Translate this page into:
Evaluation of antimicrobial activity of certain combinations of antibiotics against in vitro Staphylococcus epidermidis biofilms
Reprint requests: Dr. Rosário Oliveira, IBB-Institute for Biotechnology & Bioengineering, Centre of Biological Engineering, University of Minho, Campus de Gualtar, 4710-057, Braga, Portugal e-mail: roliveira@deb.uminho.pt
-
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Staphylococcus epidermidis is the most common pathogen associated with infections of surgical implants and other prosthetic devices owing to its adhesion and biofilm-forming ability on biomaterials surfaces. The objective of this study was to compare susceptibilities of biofilm-grown cells to single antibiotic and in combination with others to identify those that were effective against S. epidermidis biofilms.
Methods:
Biofilms were grown in the MBEC™ assay system. The use of this methodology allowed a rapid testing of an array of antibiotics alone (eight) and in combination (25 double combinations). The antibacterial effect of all treatments tested was determined by colony forming units (cfu) enumeration method.
Results:
The MBEC™ assay system produced multiple and reproducible biofilms of S. epidermidis. Although none of the antibiotics tested have demonstrated an antimicrobial effect (log reduction >3) against all S. epidermidis isolates biofilms, but combinations containing rifampicin showed in general a broader spectrum namely rifampicin-gentamicin and rifampicin-clindamycin. Levofloxacin in combination with rifampicin showed a killing effect against three isolates but failed to attain a bactericidal action against the other two.
Interpretation & conclusions:
Our findings showed that rifampicin should be a part of any antibiotic therapy directed against S. epidermidis biofilms. However, the efficient antibiotics combination might be dependent on S. epidermidis isolate being tested.
Keywords
Antibiotics susceptibility
biofilm
MBEC™ assay
Staphylococcus epidermidis
Previously regarded as an innocuous commensal microorganism on the human skin, Staphylococcus epidermidis is now seen as an important opportunistic pathogen1–3. This bacterium has become the leading cause of infections related to indwelling medical devices such as vascular catheters, prosthetic joints and artificial heart valves, mainly due to its capacity to form biofilms on such materials thus causing persistent or recurrent infections45. Infections of medical implants material are associated with considerable morbidity and costs4. These infections are very difficult to eradicate since bacteria in biofilms can be up to 1,000-fold more resistant to antibiotic treatment than the same organism growing planktonically6–8. Another problem is the ability of bacteria to acquire resistance to antibiotics therapy. This arises from the frequent use of antibiotics and mainly those of broad-spectrum. Only a few antibiotics are relatively active against S. epidermidis biofilms, and rifampicin, a transcription inhibitor, is among the most effective molecules for treating biofilm-related infections9. However, in a study where the prevalence of drug resistance among clinically significant blood isolates of S. epidermidis (n = 464) and consumption of antibiotics at a tertiary care teaching hospital (Meilahti Hospital, Helsinki) were analysed for the period 1983-1994, a remarkable increase was found in resistance to rifampin (from 0 to 23%) despite the low usage of this agent10. Accordingly, since rifampicin demonstrated a high risk of rapid development of resistance, it should not be used as monotherapy1.
Taking this fact into account, antibiotic combinations are often necessary in the treatment of S. epidermidis infections and these combinations are used involving antibiotics like rifampicin to avoid the appearance of antimicrobial resistance111. Moreover, the combinations can also enhance the effects of individual antimicrobial agents by synergic action. Another alternative to overcome the resistance problem in Staphylococci is the use of novel antibiotics such as linezolid, daptomycin, tigecycline and quinupristin/dalfopristin that have been developed and claimed to be 100 per cent efficient12. Some of the newer antimicrobial agents may provide alternatives for monotherapy or combination therapy with rifampicin1. However, this new antibiotic generation is very expensive, so the use of conventional antibiotics or antibiotic combinations still represents a valid therapeutic option.
