Translate this page into:
Evaluation of carotid artery dynamics & correlation with cardiac & hepatic iron in β-thalassaemia patients
Reprint requests: Dr Rashid H. Merchant, 501, Rangmahal 5th floor, 2 Mount Mary Road, Bandra (West), Mumbai 400 050, Maharashtra, India e-mail: deandoc2000@hotmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Early atherosclerosis and vascular complication have been described in thalassaemia patients. There is lack of data or guidelines regarding monitoring of vascular health in thalassaemia. This study was conducted to compare carotid artery structural and functional indices such as carotid artery intima-media thickness (CIMT), stiffness index (SI) and Young's elastic modulus (YEM) in β-thalassemia patients with age and sex matched controls, and to correlate these parameters with serum ferritin, cardiac iron, and hepatic iron.
Methods:
This cross-sectional study included 53 β-thalassaemia patients receiving regular blood transfusions. Carotid artery indices such as CIMT, SI, and YEM were calculated by duplex ultrasound and colour Doppler. Serum ferritin levels were measured by chemiluminescence. Cardiac and hepatic iron estimation were done using MRI T2* sequences analyzed by a special thalassaemia software.
Results:
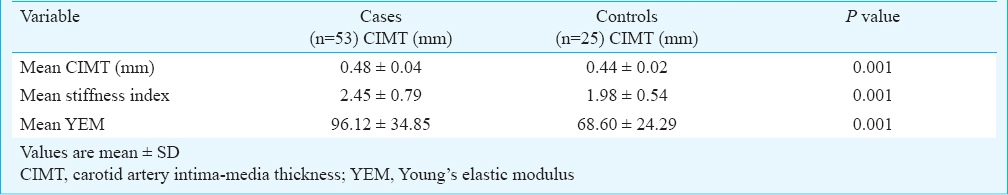
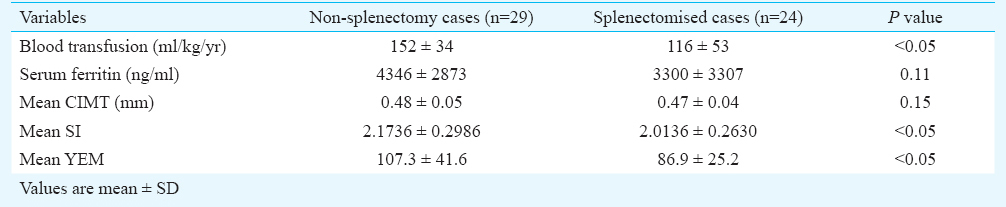
Mean CIMT of cases and controls were 0.48 ± 0.04 and 0.44±0.02 mm, respectively and these were significantly different (P<0.001). Similarly significant differences were noted in SI and YEM of cases (2.45±0.79 and 96.12±34.85, respectively) as compared to controls (1.98±0.54 and 68.60±24.29, respectively) (P<0.001). There was significant inverse correlation between stiffness index and cardiac iron overload assessed by MRI cardiac T2* (P=0.03). Mean SI and YEM of cases were (2.1736 ± 0.2986 and 107.3± 41.6, respectively) significantly higher among non-splenectomized patients compared to splenectomized patients (2.0136 ± 0.263 and 86.9 ± 25.2, respectively) (P<0.05).
Interpretation & conclusions:
CIMT and arterial stiffness indices were significantly increased in β-thalassaemia patients compared to controls which was indicative of early atherogenic changes. This study supports the hypothesis that iron overload is a risk factor for early atherosclerosis and cardiovascular disease.
Keywords
Atherosclerosis
β-thalassaemia
carotid duplex ultrasound
carotid intime-media thickness
Cardiac T2*
stiffness index
Young's elastic modulus (YEM)
The prevalence of beta-thalassaemia is estimated to be 3-4 per cent in India and about 10,000 thalassaemic infants are born annually in India12. With advances in the quality of blood transfusion their life expectancy has increased, and more thalassaemics are entering adolescence and adulthood. Iron overload, an inevitable consequence of transfusion therapy, manifests as cardiovascular, endocrine, hepatic and other complications. Cardiac iron overload is the leading cause of mortality in thalassaemic patients and is measured non-invasively with magnetic resonance imaging T2* (MRI T2*)3. However, much less is known about the degree of vascular affection and impact of iron toxicity on the cardiovascular system as a whole.
