-associated antigen-1 (LFA-1)6. The interaction of the activated LFA-1 with its ligand on the endothelial cells causes the firm adhesion of leucocytes to the endothelial surfaces. Leucocytes then transmigrate between endothelial cells to reach extravascular tissues7. These steps are essential for leucocyte migration towards the inflammatory focus and if one step is suppressed, leucocyte extravasation does not occur properly8.
Ankylosing spondylitis (AS) is a chronic debilitating and inflammatory disease belonging to the group of seronegative spondyloarthritis. AS is described by inflammation of the sacroiliac joints, the spine and enthuses. Although the etiology and the pathogenesis of AS are not yet fully understood, the environmental, genetic and immunologic factors are important in the development and progression of AS9,10. Chronic inflammation, bone erosion and new bone formation are important features of AS that lead to patient disability.
The nonsteroidal anti-inflammatory drugs (NSAIDs) are the first line treatment for AS patients for pain control and to reduce inflammation. Although the major anti-inflammatory mechanism of action of NSAIDs is inhibition of prostaglandin (PG) synthesis, data indicate to the existence of additional mechanisms11. NSAIDs also block initial steps of adhesion cascade of leucocytes on endothelial surfaces. It has been found that a group of NSAIDs such as mefenamic acid, Indomethacin and diclofenac induce the L-selectin (CD62L) shedding, whereas another group such as aspirin, piroxicam and meloxicam modulate the activation and expression of the integrins on leucocytes12,13. However, clinical and experimental data show that the use of NSAIDs may lead to potential side effects, including cardiovascular, renal and gastrointestinal (GI) toxicities that limit their use14.
Studies have revealed that the β-D-mannuronic acid (M2000), a novel NSAID with immunoregulatory property, is safe with no measureable toxicity on liver, kidney and GI tract function15,16. It has also revealed its therapeutic efficacy with high biocompatibility and tolerability in different animal models and in patients with rheumatoid arthritis (RA) and AS17-19. Previous studies have shown that the treatment of experimental autoimmune encephalomyelitis with β-D-mannuronic acid could significantly suppress disease development. Clinical improvement was associated with a notable decrease in mean number of vessels with perivascular cellular infiltration in β-D-mannuronic acid-treated rats compared with untreated controls16. Mirshafiey et al18 found that oral administration of β-D-mannuronic acid in arthritic rats caused a remarkable decrease in paw edema compared to the paw volume of vehicle-treated rats. Histological evaluation revealed a reduced cell infiltrate in the joints of β-D-mannuronic acid-treated rats as well as a reduced number of osteoclasts in the subchondral bone18. Previous results have shown that the β-D-mannuronic acid has potency in the treatment of experimental model of glomerulonephritis. Treatment with β-D-mannuronic acid caused a remarkable decrease in the urinary protein excretion, anti-BSA antibody titer, glomerular immune complex deposition and leucocyte infiltration in treated rats versus untreated controls18.
In this study, the effect of β-D-mannuronic acid was investigated on L-selectin shedding and LFA-1 gene expression as a possible anti-inflammatory mechanism of action that this compound exerts in clinical settings. This was a part of phase I/II, multicenter, randomized, placebo-controlled trial (IRCT2013062213739N1) to evaluate the efficacy and safety of β-D-mannuronic acid in AS patients.
Material & Methods
This study was conducted in the department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran. The study included patients with ankylosing spondylitis (AS) presenting at the Shariati Hospital, affiliated with Tehran University of Medical Sciences and the Iranian AS Society, Tehran, Iran. All cases and healthy controls signed written informed consent before participation in the study. The study protocols were approved by the Ethics Committee of Tehran University of Medical Sciences, which were conducted in accordance with guidelines established by the Declaration of Helsinki and the ASAS.
