Translate this page into:
Evaluating different samples & techniques for hr-HPV DNA genotyping to improve the efficiency of risk profiling for oral & cervical cancers in Sikkim, India
For correspondence: Dr Mingma Lhamu Sherpa, Department of Biochemistry, Sikkim Manipal Institute of Medical Sciences, Sikkim Manipal University, Gangtok, Sikkim 737 102, India e-mail: mingmals@yahoo.com
-
Received: ,
Accepted: ,
Abstract
Background & objectives
Oral and genital HPV infection in men may be a source of cervical diseases in their women partners as well as disease in themselves. This study aimed to evaluate and compare the performance of Hybrid Capture 2 (HC2) in physician-collected cervical samples and qPCR in self-collected urine and oral gargle samples of women and men, respectively, for hr-HPV infection status and genotyping.
Methods
One thousand and two hundred biological samples were collected from 200 women (urine, oral gargle, and cervical smear) and 200 men (urine and oral gargle) visiting a referral hospital in the remote Himalayan State of Sikkim. The extracted genomic DNA from urine and gargle samples were profiled for hr-HPV genotypes using quantitative polymerase chain reaction (qPCR) and HC2 for cervical samples.
Results
In women, hr-HPV was detected in 17.5 per cent of cervical samples by HC2, 25.5 per cent of urine, and 7 per cent of gargle samples by qPCR. For men, hr-HPV was detected in 8 per cent urine and 5 per cent gargle samples by qPCR. Among the HPV-positive women, 56 per cent of urine samples and 20 per cent of oral samples showed single-genotype infection, while the remaining had multiple genotypes. Amongst the HPV-positive men, 62.7 per cent of urine samples and 85.7 per cent of oral samples showed single-genotype infection while the remaining had multiple genotypes. Compared to Pap, the area under ROC was good for HC2 (AUC=0.89) and for qPCR (AUC= 0.852).
Interpretation & conclusions
HC2 for cervical and qPCR-based HPV DNA assay for urine and gargle sample is suitable for risk profiling for cervical cancer (CC) and oral cancer (OC) screening programmes.
Keywords
Cervical
high-risk human papillomavirus
hybrid capture 2
men
oral
real-time qPCR
urine
women
Infection caused by Human papillomavirus (HPV) DNA is the most prevalent sexually transmitted infection (STI) around the globe1. HPV infection and its persistence is a recognised source and etiologic pathogen for both precancerous and cancerous lesions of the cervix uteri (99%), penis, vulva, vagina, anus, and oropharynx (47%) as reported by International Agency for Research on Cancer (IARC) and Centers for Disease Control and Prevention (CDC)2-5.
Early detection and management are crucial, but challenges persist due to limited testing platforms in remote areas, inadequate information, and communication on HPV and cervical cancer. Oral cancer screening is primarily through oral examination and biopsy6. Cervical cancer screening with Pap-cytology and visual inspection with acetic acid (VIA) has shifted to HPV-DNA assay as the preferred method since 20206,7. Researchers identified several gaps in HPV research wherein very few studies had used urine and oral samples for HPV-DNA assay, lack of efficient approaches for isolation and HPV detection in urine and oral sample had restricted its potential clinical use, the poor acceptance of Pap as a screening tool, and HC2 was qualitative and limited to cervical sample testing only. Furthermore, HC2 test was not available in Sikkim at the time of study conception and is currently available only for research. To overcome the multidimensional challenges of existing screening modalities, there is a need for prioritisation of better method(s) for equitable screening and treatment8, by exploring more accessible and acceptable samples like urine and oral gargle to mitigate and improve acceptance to screening.
The cervical sample is now routinely used for HPV-DNA assays, and this was included with cytology to assess the efficacy and validation of two alternative samples, urine and oral gargle. Collection of these two biological samples, are more acceptable to patients, easier to obtain, viable, and non-invasive in comparison to cervical samples and oral biopsy which could enhance their uptake for screening. This study aimed to evaluate the performance of HC2 for physician-collected cervical samples, and qPCR for hr-HPV detection in urine and oral gargle samples of men and women. Positive findings in the urine and oral gargle samples implicate genital and oral HPV infection, respectively. HPV-DNA positive individuals would be advised to retest in one yr, colposcopy, treatment, or surveillance as per standard clinical practice2,3. This study aimed to assess and compare HPV infection status and genotype in urine, oral, and cervical samples of men and women of 18 yr of age and older in Sikkim.