Several cross-sectional control-based follow up studies have shown a strong and graded association between atherosclerosis (measured by carotid intima-media thickness CIMT) and increased incidence of atherosclerosis, myocardial infarction and stroke. Iron overload has been documented to cause endothelial dysfunction via oxidative damage, causing early and accelerated atherosclerosis45678. Vascular events such as thrombotic occlusions of the cerebral, portal, retinal, and coronary circulations are described in patients with excess iron, although there are currently sparse data and a lack of Indian studies regarding the mechanism of such events789. The present study was aimed to evaluate the vascular integrity and various stiffness indices in thalassaemic patients compared to age matched healthy controls, and correlate these indices with cardiac and hepatic iron status.
Material & Methods
In this cross-sectional study, a total of 53 consecutive (33 males and 20 females) β-thalassaemia major patients receiving regular blood transfusions at Nanavati Hospital, Mumbai, Maharashtra, India, were enrolled according to inclusion and exclusion criteria between November 2011 and November 2012 and compared with a control group of 25 age-matched, healthy volunteers (16 males and 9 females). Individuals with compounding risk factors for atherosclerosis like hyperglycaemia, hypertension, hyperlipidaemia, smoking, or obesity were excluded from both study and control groups. Informed written consents were taken from patients after receiving ethical clearance from the hospital ethics committee.
Serum ferritin level was determined by chemiluminescence (Elecoys Ferritin, Roche, Germany). Liver and cardiac iron levels were estimated using magnetic resonance images (MRI T2*) with a 1.5-Tesla Siemens Sonata machine using CMR Tools developed by Imperial college Siemens, Germany. Cardiac iron was defined by MRI T2* values of 20-10 ms as moderate and <10 ms as severe910. Similarly hepatic iron overload was defined by MRI T2* values as mild (6.3-2.7 ms), moderate (2.7-1.4 ms) or severe (<1.4 ms)11
Carotid artery ultrasound and Doppler studies71213141516: B-mode and colour coded duplex sonography of common carotid arteries was performed in all participants using Philips IU 22 X matrix machine, USA with a high frequency L12-5 MHz probe using the Q Lab IMT software, USA. All ultrasound examinations were performed by a single experienced radiologist who was unaware of the clinical details of cases and controls.
The common carotid vessels were evaluated for subintimal lucency, atherosclerotic plaques, and intima-media thickness (CIMT). CIMT was defined as the distance between the junction of the lumen and intima and that of the media and adventitia. CIMT was measured in a 1cm segment proximal to the bifurcation of the artery and expressed in millimeters. For each subject, measurements on both sides were obtained and the average of two sides was considered the patient's mean CIMT.
The carotid artery diameters, systolic diameter (Ds) and diastolic diameter (Dd), were measured by echo tracking of arterial wall motion during systole and diastole. The diameter-change waveform was calculated by subtracting the distance of the near wall from that of the far wall. D = mean (average) diameter was calculated manually. Diameters were calculated in centimeter and expressed in millimeter for uniformity of data information.
By using CIMT, Ds, Dd and blood pressure indices for arterial stiffness were calculated1718.
(i) Stiffness index (SI): indicates rigidity of arterial wall and is calculated as
SI = Ln (SBP/DBP) / ((Ds-Dd)/D),
SBP, systolic blood pressure; DBP, diastolic blood pressure
Ln, natural logarithmic value; SI, ratio of natural logarithm (systolic blood pressure/diastolic blood pressures) to relative change in diameter, and expresses local stiffness of the arterial wall obtained from the relationship between vessel diameter and artery pressure values.
(ii) Young's elastic modulus (YEM): a measure of elasticity or pressure change required to theoretically stretch the artery 100 per cent from baseline per unit area1718.
YEM is calculated as: [(SBP - DBP) x Dd)] / (Ds – Dd) /IMT
The average of both sides was taken as mean SI and mean YEM.
The sample size was calculated based on two sample size mean. It was hypothesised that a difference of 0.04 mm CIMT was clinically significant based on our internal data. For an alpha of 0.5 and beta power of 0.1 and expected variance of 0.0016, we calculated sample size of 22 in each arm, but enrolled more cases than control in almost 2:1 ratio.
Statistical analysis: The data were analyzed using SPSS software (version 13) (SPSS Inc, Chicago, USA). The correlation between variables (cardiac T2*, liver T2*, serum ferritin, CIMT, SI, YEM) were analyzed using Pearson's coefficient of correlation. To compare variables between thalassaemic patients and healthy controls, unpaired t test was applied, and where normality test failed, Mann-Whitney test was applied.