The aim of the present work was to investigate the antimicrobial activity of some of the most common antibiotics alone and in combination against in vitro S. epidermidis biofilms.
Material & Methods
Bacterial isolates & antibiotics: In this study, previously well characterized biofilm-producing S. epidermidis isolates were used: 117977, 132034, 150571, 1457 and 9142. The first three were obtained from the Department of Biological Sciences, University of Calgary, Calgary, Canada and the last two were provided by Dr G.B. Pier, Channing Laboratory, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, USA. These were clinical isolates (isolated from infected catheters) and were stored at -80°C. All the assays were performed using brain heart infusion (BHI) medium, tryptic soy broth (TSB) and tryptic soy agar (TSA) [Merck, Germany], prepared according to the manufacturer's instructions. This study was done in Ceri lab, Department of Biological Sciences, University of Calgary, Calgary, Alberta, Canada.
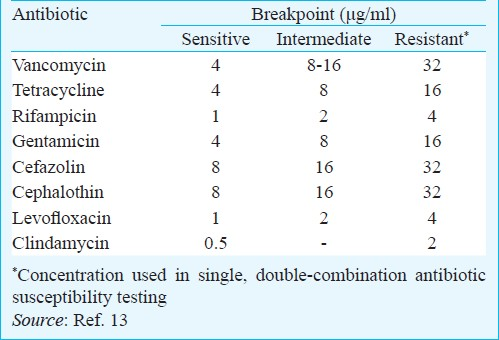
Antibiotics tested were vancomycin, tetracycline, rifampicin, gentamicin, cefazolin, cephalotin, levofloxacin and clindamycin. All antibiotics were purchased from Sigma Chem. Co., USA. The concentrations used to test the susceptibility of biofilm-grown S. epidermidis to single and double-combinations of antibiotics are presented in (Table I).
Biofilm formation: Biofilms were grown in a Calgary biofilm device (CBD) [commercially available as the MBEC physiology and genetics assay (Innovotech Inc., Edmonton, AB, Canada)], as originally described by Ceri et al14. The CBD consists of two-part reaction vessel. The top component of the device is a polystyrene lid with 96 identical pegs. The lid is inserted into the bottom piece of the device- a microtiter plate into which the inoculated growth medium is placed. The CBD is then placed on a gyro rotary shaker in an incubator which provides a shear force against the pegs, this facilitates the formation of 96 statistically equivalent biofilms on the surface of the pegs14–16.
In brief, several colonies of the isolates grown on TSA plates were suspended in saline (0.9% NaCl) to a density of 1.0 on the McFarland scale, as indicated by the manufacturer. The bacterial suspension was resuspended in medium to obtain a cellular concentration of circa 1 × 107 colony forming units (cfu)/ml. This solution was used as inoculum for the MBEC™ device (MBEC™ Biofilm Technologies Ltd. Calgary, Alberta, Canada). The biofilms were grown during 48 h, at 37°C at 150 rpm and on a rocking platform where the shear force was created against the pegs forming 96 equivalent biofilms. To enumerate the biofilm cfu on individual control pegs, pegs were broken off the MBEC peg lid using sterile forceps, placed into 200 μl of sterile saline and sonicated for 8 min. Bacteria were then enumerated by serial dilution plating. Colony forming units/peg counts were determined from at least three independent experiments.
This protocol was performed with three different biofilm growth media: TSB, TSB + 0.25% glucose and BHI medium. After selecting the medium that allowed the highest biofilm formation (cfu per peg >6 log), the previous procedure was repeated with the selected medium.