Material & Methods
Study design and sampling technique
This is a cross-sectional study conducted from November 2018 to February 2022 (39 months) at the Central Referral Hospital, Gangtok, Sikkim. A convenient (non-probability) approach was used for sampling. Sample size (n=400) was calculated using Epi info, version 7.2.1.0, assuming 95 per cent confidence interval (CI) and five per cent acceptable margin of error (α=0.05).
Study population
A total of 1200 samples of three different types; (cervical, oral gargle and urine from 200 women and oral gargle and urine from 200 men) were collected from consenting participants between 18-65 yr of age following clearance from the Institutional Ethics Committee. The enrolled participants were volunteers and patients from outpatient departments (OPD) of Otorhinolaryngology (ENT) and Obstetrics and Gynaecology (OBG) during routine cytological screening.
Inclusion and exclusion criteria
Men and women of 18 yr of age and older were included for urine and oral samples. Women of 18 yr of age or above, who were married/sexually active and those clinically indicated as standard of care determined by the treating physician, were included for cervical sample collection. Pregnant women, non-consenting cases, immunocompromised individuals, women with history of total hysterectomy, radical trachelectomy and patient on radiotherapy were excluded.
Sample collection and processing
Paired urine and oral gargle samples from 200 men and women, and physician-collected cervical smear for HPV-DNA assay and cytology were collected from women participants (n=200). The cervical cells used for cytology and digene specimen transport medium (STM) vials were stored at -20°C, till hybridization with HC2. First voided morning urine, and gargle samples were collected before brushing, in 50 ml sterile containers and stored at 4-6°C for 4-6 hrs before processing. The urine and gargle samples were stored in 1X Phosphate Buffer Saline (PBS) at -20°C for batch-wise gDNA extraction.
DNA extraction
gDNA was extracted from all the 800 urine and oral gargle samples by standard Proteinase K digestion followed by phenol-chloroform method with modifications as reported by earlier, which the investigators replicated9,10. The extracted gDNA was quantified, and amplified using standard protocols and the study algorithm is elaborated in Figure 1.
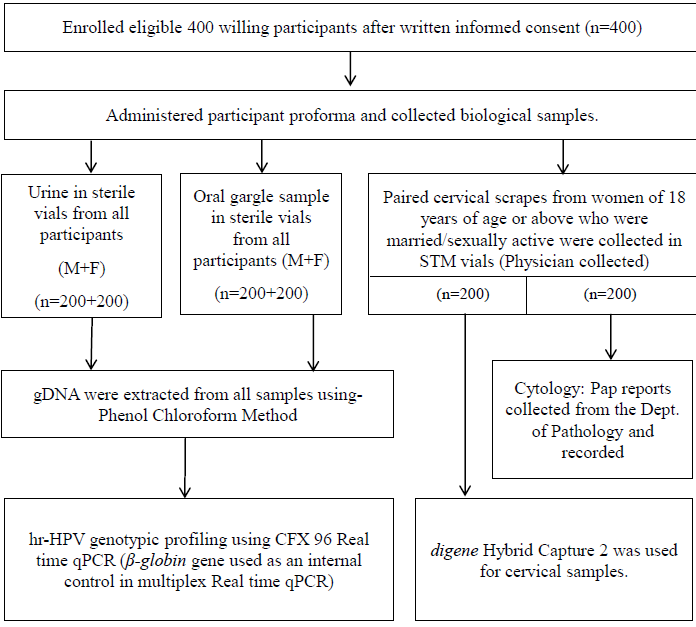
- Study algorithm. dept, department; M, male; F, female; Pap, papanicolaou test; qPCR, quantitative real time PCR.
High-risk HPV detection for cervical samples with HC2
The cervical samples were tested for 13 hr-HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) by qualitative HC2 (digene, Qiagen)11. A relative light unit per Cut-off (RLU/Co) ≥2 was taken as threshold for HC2 positivity.