Results
The cases (n=53) were between 13 and 33 yr with 20 cases (37.7%) below 20 yr, 24 cases (45.3%) between 21 to 25 yr and nine (17%) above 25 yr. Controls were equally distributed in the age groups defined above (<20 yr- 8 controls, 21-25 yr- 8 controls, >25 yr- 9 controls) and had same baseline characteristics as cases. The mean ages were 21.85±4.58 and 22.08±4.46 yr in cases and controls, respectively. All patients were on regular blood transfusion, with an average blood requirement of 134.54 ml/kg/yr. Their mean pre-transfusion Hb was 9.1g/dl. Twenty nine of 53 patients (54.7%) were splenectomized. Fifty of 53 patients (94.4%) were on various chelation therapy (compliance and dosage not assessed) with either a single chelating agent (deferasirox) or on combination therapy (deferasirox and deferiprone or desferrioxamine). The mean ferritin level was 3773.38 ng/l ± 3133.18 (range of 300 to16000 ng/l). Of the total number of patients studied, 29 (54.7%) had evidence of cardiac iron overload, 10 (18.9 %) had severe cardiac iron overload, 48 (90.6%) had evidence of liver iron overload with 39 (73.6%) showing moderate to severe liver iron overload.
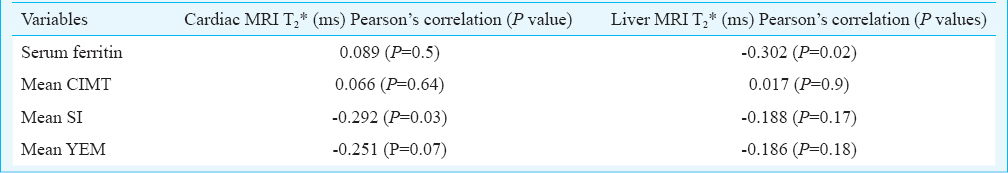
The mean CIMT of patients was (0.48±0.04mm) which was significantly higher than that of controls (0.44±0.02mm) (P<0.001). Stiffness index (SI) in patients (2.45±0.79) was significantly (P<0.001) higher than controls (1.98±0.54). Similarly YEM was also significantly (P<0.001) elevated in cases (96.12±34.85) as compared to controls (68.60±24.29) (Table I). There was a significant negative correlation between SI and cardiac iron overload with Pearson correlation coefficient value of -0.292 (P=0.03) (Table II, Figure). No correlation was found between mean CIMT, YEM, and cardiac iron overload. There was no significant correlation between hepatic iron overload and mean CIMT, SI, or YEM. Serum ferritin correlated poorly with mean CIMT, SI, and YEM. A subset analysis of comparison between splenectomized and non-splenectomized thalassaemic children is presented in Table III.
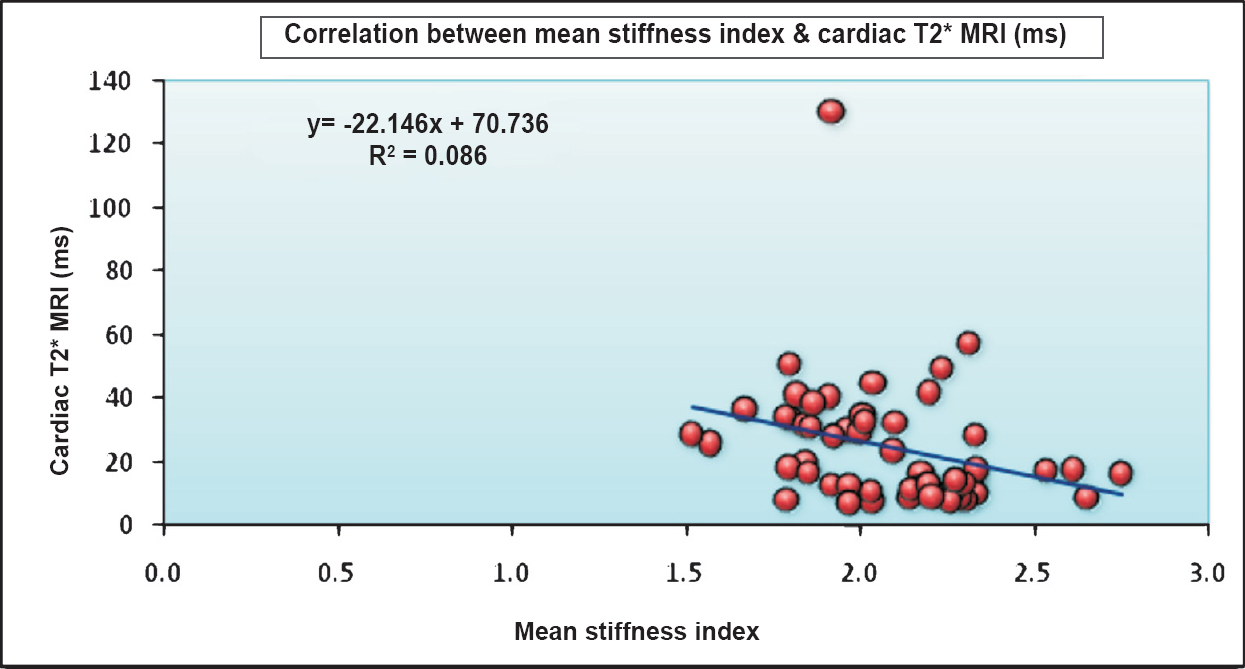
- Correlation between mean stiffness index and cardiac iron overload (cardiac MRI T2*).