Patients and controls: In total, 30 individuals with AS fulfilling the modified New York criteria10 that had active disease [Bath AS Disease Activity Index (BASDAI) score ≥4 on a 0-10, visual analogue scale (VAS)] were enrolled. The presence of axial involvement, no peripheral involvement and the need for daily treatment with NSAIDs were other relevant eligibility criteria. Additionally, if the individuals required the use of concomitant prednisolone >10 mg/day, tumour necrosis factor alpha (TNF-α) inhibitor and methotrexate >15 mg/wk, they were excluded. Treatment of the AS cases with β-D-mannuronic acid according to the standards of Assessment of Spondyloarthritis International Society (ASAS) was performed between March 21, 2014 and March 21, 2015 (clinical trial identifier: IRCT2013062213739N1). According to our clinical evaluation in RA patients (clinical trial identifier: IRCT2014011213739N2)20, a minimum dosage (18 mg/kg/d) of β-D-mannuronic acid was prescribed in gelatinized capsules (two 500 mg capsules/day) for 12 wk. Clinical outcome evaluations [BASDAI score, AS quality of life (ASQoL), BASFI score, total back pain and morning stiffness], physical examinations and medical history were conducted at baseline and week 12. Furthermore, 15 healthy, sex and age-matched individuals (4 females, 11 males) with mean age 33.1±9.2 without any background disease were recruited as healthy controls.
Total RNA extraction and complementary DNA synthesis: The fresh peripheral blood (5 ml) was collected from healthy controls and AS cases before and after β-D-mannuronic acid treatment. The peripheral blood mononuclear cells (PBMCs) were collected from freshly obtained blood samples by Ficoll-Hypaque density-gradient centrifugation (Biochrom AG, Germany). Afterward, the total RNA was extracted from ~1×107 cells using Hybrid-R™ kit (GeneAll Biotechnology, Seoul, South Korea) as per the manufacturer’s protocol. The NanoDrop 2000 spectrophotometer (Thermo Scientific, USA) was used to measure the quality and concentration of total RNA and for complementary DNA (cDNA) synthesis, (OD260/280 ratio in the range of 1.8-2.2). The oligo-dT and random hexa-mer primers in the presence of reverse transcriptase enzyme were used to the first strand cDNA synthesis using PrimeScript™ RT reagent Kit (Takara, Japan).
Quantitative real-time polymerase chain reaction (qRT-PCR): The ABI StepOnePlus™ Real-Time PCR System (Applied Biosystems Company, USA) was employed to carry out qRT-PCR using Syber premix Ex Taq II (Takara, Japan).
All samples were evaluated in triplicate. The reactions' volume were 25 μl included 12.5 μl of SYBR Premix Ex Taq II, 2 μl of cDNA, 1 μl of reverse and forward primers, 0.5 μl ROX Reference Dye, and 8.0 μl of nuclease -free water.
The qRT-PCR was performed at the following conditions: one cycle of predenaturing at 95°C for 5 sec, 40 cycles of denaturation at 95°C for 5 sec, annealing and extension at 60°C for 30 sec using the specific primer set for the LFA-1 gene previously described by Li JP, et al21. The relative mRNA levels were normalized to the endogenous control, β-Actin. The relative expression levels of the PCR product were analyzed using the 2−ΔΔCT formula.
Flow cytometry: For flow cytometric analysis of CD62L-expressing CD4+ T-cells, PBMCs suspension were prepared at a density of 2×106 cells/ml. The cells were then washed with staining buffer and subsequently stained with PE/Cy5 anti-human CD4 and PE anti-human CD62L (Biolegend, USA) at 4°C in the dark for 20 min. Isotype controls were provided to perform correct compensation and confirm antibody specificity. The prepared samples were examined using a FACS Calibur flow cytometer and Cell Quest software (BD Biosciences, San Jose, CA, USA).
Statistical analysis: The data were represented as mean ± standard error of means (SE) for numerical variables and percentages and frequencies for categorical variables. The LFA-1 gene expression and the frequency of CD62L- expressing CD4+ T cells before and after treatment with β-D-mannuronic acid were analyzed using paired Student’s t tests. All statistical data were evaluated using the SPSS software version 22 (IBM Corp, Armonk, NY, USA). A P<0.05 was considered statistically significant.
Results
Most of the cases examined in this study were male (72.3%), and positive for the human leucocyte antigen–B27 allele (71%). The mean age of the study participants was 31.5±8.1 yr (range 18-46 yr), and the mean disease duration was 10.8±8.6 yr.
