Translate this page into:
Evaluation of 75 g glucose load in non-fasting state [Diabetes in Pregnancy Study group of India (DIPSI) criteria] as a diagnostic test for gestational diabetes mellitus
Reprint requests: Dr Reva Tripathi, Department of Obstetrics & Gynaecology, Lok Nayak Hospital, New Delhi 110 002, India e-mail: revatripathi@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
There is no consensus regarding optimal standard for diagnosis of gestational diabetes mellitus (GDM). In this study, use of 75 g glucose load in non-fasting state [Diabetes in Pregnancy Study Group of India (DIPSI) criteria] as a diagnostic test for GDM in pregnant women was compared with different oral glucose tolerance tests (OGTTs).
Methods:
This prospective study included 936 pregnant women, who underwent plasma glucose evaluation two hours after the challenge of 75 g glucose load irrespective of the timing of last meal (DIPSI criteria for GDM). After three days, standard 75 g OGTT was done in all women irrespective of previous plasma glucose value. Accuracy of the first result was compared to OGTT using cut-offs as per the World Health Organization (WHO) and International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria for the diagnosis of GDM.
Results:
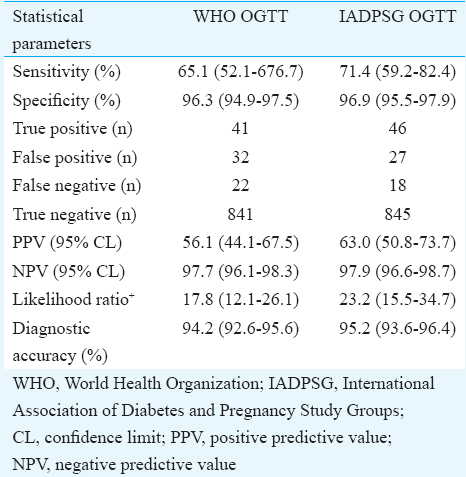
Of the total 936 pregnant women, 73 (7.8%) patients had plasma glucose value ≥140 mg/dl when measured two hours after glucose load. When comparing with the WHO and IADPSG criteria, the sensitivity values were 65.1 and 74.1 per cent, respectively, and the corresponding specificity values were 96.3 and 96.9 per cent, respectively. On comparing with the WHO OGTT, only 41 of the 73 (56.2%) were true positives, whereas when IADPSG criteria were used, true positives were 46 (63%). False negative cases were also present when classified by the WHO and IADPSG criteria though in lesser numbers than false positives. The positive predictive values (PPVs) for the WHO and IADPSG criteria were 56.1 and 63 per cent, respectively, and their corresponding negative predictive values were 97.7 and 97.9 per cent, respectively.
Interpretation & conclusions:
Our findings showed that when 75 g glucose load in non-fasting state was used as a diagnostic test for GDM, almost one quarter of patients with GDM escaped diagnosis as sensitivity values were low. On the other hand, some GDM cases were falsely labelled as normal as this test did not account for cases of fasting hyperglycaemia. In addition, comparison with other OGTTs showed low PPVs. Hence, use of DIPSI criteria for diagnosing GDM must be reconsidered till further validation.
Keywords
75 g non-fasting glucose load
diagnostic test
gestational diabetes
oral glucose tolerance tests
pregnancy
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance which is diagnosed for the first time during pregnancy, irrespective of treatment with diet or insulin1. Depending on population sample and diagnostic criteria used, prevalence ranges from 1 to 20 per cent2. There are varied recommendations for screening and diagnostic tests for GDM and no uniform standard has yet been established.
Currently, the diagnostic test for GDM recommended by the Diabetes in Pregnancy Study Group of India (DIPSI) is an evaluation of plasma glucose after two hours of ingestion of 75 g glucose load irrespective of meal timings. This has also been included in the guidelines issued by the Ministry of Health and Family Welfare, Government of India3. The American Diabetes Association (ADA) had recommended a 2-step approach to identify GDM with an initial glucose challenge test (GCT) with 50 g glucose load followed by a diagnostic oral glucose tolerance test (OGTT) with 100 or 75 g glucose load1. According to the World Health Organization (WHO) criteria, GDM is diagnosed using 75 g OGTT with fasting plasma glucose value >126 mg/dl or two-hour >140 mg/dl4.