Discussion
Elasticity is primarily determined by the structural component of the arterial wall, vascular smooth muscle tone and transmural distending pressure1920. Alterations of arterial structures with disruption of elastic tissue and calcification have been demonstrated in patients with β-thalassaemia major and may translate functionally into alteration of arterial stiffness in vivo. Arterial stiffness and Young's elastic modulus are important mechanical properties, because these are related to vascular impedance and in turn to the afterload that is presented to the left ventricle. It is presumed that changes in elasticity / stiffness can be detected before clinically apparent vascular disease, and act as early marker for development of atherosclerosis.
Various theories have been proposed for early atheromatous changes associated with thalassaemia. Myocardial parenchymal damage generally occurs secondary to iron deposition, atherogenic vascular complications described in β-thalassaemia patients have been largely attributed to an increase in lipid peroxidation caused by toxic hydroxyl radicals generated by the Haber-Weiss reaction19. Free heme and iron produced during haemolysis decrease production of nitrous oxide causing vasoconstriction, endothelial dysfunction and diffuse vascular elastic tissue injury. Duffy et al21 have shown that iron chelation with desferrioxamine in adults with coronary artery disease improves endothelium dependent vasodilatation. Moreover, hypercoagulability is also a well described entity in thalassaemia22. Oxidative damage due to iron overload leads to endothelial dysfunction and overexpression of adhesion molecules (ICAM, VCAM) and along with increased platelet aggregation and membrane abnormality, leads to early atherosclerosis and vascular damage21. Pathologically it is reflected as psudoxanthoma like changes in vessels wall, i.e. Elastorhexis, thickening and calcification of elastic tissue of vessel wall before overt atherosclerosis is established23. Anaemia is now considered to be one of the independent risk factors responsible for early atherosclerosis24.
In accordance with other studies, we also noted that CIMT increased with advancing age of thalassaemia patients. Patients were divided in age groups of <20 yr, 21 to 25 yr and >25 yr and the mean CIMT values were 0.47, 0.48 and 0.49 mm, respectively. Also, CIMT was significantly higher in thalassaemic patients as compared to age and sex matched controls indicating early onset of atherosclerosis.
Our study showed a correlation between cardiac siderosis and arterial stiffness and an absence of correlation between hepatic siderosis and arterial stiffness. Cusma Piccione et al25 studied the correlation between cardiac iron sores and arterial stiffness. This may be due to different iron kinetics in the liver and the cardiovascular system. Cardiomyocytes may also be damaged by non-transferrin bound iron (NTBI) without overt iron overloading which may indicate a higher sensitivity of the cardiomyocytes and vascular endothelial cells to the oxidant effect of iron26. As iron chelators remove iron more readily from the liver than from the heart, vascular system involvement may need intense chelation therapy for prevention of vascular complications. In the present study no attempt was made to evaluate effect of iron chelation on CIMT or stiffness indices.
In our study there was no correlation between CIMT and cardiac or hepatic iron overload, perhaps because of the relatively young patients and small sample size. The cases had a significantly higher stiffness index than controls and were at an increased risk of early atherosclerosis and hence at risk for cardiovascular morbidity. YEM was significantly higher in cases as compared to controls, indicating impaired distensibility, but it did not correlate with cardiac and hepatic iron overload. The stiffness index correlated with cardiac iron load. Our observations demonstrated that carotid artery stiffness increased with an increase in cardiac iron overload and that the cardiac MRI T2* not only reflected the severity of cardiac iron overload but also impaired arterial elasticity. Our findings show that cardiac MRI T2* can also be a useful marker for overall cardiovascular health and that serum ferritin does not correlates with CIMT or SI and YEM, indicating that serum ferritin alone is a poor marker of vascular health.