High-risk HPV detection from urine and oral gargle samples of men and women using qPCR
hr-HPV DNA from both urine and oral gargle samples were amplified by multiplex real time qPCR for14 hr-HPV genotypes (16, 18, 31, 39, 45, 59, 33, 35, 56, 68, 51, 52, 58, 66). A Cycle threshold (Ct) value of 30 was taken as a positive cut-off value for qPCR and housekeeping gene (β-globin) was used as internal amplification control (Amplisen, Biorad CFX 96 thermocycler12. Two sets of positive calibrators, one set of negative calibrators, and two positive control samples from cancer cell lines (SiHa and HeLa procured from the Amity University, Noida) were used as controls.
Data analysis
HPV in different specimen(s) were reported in terms of frequency and percentages. The performance characteristics were compared and analyzed. Sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV) and accuracy were calculated for different samples screened in qPCR to compare and validate one test vs. another, and one sample vs. another. The degree of agreement was evaluated with Cohen’s kappa constant (κ) for paired samples as follows: very good (0.81-1); good (0.61-0.81); moderate (0.41-0.6); fair (0.21-0.4); and poor (<0.2). A multi-layered comparison model was used to compare hr-HPV testing by HC2 with Paps, the only available gold standard or cervical cancer (CC) risk screening (Fig. 2). For technique comparison the qPCR of urine sample was then compared with HC2 of cervical samples, and qPCR for oral sample was then compared to qPCR for urine for the same individual. The area under the curve (AUC) for Receiver Operating Characteristic (ROC) with 95 per cent confidence interval (CI) was used for measuring the performance accuracy of the above algorithm. AUC with a value of one or near one was considered as an ideal and good measure of the screening model, whereas AUC<0.5 reflected poor measure of the study model. Data was recorded in excel and the statistical analysis was performed in SPSS, version 27.
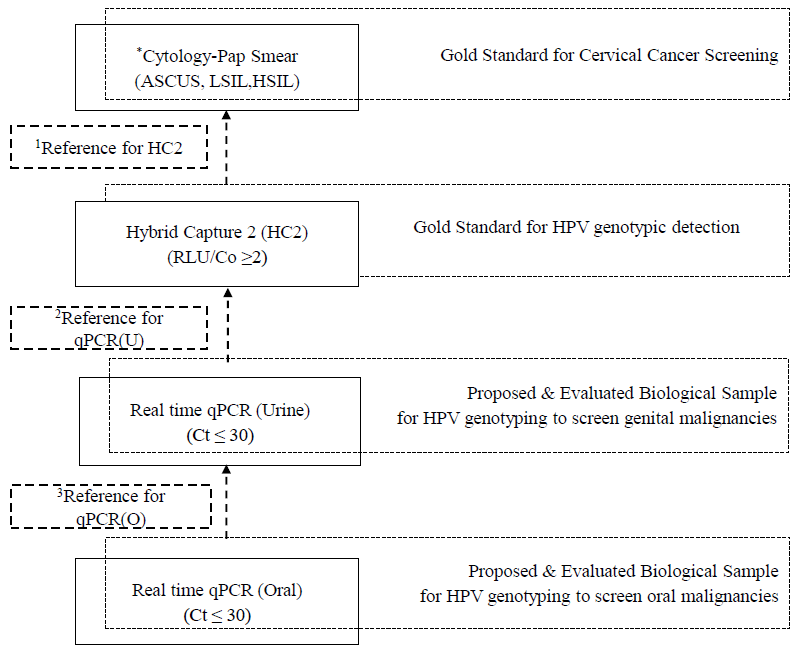
- Algorithm for performance characteristics of different techniques for HPV DNA Assay and its matrix for understanding the comparison between samples and between techniques. ASCUS, atypical squamous cells of undetermined significance; LSIL, low grade squamous intraepithelial lesion; HSIL, high grade squamous intraepithelial lesion; U, urine; O, oral, Ct, cycle threshold, RLU/Co, relative light unit per cut off. *Pap positive was any sample with cytology reports including ASCUS, LSIL and HSIL, 1Pap used as reference gold standard for comparing the performance of HC2. 2HC2 taken as reference gold standard for comparing the performance of qPCR(U). 3qPCR(U) taken as reference point for comparing the performance of qPCR(O). Note: *Pap is the only gold standard technique used as single reference point in ROC to assess the performance of HC2, qPCR(U) and qPCR(O).