Clinical outcome: The improvement in the AS cases was observed after two weeks post-treatment and also continued upto the 12 wk treatment period. The mean scores BASFI, ASQoL, BASDAI, and morning stiffness total back pain showed a remarkable decrease at the end of 12 wk of treatment (Table).
| Clinical outcome | Before treatment | After treatment | P |
|---|---|---|---|
| BASDAI score (0-10 cm VAS) | 5.9±1.3 | 4.0±1.9 | 0.002 |
| BASFI score (0-10 cm VAS) | 4.3±2.0 | 2.8±1.8 | 0.013 |
| ASQoL (range 0-18) | 9.9±4.5 | 6.7±4.6 | 0.031 |
| Morning stiffness (0-10 cm VAS) | 6.1±1.9 | 3.8±2.2 | 0.011 |
| Total back pain (0-10 cm VAS) | 5.6±1.2 | 3.7±2.1 | 0.002 |
Values are shown as mean±SE. ASQoL, ankylosing spondylitis quality of life; BASDAI, bath ankylosing spondylitis disease activity index; BASFI, bath ankylosing spondylitis functional index; VAS, visual analogue scale
The effect of β-D-mannuronic acid on LFA-1 gene expression: To study the underlying molecular consequences of β-D-mannuronic acid on LFA-1 gene, we examined its relative gene expression in 30 active AS cases before and after 12 wk treatment with β-D-mannuronic acid, and in 15 normal, individuals as healthy controls.
Our findings indicated that the level of LFA-1 gene expression in cases with AS was higher than controls (1.52 fold, P=0.046). The results also demonstrated that the level of LFA-1 gene expression after a 12 wk therapy with β-D-mannuronic acid was significantly reduced (1.6 fold) and the difference of LFA-1 gene expression before and after treatment with β-D-mannuronic acid was significant (P=0.01). Furthermore, there was no significant difference between treatment group and controls in terms of LFA-1 gene expression (P=0.615; Fig. 1).
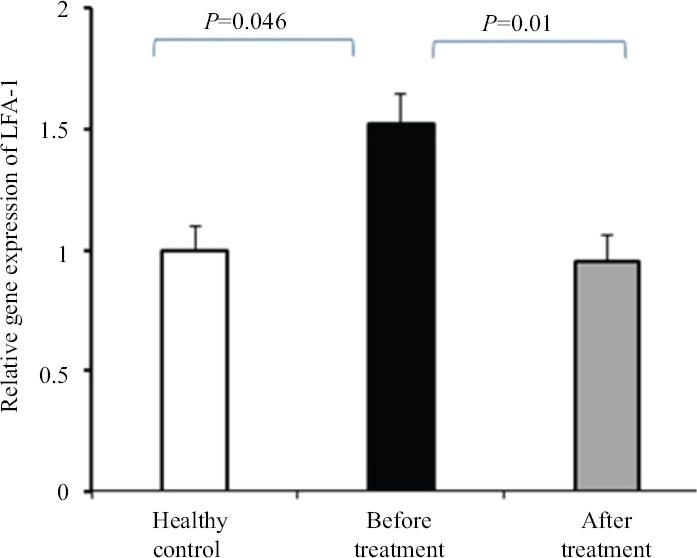
- Effect of β-D-mannuronic acid on leucocyte function-associated antigen (LFA)-1 gene expression. Data presented as mean ± SEM of triplicate samples from 30 patients with active ankylosing spondylitis before and after 12 wk treatment with β-D-mannuronic acid and 15 healthy controls. SEM, standard error of the mean.
The effect of β-D-mannuronic acid on L-selectin shedding: To find out whether β-D-mannuronic acid is able to exert its blocking effect on leucocyte-endothelial cell adhesion by shedding of L-selectin, we first examined the frequency of CD62L-expressing CD4+ T-cells in patients with AS before and after therapy with β-D-mannuronic acid in comparison to healthy controls. Flow cytometric analysis revealed that the baseline frequency of CD62L-expressing CD4+ T-cells were not considerably different between cases (with AS) and controls (53.8%±18.7% vs. 56.2%±12.8%; P=0.7) (Fig. 2). Furthermore, after 12 wk therapy with β-D-mannuronic acid, the frequency of CD62L-expressing CD4+ T-cells in as the cases, was not significantly changed, compared to its frequency in the patients before therapy (50.3%±10.2% vs. 53.8%±18.7%; P=0.5) (Fig. 2).