Limitations of the study included descriptive, cross-sectional nature with no follow up, smaller sample size and heterogeneity of thalassaemia population. Though anaemia is one of the confounder in atherosclerosis, its effect was minimised due to high pre-transfusion Hb levels. Nitric oxide is a protective factor that requires arginine for production in the endothelium. Haemolysis itself leads to decreased arginine and increased arginase levels. This leads to decreased nitric oxide, which normally opposes vasoconstriction and LDL oxidation27. We have not evaluated effect of NO on atherosclerosis. It is possible that some changes in carotid artery dynamics in our patients may be related to decreased nitric oxide due to haemolysis, in addition to iron overload. We did not evaluate the effect of chelation on arterial flow dynamics which would have been more clinically relevant.
In conclusion, our study showed that the CIMT increased in thalassaemia patients, as compared to age and sex matched controls, indicating early atherosclerosis. Further, arterial stiffness increased significantly as cardiac iron overload increased, but there was no significant correlation between CIMT and cardiac or hepatic iron overload nor was there any correlation between arterial stiffness and hepatic iron overload. Further studies will be required to see if these early changes of atherosclerosis in thalassaemia patient are reversible with intensive chelation therapy to prevent vascular events.
Acknowledgment
Authors thank Drs Deepak Langde and N.S. Kabra for statistical inputs.
Conflicts of Interest: None.
References
- Prevalence of β-thalassemic and other hemoglobinopathies in six cities in India: A multicentric study. J Community Genet. 2013;4:33-42.
- [Google Scholar]
- Efficacy of deferasirox in reducing and preventing cardiac iron overload in β-Thalassemia. Blood. 2010;115:2364-71.
- [Google Scholar]
- Ventriculo-vascular interactions in patients with beta thalassaemia major. Heart. 2005;91:769-73.
- [Google Scholar]
- Carotid intima-media thickness is increased and related to arterial stiffening in patients with beta-thalassaemia major. Br J Haematol. 2006;135:732-4.
- [Google Scholar]
- Global vasomotor dysfunction and accelerated vascular aging in beta-thalassemia major. Atherosclerosis. 2008;198:448-57.
- [Google Scholar]
- Carotid intimomedial thickness - a non-invasive index of vascular health. J Assoc Physicians India. 2008;56:577-8.
- [Google Scholar]
- Measurement of intima-media thickness of common carotid arteries with high-resolution B-mode ultrasonography: inter- and intra-observer variability. Ultrasound Med Biol. 1991;17:225-30.
- [Google Scholar]
- Effect of deferasirox chelation on liver iron and total body iron concentration. Indian J Pediatr. 2013;80:655-8.
- [Google Scholar]
- Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459-67.
- [Google Scholar]
- Assessment of intima-media thickness of carotid arteries: evaluation of an automated computer software. Neuroradiology. 2008;50:849-53.
- [Google Scholar]
- Association of iron overload based quantitative MRI T2* technique and carotid intima-media thickness in patients with beta-thalassemia: a cross-sectional study. BMC Cardiovasc Disord. 2010;10:62.
- [Google Scholar]
- Experimental and clinical validation of arterial diameter waveform and intimal media thickness obtained from B-mode ultrasound image processing. Ultrasound Med Biol. 1999;25:1353-63.
- [Google Scholar]
- Common carotid intima-media thickness and arterial stiffness: indicators of cardiovascular risk in high-risk patients. The SMART Study (Second Manifestations of ARTerial disease) Circulation. 1999;100:951-7.
- [Google Scholar]
- Arterial stiffness in the young: assessment, determinants, and implications. Korean Circ J. 2010;40:153-62.
- [Google Scholar]
- Iron, atherosclerosis and ischemic heart disease. Arch Intern Med. 1999;159:1542-8.
- [Google Scholar]
- Premature atherosclerosis in non-transfusion-dependent beta-thalassemia intermedia. Cardiology. 2011;118:159-6.
- [Google Scholar]
- Iron chelation improves endothelial function in patients with coronary artery disease. Circulation. 2001;103:2799-804.
- [Google Scholar]
- Elastic tissue abnormalities resembling pseudoxanthoma elasticum in thalassemia and the sickling syndromes. Blood. 2002;99:30-5.
- [Google Scholar]
- Anemia as a risk factor for cardiovascular disease in The Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol. 2002;40:27-33.
- [Google Scholar]
- Early identification of cardiovascular involvement in patients with β-thalassemia major. Am J Cardiol. 2013;112:1246-51.
- [Google Scholar]
- Evalvation of cardiac iron load by cardiac magnetic resonance in thalassemia. Indian Pediatr. 2011;48:697-701.
- [Google Scholar]
- Nitric oxide and arginine dysregulation: A novel pathway to pulmonary hypertension in hemolytic disorders. Curr Mol Med. 2008;8:620-32.
- [Google Scholar]