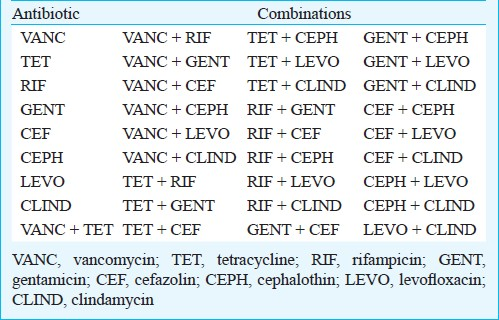
Biofilm challenge and recovery: The challenge plates were prepared using the antibiotics at break-point concentrations (Table I) alone and in all possible double combinations (Table II). The biofilms formed on the lid of the MBEC™ were rinsed twice with 0.9 per cent saline and placed into the challenge plate overnight at 37°C, at 150 rpm, on a rocking platform and 95 per cent relative humidity. After that the challenged biofilms were rinsed twice in saline and were tranferred to a recovery plate that consisted of TSB medium plus (1% v/v) Tween 80. Biofilms were removed from all pegs at once, by sonication for 8 min on high with an Aquasonic sonicator (model 250HT, VWR Scientific, Mississauga, ON, Canada)14. The vibration disrupted biofilms from the surface of the 96 pegs into the recovery plate. Then, colony forming units were determined as follows: the recovery medium (containing the sonicated biofilms) was serially diluted. The biofilm cultures (10-fold diluted) were spotted on TSA plates, the plates were incubated for 48 h at 37°C to ensure maximum recovery of the surviving microorganisms and after that the cfu counted.
Results
In this study, eight antibiotics, commonly used in the treatment of Gram-positive infections, were tested at their breakpoint concentrations. The effect of these antibiotics combined in pairs (Table II) was also assessed. TSB without glucose stimulated more biofilm formation (data not shown), forming at the end of the incubation period (48 h), a biofilm of approximately 6 log cfu per peg. Thus, this medium was selected for the subsequent antibiotic susceptibility tests on S. epidermidis biofilms. The amount of glucose (0.25% w/v) used to promote the formation of S. epidermidis biofilms in traditional 96-well plates617 was not favourable to biofilm formation in CBD.
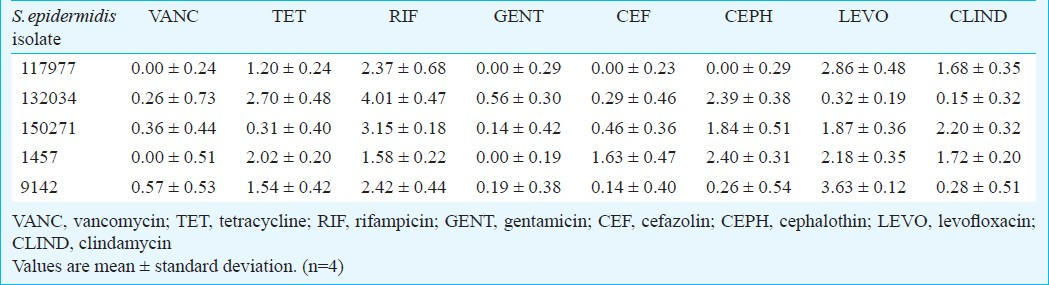
The effect of the tested antibiotics alone was evaluated against the biofilms of the five clinical isolates of S. epidermidis assayed. The results obtained are expressed as reduction in treated biofilms compared to untreated controls (Table III). In general, none of the antibiotics tested was effective against S. epidermidis biofilm. Only rifampicin was effective against S. epidermidis isolates 132034 and 150271 as well as levofloxacin against isolate 9142 (Table III) because the log10 cfu reduction observed was higher than 3 log. Although the reduction caused by rifampicin and levofloxacin is mostly inferior to 3 log, these were the antibiotics having the broadest and highest antimicrobial effect against all S. epidermidis isolates tested.