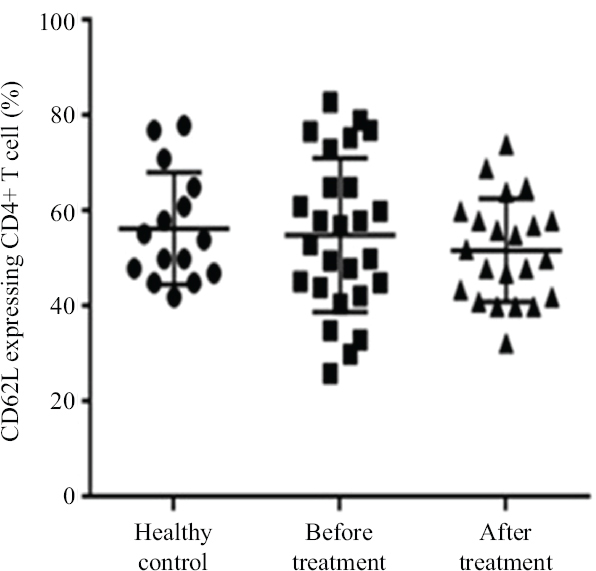
- The frequency of CD62L-expressing CD4+ T-cells in ankylosing spondylitis patients before and after treatment with β-D-mannuronic acid and healthy controls.
In the next step, we investigated the status of L-selectin expression on CD4+ T-cells based on mean fluorescence intensity. The baseline L-selectin expression levels on CD4+ T-cells were not significantly different between individuals with AS and healthy controls (91.3±15.1 vs. 100.4±19.1; P=0.5) (Fig. 3). Also, after 12 wk treatment with β-D-mannuronic acid, L-selectin expression levels on CD4+ T-cells in individuals with AS, were not considerably changed, as compared to the levels before treatment (83.0±14.2 vs. 91.3±15.1; P=0.2) (Fig. 3).
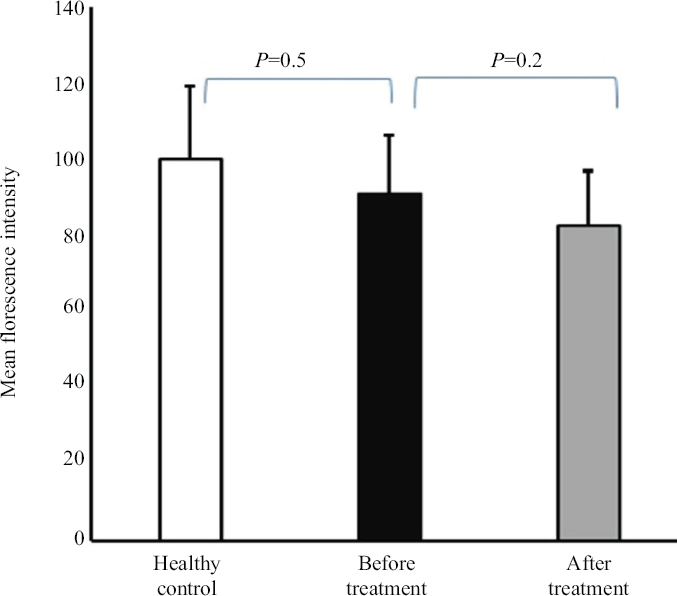
- The expression of L-selectin (mean fluorescence intensity) in ankylosing spondylitis patients before and after treatment with β-D- mannuronic acid and in healthy controls.
Discussion
Studies have shown that NSAIDs have the anti-inflammatory mechanism of action other than the suppression of COX enzymes8. A key part of the process of inflammation is the migration of leucocytes from the bloodstream to sites of tissue injury and their infiltration in inflamed tissues. It is multistep so that if one step is inhibited, leucocyte extravasation does not occur correctly and overall inflammation will decrease5. The CAMs have been considered as a possible source of potent and novel targets for the treatment of inflammation. In recent years, many efforts have been made aiming to develop antagonists of CAMs22,23. One of the most important prostaglandin (PG) independent anti-inflammatory mechanisms of NSAIDs is the induction of L-selectin shedding not only in vitro but also in vivo in both humans and mice24. The L-selectin plays an essential role in constitutive lymphocyte trafficking and directing leucocytes to sites of inflammation. Therefore, it represents an important target for therapeutic interventions.