After publication of results of the large, multicentric, international Hyperglycaemia and Pregnancy Outcome (HAPO) study5, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) recommended a single-step approach to the diagnosis of GDM in 2010. They recommended 75 g OGTT for all pregnant women and diagnosed GDM if one or more plasma venous glucose values exceeded the following thresholds: fasting >92 mg/dl, one hour ≥180.0 mg/dl, or two hour ≥153 mg/dl6. The WHO (2013)7 and ADA (2014)8 have accepted the IADPSG criteria, but this is not accepted by the American College of Obstetricians and Gynecologists (ACOG) who still continue to recommend two-stage testing for diagnosis9.
According to the ADA, WHO and IADPSG recommendations, patients are required to come to the antenatal clinic in the fasting state and subsequently undergo multiple blood sampling for testing. On the other hand, the DIPSI recommends a two-hour plasma glucose evaluation after the use of 75 g glucose load in non-fasting state irrespective of the timing of last meal10 as a simple, economical and feasible single-step procedure for the diagnosis of GDM. As per the recommendations of the DIPSI, GDM is diagnosed if the venous plasma glucose value exceeds 140 mg/dl. As this is certainly a much simpler test and more patient friendly and also minimizes the inconvenience to pregnant women, it is being widely used. This test is claimed to serve both as a screening and a diagnostic procedure11. This has led us to conduct this study to validate the use of 75 g glucose load in non-fasting state as a one-step procedure for the diagnosis of GDM.
Material & Methods
The study was conducted between October 2011 and February 2013 on pregnant women attending the antenatal clinic of the department of Obstetrics and Gynaecology, Maulana Azad Medical College and associated Lok Nayak Hospital, New Delhi, India. A total of 1000 women were enrolled and 936 of them successfully completed the study. Consecutive women who could be tested before 28 completed weeks of gestation were included after taking informed written consent; known diabetics were excluded from the study. The study was approved by the Institutional Ethical Committee.
Women were included at the first antenatal visit which was either during the first or second trimester. All women underwent a non-fasting plasma glucose evaluation two hours after giving 75 g of glucose load between 24 and 28 wk of gestation. The 75 g glucose load was given irrespective of time of previous meal and venous sample was drawn after two hours. The recommended cut-off value of this 75 g non-fasting DIPSI test is >140 mg/dl11.
Subsequently, all women, irrespective of the two-hour value in non-fasting test, underwent standard OGTT with 75 g of glucose after three days of initial testing. The mean time interval between the non-fasting test and fasting OGTT was 3.5±0.4 days. All women were advised an unrestricted diet for the intervening days prior to OGTT. A venous blood sample (2 ml) was taken after overnight fasting of 8-14 h. A solution containing 75 g of glucose was then given orally to all women and two venous blood samples were subsequently collected at hourly intervals. Plasma glucose estimation was done by hexokinase method on an autoanalyzer12. The machine used was Cobas C501 (Roche Diagnostics, USA). Calibration of the analyzers was done as per the directions in the Kit Inserts for the specific tests. Two levels of internal controls were run twice a day. Levey–Jennings charts12 were prepared on the basis of laboratory-derived mean and standard deviation. Suitable corrective action was taken for any violation of the Westgard Rules12. The laboratory also participates in the External Quality Assessment Scheme (EQAS) of Randox Laboratories, UK. Samples are received from EQAS co-ordinator and analyzed in the laboratory every month and accuracy was compared with the standard.
In this study, the 75 g non-fasting DIPSI criteria were compared with the WHO41999 and IADPSG6 criteria. As varied cut-offs were used for the diagnosis of GDM, the same values were designated as normal or abnormal depending on the diagnostic criteria utilized. Comparative analysis was made to estimate the accuracy of diagnosis. Statistical analysis was performed using Stata 11.0 (College Station, Texas, USA) and graphics were done using R software, version 3.2.2 (The R Foundation, https://www.r-project.org/).