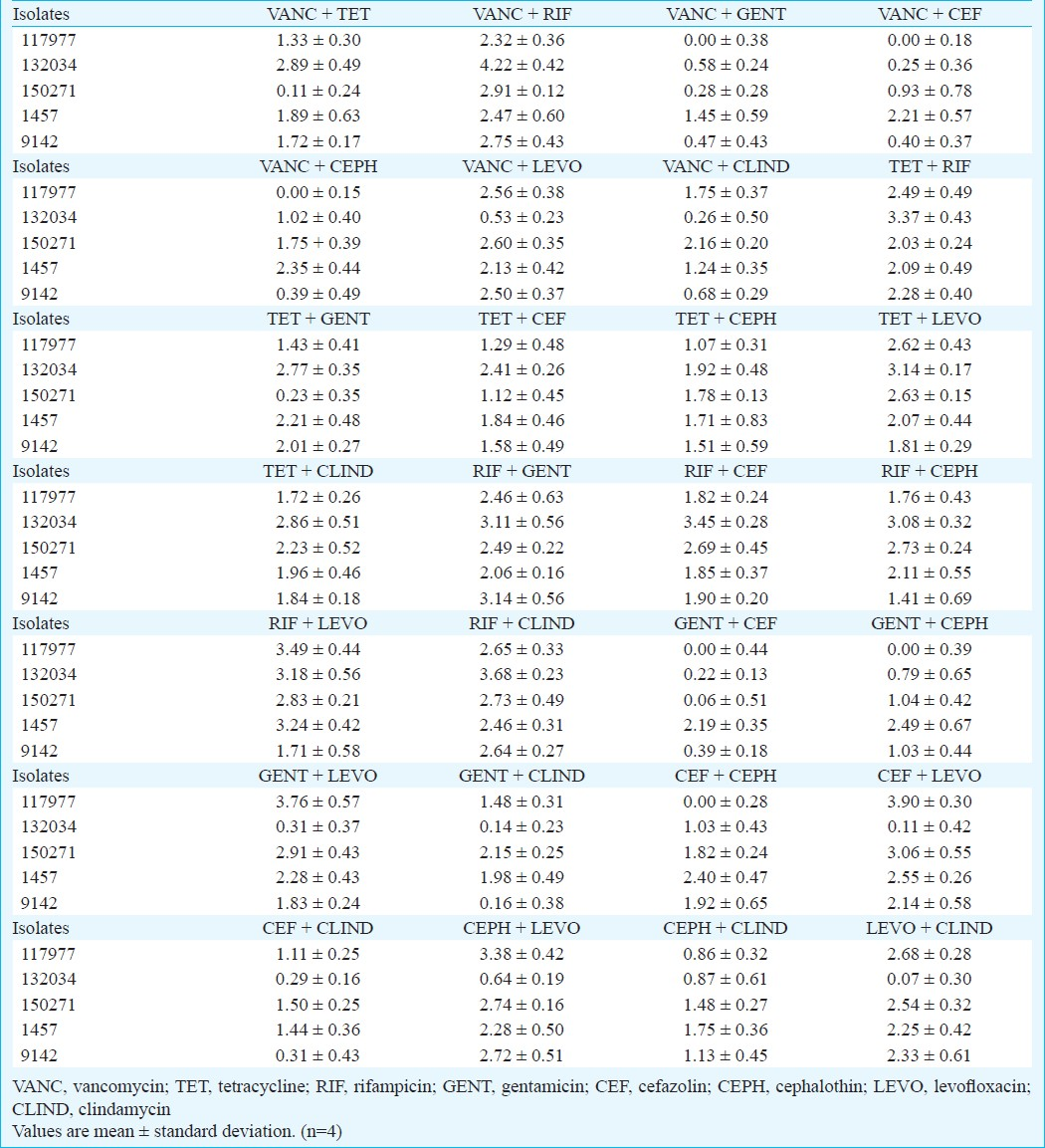
The results presented in Table IV show the reduction in biofilms log10 cfu for all combinations of antibiotics tested. Most combinations tested did not promote a 3 log reduction in bacterial counts. Nevertheless, and as it could be expected, most of those containing rifampicin were able to reach at reasonable levels of bactericidal effect. Examples are rifampicin-clindamycin and rifampicin-gentamicin, the former promoting reductions above 2.5 log in biofilm cell counts for all isolates tested. Notably, the combination rifampicin-levofloxacin displayed a high killing effect specifically against three isolates but against 9142 the log reduction was below 2.0.