Previous studies have shown that several NSAIDs such as indomethacin, mefenamic acid, diclofenac and aceclofenac inhibit the first step of the adhesion cascade mediated by L-selectin. This effect is due to rapid cleavage and shedding of L-selectin from the cell surface8. Other NSAIDs such as piroxicam and meloxicam neither reduce L-selectin expression nor inhibit the first step of the leucocyte-endothelial attachment. In the present study, we found that β-D-mannuronic acid could not induce shedding of L-selectin from leucocyte surfaces in individuals with AS. Structure-function relationship studies have demonstrated that the capability of some NSAIDs to shed L-selectin from leucocyte surfaces, requires the presence of N-phenylanthranilic acid or diphenylamine in the NSAID structural core8,13,25. N-phenylanthranilic acid and diphenylamine neither affect non-specific activation of leucocytes nor prostaglandin inhibition26. The exact mechanism by which diphenylamine-based compounds mediate the activation-independent downregulation of L-selectin in leucocytes remains unknown. It seems that these compounds modify the ATP levels in leucocytes and a disintegrin and metalloproteinase (ADAM)-17 may be stimulated in an ATP-dependent manner. The most important function of catalytically active ADAM-17 is to cleave ectodomains of various transmembrane proteins such as L-selectin27. It should be noted that piroxicam and meloxicam as NSAIDs neither reduce L-selectin expression nor block the process of the leucocyte-endothelial cell attachment8. These NSAIDs interfere with other steps in the leucocyte adhesion cascade including inhibition of integrin activation and expression on leucocyte surfaces. In addition, studies have shown that high-dose aspirin can diminish the surface expression of Mac-1 (CD11b/CD18) and LFA-1 (CD11a/CD18) on diabetic leucocytes to similar levels in nondiabetic animals12.
In this study, we found that β-D-mannuronic acid exerted anti-adhesive properties. It significantly reduced LFA-1 expression in individuals with AS in comparison with controls. Consistent with these results, Hosseini et al28 indicated that in vitro treated 4T1 cell line with β-D-mannuronic acid significantly decreased the matrix metalloproteinase-9 (MMP-9), MMP-2 (gelatinases) functions and adhesion of 4T1 cells to laminin, fibronectin and collagen I without any detectable cytotoxicity.
Furthermore, we demonstrated β -D-mannuronic acid as a potentially novel NSAID which had a pivotal role in AS management by decreasing pain, disease activity and inflammation. In line with these results, Fattahi et al19 found that β-D-mannuronic acid was effective, safe and generally well tolerated in cases of AS. It provided acceptable benefits to individuals with AS by improving multiple disease features including pain, inflammation and disease activity. In another study, Ahmadi et al17 revealed that β-D-mannuronic acid not only has a potent anti-inflammatory effect in RA patients but also could reduce the anti-dsDNA antibodies, RF, the serum levels of anti-CCP, erythrocyte sedimentation rate and C-reactive protein significantly.
According to its molecular structure, it can be predicted that β-D-mannuronic acid binds to the Toll-like receptors (TLRs) on cell surfaces. It has been found that β-D-mannuronic acid interferes with the TLR signalling pathway through suppressing the transcription factor NF-κB, the adaptor molecules TRAF6, IRAK1 and miR-146a29,30. In addition, it has been demonstrated that β-D-mannuronic acid has multiple molecular mechanisms for its anti-inflammatory effects. A recent study revealed that β-D-mannuronic acid has anti-inflammatory and immunoregulatory effects in inflammatory bowel disease patients. It significantly reduced IL-17 and TNF-α gene expression, while the expression of the FOXP3 gene was found to be upregulated31. Barati et al20 found that β-D mannuronic acid has a suitable efficacy in RA patients, so that it could not only considerably downregulate RORγt and IL17 gene expressions but also increased GATA3 and IL4 gene expressions after 12 wk of therapy in these individuals.
The present study had a few limitations. First, our sample size was relatively small. Second, we used the qRT-PCR technique for the detection of LFA-1 gene expression. The correlation between mRNA levels and protein abundance is attributed to complex post-transcriptional regulation, therefore, gene expression should be confirmed at protein level by immunohistochemistry and flow cytometry techniques.
Overall, developing an anti-adhesion molecule therapy is a key effort for inhibiting and controlling the development of inflammatory and autoimmune disorders. This study for the first time indicated that β-D-mannuronic acid can interfere with events of adhesion cascade through inhibition of LFA-1 gene expression. In addition, β-D-mannuronic acid presented acceptable benefits to AS cases by also improving multiple disease features including pain, disease activity and inflammation. Based on these results, oral administration of β-D-mannuronic acid as a novel NSAID with immunoregulatory characteristics could aid in the process of AS management.
Financial support and sponsorship
None.
Conflicts of interest
None.
References
- Vascular cell adhesion molecule 1, intercellular adhesion molecule 1, and cluster of differentiation 146 levels in patients with type 2 diabetes with complications. Endocrinol Metab (Seoul) 2017. ;32:99-105.