Results
A total of 1000 women were included and 936 of them were successfully followed up and were included for final analysis. Of the total 936 pregnant women who were tested between 24 and 28 wk of gestation, 826 women (88.25%) tested negative for GDM by all methods. The remaining 110 women had one or other tests for GDM positive and hence could be labelled as GDM.
Using the DIPSI criteria, 73 women (7.8%) had a two-hour plasma glucose value ≥140 mg/dl and were therefore labelled as having GDM, whereas when the IADPSG criteria were used, only 64 (6.8%) women were diagnosed as GDM. However, these were not all amongst 73 women having abnormal DIPSI value. Using the WHO criteria, 63 (6.7%) women had abnormal OGTT, but here also these women were different from those identified abnormal as per the DIPSI criteria. The Figure shows the diagnostic variability of these tests. Only 35 women could be labelled as having GDM by all the three methods (DIPSI, IADPSG and WHO).
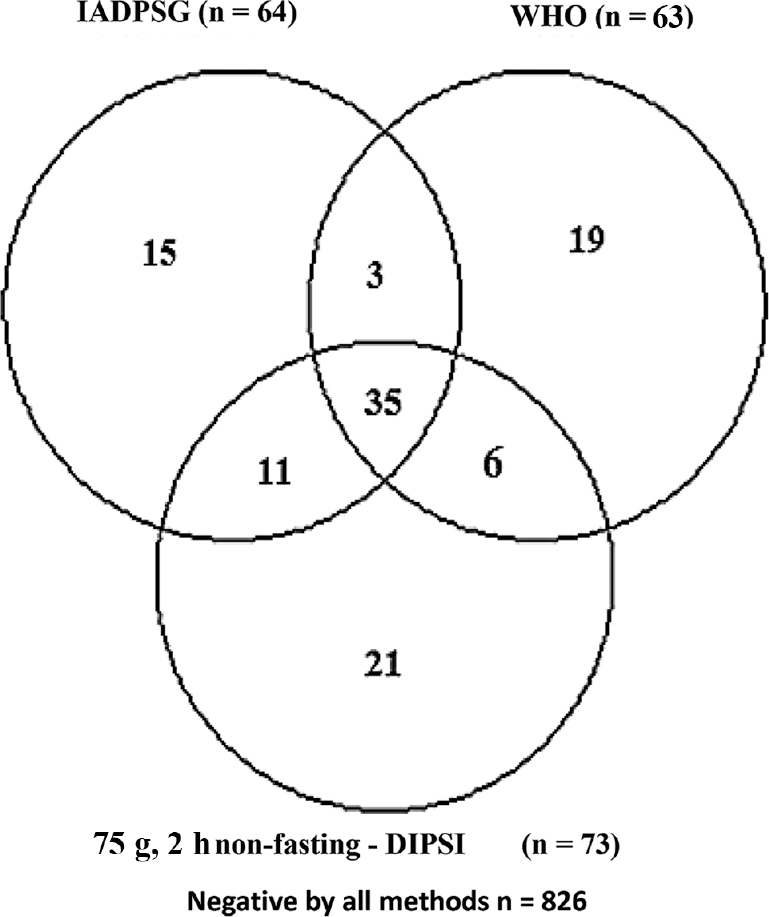
- Venn diagram showing detailed breakup of patients diagnosed as gestational diabetes mellitus by different methods along with their concordance/discordance. WHO, World Health Organization; IADPSG, International Association of Diabetes and Pregnancy Study Groups; DIPSI-Diabetes In Pregnancy Study Group of India.
It was also observed that the DIPSI test was positive in 21 women who had normal OGTT by both other criteria (WHO & IADPSG). This reiterates the problem of false-positive cases diagnosed by the DIPSI criteria. The detailed comparison of 75 g non-fasting test with different OGTTs is shown in the Table. On comparing with the WHO OGTT, sensitivity of 75 g non-fasting DIPSI test was 65.1 per cent and specificity was 96.3 per cent. True positives were 41; false negatives and false positives were 22 and 32, respectively, whereas diagnostic accuracy was 94.2 per cent. When using the IADPSG as diagnostic standard, sensitivity, specificity and diagnostic accuracy of 75 g, non-fasting test was 71.4, 96.9 and 95.2 per cent, respectively.