Discussion
Standard antibiotic therapy is only able to eliminate planktonic cells, leaving the sessile forms to propagate within the biofilm and to continue to disseminate when therapy is terminated. In biofilms, microbes are protected from antimicrobial agents and the host immune system18. In fact, increasingly microorganisms have the ability to withstanding the effect of antibiotics and individual antibiotics are generally ineffective against bacteria biofilms. To overcome such problems, combination of antibiotics is a possible alternative to threat staphylococcal biofilm infections. Previous studies have also demonstrated impressive results with rifampicin, however, the risk of rapid development of resistance is a major problem, and rifampicin should not be used as monotherapy11920. It has been considered that combinations of rifampicin with other anti-staphylococcal agents such as quinolones or fusidic acid could prevent the emergence of rifampicin resistance during therapy1921.
Since antibiotics alone were generally not effective against S. epidermidis biofilms and taking into consideration the strategy of combined therapy to avoid resistance, the double combinations of the antibiotics were tested against the same biofilms. In a previous study22, where some double and triple combinations of antibiotics were studied, several triple combinations, all containing rifampicin were active against S. epidermidis and only one double combination vancomycin - rifampicin was reported to be active. In this study, 17 S. epidermidis isolates were assessed and the susceptibility to antibiotics was tested in terms of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC). However, a triple combination may be an overload of antibiotics and more prone to the development of secondary effects.
Monzón et al11 also tested some double combinations of antibiotics against four S. epidermidis isolates and the highest reduction they observed was 2.19 log obtained with the combination vancomycin-rifampicin and only against one specific isolate, using both antibiotics at 4 × MIC.
In this study, we tested the effect of traditional antibiotics alone and in combination, in S. epidermidis biofilm cells, using breakpoint concentrations1322. The Calgary Biofilm Device (CBD) was used for biofilm formation because, despite being expensive, it is a high-throughput methodology with several advantages, namely its multiple equivalent biofilms that can be used for testing. Its use can avoid the need to repeat assays, which is usually necessary when using standard plates. An array of antimicrobial compounds with varying concentrations can easily be assessed. This allows rapid testing of compounds for antibiofilm activity. These advantages can overcome the cost associated problems of this methodology.
Our results demonstrated the advantage of using antibiotic combinations in the treament of S. epidermidis infections. However, the effect of these combinations is highly strain-dependent. Alternative agents are novel antibiotics such as linezolid, tigecycline and daptomycin claimed to be highly effective against biofilms but these agents have some disadvantages. Apart from their high cost, these have been in clinical use for a short time only and the extent of their toxicity is yet to be experienced. Hajdu et al4 observed no significant reduction in S. epidermidis biofilms cfu with daptomycin and tigecycline, not even at the highest concentrations tested (128 × MIC). Generally these concentrations are far beyond any concentration that can be achieved after administration of standard therapeutic doses4. Aslam et al23 also tested the effect of tigecycline and after 12 h of treatment only a mean reduction of the bacterial growth by 2log10 counts was obtained, notably using a concentration of 1 mg/ml (1,000 fold higher than its MIC for the organisms tested in the planktonic phase). In this case, the concentration of tigecycline expected to be in human serum after standard dosing is 2 mg/l4. Utilizing high doses of antimicrobials to eradicate biofilm has had limited successs in the clinical setting23.
In conclusion, there are some combinations of more traditional antibiotics that can be strongly considered as therapeutic strategies for an efficient control of S. epidermidis biofilms associated infections. Rifampicin combined with clindamycin or with gentamicin showed to have the broadest range of action, although rifampicin in combination with levoflxacin displayed a higher killing effect against three out of the five isolates. As an alternative to monotherapy, these combinations can be advantageous avoiding the likehood of resistance development.