- [Google Scholar]
- Cell adhesion molecules in Alzheimer's disease. Degener Neurol Neuromuscul Dis. 2012;2:65-77.
- [Google Scholar]
- Leukocyte recruitment in inflammation:basic concepts and new mechanistic insights based on new models and microscopic imaging technologies. Cell Tissue Res. 2014;355:647-56.
- [Google Scholar]
- Anti-adhesion molecule therapy for inflammatory bowel disease. Therap Adv Gastroenterol. 2010;3:239-58.
- [Google Scholar]
- T lymphocyte subset imbalances in patients contribute to ankylosing spondylitis. Exp Ther Med. 2015;9:250-56.
- [Google Scholar]
- The classification and diagnostic criteria of ankylosing spondylitis. J Autoimmun. 2014;48-49:128-33.
- [Google Scholar]
- Diclofenac:an update on its mechanism of action and safety profile. Curr Med Res Opin. 2010;26:1715-31.
- [Google Scholar]
- Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. Faseb J. 2002;16:438-40.
- [Google Scholar]
- Structure-function relationship and role of tumor necrosis factor-alpha-converting enzyme in the down-regulation of L-selectin by non-steroidal anti-inflammatory drugs. J Biol Chem. 2002;277:38212-21.
- [Google Scholar]
- Benefits and risks of ankylosing spondylitis treatment with nonsteroidal antiinflammatory drugs. Arthritis Rheum. 2008;58:929-38.
- [Google Scholar]
- Preclinical assessment of β-d-mannuronic acid (M2000) as a non-steroidal anti-inflammatory drug. Immunopharmacol Immunotoxicol. 2015;37:535-40.
- [Google Scholar]
- Novel immunosuppressive therapy by M2000 in experimental multiple sclerosis. Immunopharmacol Immunotoxicol. 2005;27:255-65.
- [Google Scholar]
- The potent inhibitory effect of β-D-mannuronic acid (M2000) as a novel NSAID with immunosuppressive property on anti-cyclic citrullinated peptide antibodies, rheumatoid factor and anti-dsDNA antibodies in patients with rheumatoid arthritis. Curr Drug Discov Technol. 2017;14:206-14.
- [Google Scholar]
- Evaluation of the efficacy and safety of β-d-mannuronic acid in patients with ankylosing spondylitis: A 12-week randomized, placebo-controlled, phase I/II clinical trial. Int Immunopharmacol. 2018;54:112-17.
- [Google Scholar]
- Effects of β-d-mannuronic acid, as a novel non-steroidal anti-inflammatory medication within immunosuppressive properties, on IL17, RORγt, IL4 and GATA3 gene expressions in rheumatoid arthritis patients. Drug Des Devel Ther. 2017;11:1027-33.
- [Google Scholar]
- Interleukin-27 as a negative regulator of human neutrophil function. Scand J Immunol. 2010;72:284-92.
- [Google Scholar]
- Review article:anti-adhesion therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:579-94.
- [Google Scholar]
- In vivo modulation of the inflammatory response by nonsteroidal antiinflammatory drug-related compounds that trigger L-selectin shedding. Eur J Immunol. 2013;43:55-64.
- [Google Scholar]
- Down-regulation of L-selectin expression in neutrophils by nonsteroidal anti-inflammatory drugs:role of intracellular ATP concentration. Blood. 2000;96:3592-3600.
- [Google Scholar]
- L-selectin shedding by NSAIDs:old friends in new dresses. Eur J Immunol. 2013;43:50-4.
- [Google Scholar]
- Targeting of crosstalk between tumor and tumor microenvironment by β-D mannuronic acid (M2000) in murine breast cancer model. Cancer Med. 2017;6:640-50.
- [Google Scholar]
- Pharmacological effects of β-d-mannuronic acid (M2000) on miR-146a, IRAK1, TRAF6 and NF-κB gene expression, as target molecules in inflammatory reactions. Pharmacol Rep. 2017;69:479-84.
- [Google Scholar]
- M2000 (β-D-Mannuronic Acid) as a novel antagonist for blocking the TLR2 and TLR4 downstream signalling pathway. Scand J Immunol. 2017;85:122-29.
- [Google Scholar]
- Immunomodulatory effects of M2000 (β-D-Mannuronic acid) on TNF-α, IL-17 and FOXP3 gene expression in patients with inflammatory bowel disease. Int Immunopharmacol. 2017;51:107-13.
- [Google Scholar]