Discussion
Women with a history of GDM are at an increased risk of developing type 2 diabetes mellitus in future and their children are also at risk for the same13. Women with GDM require intensive monitoring during pregnancy to offset the potential complications and hence accuracy of diagnosis is important. Earlier, two-step approach was followed for the diagnosis of GDM, which used initial screening with the 50 g GCT, followed by OGTT in patients with abnormal GCT. The HAPO study5 and the subsequent IADPSG criteria6 suggested universal use of OGTT for the diagnosis of GDM. However, this appeared to be an impractical exercise, especially in developing countries, and hence the DIPSI recommended 75 g non-fasting test as a simple, economical and feasible single-step procedure for the diagnosis of GDM. The National Institute for Health and Clinical Excellence (NICE)14 has recommended different values as cut-offs but these are already being questioned15.
In the present study, 75 g, two-hour non-fasting DIPSI test was done and followed by OGTT. Using OGTT as per the WHO and IADPSG criteria as gold standard, the sensitivity of 75 g non-fasting test was low. With this low sensitivity, about one quarter of women with GDM were missed. Missing such a large number is not acceptable for a diagnostic test, especially as GDM is associated with both maternal and perinatal complications.
There were a substantial number of women (28.8%) who were positive by DIPSI method but were not confirmed by any type of fasting OGTT with low PPV. Overdiagnosing such a large number of women may lead to unnecessary increase in surveillance both clinical and ultrasonographic, induction of labour, need for surgical interventions and psychological stress to pregnant women who are falsely labelled as having GDM. Many of the women who are falsely labelled as normal are those who have derangement of fasting sugar values and this may impact obstetrical outcomes.
It is not only that the numbers are different and depend on the criteria used, but the identification of patients is also different as some patients test positive by one method but are negative when other criteria are applied. Since the negative predictive value of the DIPSI test is high, it shows that if values do not meet designated levels, a patient is unlikely to be a case of GDM.
A study done by Anjalakshi et al11 on south Indian population showed 100 per cent sensitivity and 100 per cent specificity of 75 g, two-hour non-fasting DIPSI test when compared with the WHO-recommended 75 g OGTT7 for the diagnosis of GDM. They concluded that there was no significant difference between the two tests in identifying women with GDM. Another study conducted on Indian population had shown similar results16. However, Mohan et al17 have shown a very low sensitivity of 'non-fasting OGTT as compared to the fasting OGTT'. These authors further reported that DIPSI test when compared to the WHO criteria had sensitivity of 27.7 per cent and specificity of 97.7 per cent and when compared to the IADPSG criteria had sensitivity of 22.6 per cent and specificity of 97.8 per cent17. This should be considered significant as it deals with a population similar to the one which was utilized to determine the DIPSI cutoff11. In another Indian study conducted in the State of Maharashtra18, GDM was reported in only 6.52 per cent cases and they suggested that this low prevalence might be due to 'less sensitivity of DIPSI criteria'. Another study, though conducted in smaller numbers19 had stated that 22.36 per cent of cases of GDM were not diagnosed by the DIPSI criteria. A study on Srilankan women has also concluded that 'GCT with two-hour cut-off value ≥140 mg/dl is not sensitive enough to diagnose GDM recognized by GTT'20. This study analyzed only 274 women, but prevalence rate of GDM in Srilanka study20 was 22 per cent and therefore, their results should be considered significant. However, Magon et al21 had recommended the DIPSI test for universal use in India.
The problem of accuracy of the 75 g, two-hour non-fasting DIPSI test is further highlighted by showing low positive predictive value (PPV) in comparison with various types of OGTT. Low PPV implies that many women labelled as having GDM by this method are not corroborated by other methods and are unlikely to be truly cases of GDM. The negative predictive value of the test is very high and hence shows that, if values do not meet designated levels, a woman is unlikely to be a case of GDM.
Though the diagnostic accuracy of the DIPSI test was about 94 per cent, but remaining 5-6 per cent women were incorrectly diagnosed. This group comprised both, those who were normal but diagnosed as diabetic and also those who were abnormal (diabetic) but were falsely labelled as normal. When it is considered that India has 25.6 million numbers of births annually22, this translates into approximately 1.2-1.5 million incorrectly diagnosed cases of GDM. This appears too large a number of cases to be ignored.