References
- Antibiotic susceptibility among Staphylococcus epidermidis isolated from prosthetic joint infections with special focus on rifampicin and variability of the rpoB gene. Clin Microbiol Infect. 2009;15:238-44.
- [Google Scholar]
- Staphylococcus epidermidis- the “accidental” pathogen. Microbiology. 2009;7:555-67.
- [Google Scholar]
- Quorum-sensing control of biofilm factors in Staphylococcus epidermidis. J Infect Dis. 2003;188:706-18.
- [Google Scholar]
- Effects of vancomycin, daptomycin, fosfomycin, tigecycline, and cefriaxone on Staphylococcus epidermidis biofilms. J Orthop Res. 2009;27:1361-5.
- [Google Scholar]
- Minimal attachment killing (MAK): a versatile method for susceptibility testing of attached biofilm-positive and -negative Staphylococcus epidermidis. Med Microbiol Immunol. 2002;191:107-14.
- [Google Scholar]
- Low concentration of vancomycin stimulate biofilm formation in some clinical isolates of Staphylococcus epidermidis. J Clin Pathol. 2010;62:1112-6.
- [Google Scholar]
- Mechanisms of biofilm resistance to antimicrobials agents. Trends Microbiol. 2001;9:34-9.
- [Google Scholar]
- In vitro activity of a new antibacterial rhodanine derivative against Staphylococcus epidermidis biofilms. J Antimicrob Chemother. 2006;58:778-83.
- [Google Scholar]
- Increased resistance among Staphylococcus epidermidis isolates in a large teaching hospital over a 12-year period. Eur J Clin Microbiol Infect Dis. 1996;15:133-8.
- [Google Scholar]
- Synergy of different antibiotic combinations in biofilms of Staphylococcus epidermidis. J Antimicrob Chemother. 2001;48:793-801.
- [Google Scholar]
- Role of coagulase-negative staphylococci in human disease. Vet Microbiol. 2009;134:45-54.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. In: Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically (8th ed). Wayne, PA, USA: Approved Standard M7-A8. CLSI; 2010.
- [Google Scholar]
- The Calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities in bacterial biofilms. J Clin Microbiol. 1999;37:1771-6.
- [Google Scholar]
- Characterization of biofilm growth and biocide susceptibility testing of Mycobacterium phlei using MBEC™ assay system. FEMS Microbiol Lett. 2001;203:263-7.
- [Google Scholar]
- Copper and quaternary ammonium cations exert synergistic bactericidal and antibiofilm activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2008;52:2870-81.
- [Google Scholar]
- Comparative assessment of antibiotic susceptibility of coagulase-negative staphylococci in biofilm versus planktonic culture as assessed by bacterial enumeration or rapid XTT colorimetry. J Antimicrob Chemother. 2005;56:331-6.
- [Google Scholar]
- Effect of berberine on Staphylococcus epidermidis biofilm formation. Int J Antimicrob Agents. 2009;34:60-6.
- [Google Scholar]
- Molecular characterization of resistance to rifampicin in an emerging hospital-associated methicillin-resistant Staphylococcus aureus clone ST228, Spain. BMC Microbiol. 2010;10:68.
- [Google Scholar]
- Reconsideration of rifampin: a unique drug for a unique infection. In: JAMA. Vol 279. 1998. p. :1575-7.
- [Google Scholar]
- Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46:1032-7.
- [Google Scholar]
- Multiple combination bactericidal testing of Staphylococcal biofilms from implant-associated infections. Antimicrob Agents Chemother. 2006;50:55-61.
- [Google Scholar]
- Combination of tigecycline and N-acetylcysteine reduces biofilm-embedded bacterial on vascular catheters. Antimicrob Agents Chemother. 2007;51:1556-8.
- [Google Scholar]