The precision of testing is a major concern. The laboratory standards maintained for the HAPO study as detailed elsewhere23 are unlikely to be replicated by others. In such circumstances, the validity of reports would be questionable. The issue of quality control in laboratories should not be ignored as incorrect diagnosis impacts subsequent clinical care.
The large extent of false positives, together with a smaller number of false negatives, is a major limitation of DIPSI test. The consequences of clinical interventions due to the erroneous diagnosis of GDM cannot be overlooked. Although no cost-benefit analysis has yet been conducted, the clinical, financial, psychological and logistic burden as a result of increased diagnosis of GDM cannot be negated.
In conclusion, the 75 g, two-hour non-fasting DIPSI test when used for diagnostic purposes showed poor sensitivity as well as low PPVs when compared with OGTT. This will translate into almost one quarter of patients with GDM escaping diagnosis. In addition, many women will be wrongly overdiagnosed as GDM leading to unnecessary monitoring and interventions. Hence, use of this test for diagnostic purposes needs to be further investigated in large, multicentric studies before utilizing for universal implementation in India.
Conflicts of Interest: None.
References
- IDF Diabetes Atlas 2013
- National guidelines for diagnosis and management of gestational diabetes mellitus. New Delhi. Maternal Health Division, Ministry of Health & Family Welfare. New Delhi: Government of India; 2015.
- Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539-53.
- [Google Scholar]
- IADPSG recommendation on the diagnosis and classification of hypoglycemia in pregnancy. Diabetes Care. 2010;33:676-82.
- [Google Scholar]
- World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Geneva: World Health Organization; 2013.
- Standards of medical care in diabetes-2014. Diabetes Care. 2014;37(Suppl 1):S14-80.
- [Google Scholar]
- Committee on Practice Bulletins - Obstetrics. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol. 2013;122(2 Pt 1):406-16.
- [Google Scholar]
- Gestational diabetes mellitus - Guidelines. J Assoc Physicians India. 2006;54:622-8.
- [Google Scholar]
- A single test procedure to diagnose gestational diabetes mellitus. Acta Diabetol. 2009;46:51-4.
- [Google Scholar]
- Tietz textbook of clinical chemistry and molecular diagnostics (5th ed). Philadelphia: Saunders; 2012.
- Gestational diabetes mellitus (position statement) Diabetes Care. 2002;25(Suppl 1):S94-6.
- [Google Scholar]
- 2015. Diabetes in pregnancy: Management from preconception to the postnatal period. NICE Guidelines. Available from: https://www.nice.org.uk/guidance/ng3/history
- Diagnosis of gestational diabetes mellitus: falling through the net. Diabetologia. 2015;58:2003-12.
- [Google Scholar]
- Single glucose challenge test procedure for diagnosis of gestational diabetes mellitus: a Jammu cohort study. J Assoc Physicians India. 2013;61:558-9.
- [Google Scholar]
- Comparison of screening for gestational diabetes mellitus by oral glucose tolerance tests done in the non-fasting (random) and fasting states. Acta Diabetol. 2014;51:1007-13.
- [Google Scholar]
- Screening of gestational diabetes mellitus in antenatal women using DIPSI guidelines. Int J Res Med Sci. 2016;4:446-9.
- [Google Scholar]
- Comparison of DIPSI and IADPSG criteria for diagnosis of GDM: A study in a North Indian tertiary care center. Int J Diabetes Dev Ctries. 2015;35:1-2.
- [Google Scholar]
- Is non fasting glucose challenge test sensitive enough to diagnose gestational diabetes mellitus? Int Arch Med. 2015;8(93)
- [Google Scholar]
- Diagnosing GDM: Role of simple, cost effective, and sensitive DIPSI test. J Obstet Gynaecol India. 2014;64:299-300.
- [Google Scholar]
- UNICEF, Statistics, India. Available from: http://www.unicef.org/infobycountry/india_statistics.html
- Integration of local and central laboratory functions in a worldwide multicentre study: Experience from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Clin Trials. 2006;3:397-407.
- [Google Scholar]