Results
High-risk HPV detection for physician-collected cervical samples using Pap and HC2 among women
Abnormal cervical cytology was reported in six per cent (12/200):33.3 per cent (4/12) were atypical squamous cells of undetermined significance (ASC-US), 8.3 per cent (1/12) was low grade squamous intraepithelial lesion (LSIL), and 58.3 per cent (7/12) were high grade squamous intraepithelial lesion (HSIL). hr-HPVs were detected in 17.5 per cent (35/200) by HC2. Eleven of the twelve women with abnormal cervical cytology tested positive for hr-HPV by HC2 (Table I).
| Technique | n | Positivity rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytology (Pap) | 200 | 12 (6) | ASCUS | LSIL | HSIL | ||||||
| HC 2 Assay | 200 | 35 (17.5) | 4 (33.3) | 1 (8.3) | 7 (58.3) | ||||||
| Cytology frequency (%) | Total | ||||||||||
| Pap Positive | Pap Negative | ||||||||||
| HC2 Assay | HPV Positive | 11 (5.5) | 24 (12) | 35 (17.5) | |||||||
| HPV Negative | 1 (0.5) | 164 (82) | 165 (82.5) | ||||||||
| Total | 12 (6) | 188 (94) | 200 (100) | ||||||||
| Women | Men | ||||||||||
| Technique/sample type | qPCR | ||||||||||
| Urine (n=200) | Oral (n=200) | Urine & Oralb | κ | Urine (n=200) | Oral (n=200) | Urine & Oralb | κ | ||||
| n (%)a | n (%)a | n (%)a | n (%)a | ||||||||
| Overall Hr-HPV Types | 51 (25.5) | 14 (7) | 14 | 0.501 | 16 (8) | 10 (5) | 10 | 0.754 | |||
| Single | 32 (62.7) | 12 (85.7) | - | - | 9 (56.2) | 2 (20) | - | - | |||
| Multiple | 19 (37.3) | 2 (14.2) | - | - | 7 (43.8) | 8 (80) | - | - | |||
| HPV16 | 17 (33.3) | 6 (42.9) | 1 | 0.045 | 4 (25) | 2 (20) | 1 | 0.324 | |||
| HPV18 | 4 (7.8) | - | - | - | - | - | - | - | |||
| HPV31 | 1 (2) | - | - | - | 1 (6.25) | 1 (10) | - | - | |||
| HPV39 | 10 (19.6) | 2 (14.3) | - | - | 2 (12.5) | - | - | - | |||
| HPV45 | 10 (19.6) | 2 (14.3) | - | - | 1 (6.25) | 3 (30) | - | - | |||
| HPV59 | 3 (5.9) | 1 (7.1) | - | - | 1 (6.25) | 1 (10) | - | - | |||
| HPV33 | 3 (5.9) | - | - | - | 6 (37.5) | 8 (80) | 4 | 0.556 | |||
| HPV35 | 6 (11.8) | 1 (7.1) | - | 0.009 | 6 (37.5) | 4 (40) | 2 | 0.385 | |||
| HPV56 | 2 (3.9) | - | - | - | 1 (6.25) | 2 (20) | - | - | |||
| HPV68 | 9 (17.6) | 4 (28.6) | 3 | 0.446 | 5 (31.25) | 4 (40) | - | - | |||
| HPV51 | 1 (2) | - | - | - | - | - | - | - | |||
| HPV52 | 3 (5.9) | - | - | - | - | - | - | - | |||
| HPV58 | 6 (11.8) | 1 (7.1) | - | - | - | - | - | - | |||
| HPV66 | 1 (2) | - | - | - | - | - | - | - | |||
High-risk HPV detection for urine and oral gargle sample using qPCR:
hr-HPV infection status in women: 25.5 per cent (51/200) urine samples were positive for hr-HPV and seven per cent (14/200) oral gargle samples by qPCR. Of the 51 positive samples, 62.7 per cent (32/51) were infected with only a single hr-HPV genotype, and the rest were co-infections (more than two genotypes). Among the positives in oral gargle, 85.7 per cent (12/14) were positive for single hr-HPV type and the remaining exhibited infection with multiple hr-HPV genotypes (Supplementary Fig.1). Distribution of multiple hr-HPV infection were as follows: for urine 13 (68.4%) samples had two genotypes and six samples (31.6%) had three genotypes, and for oral samples two and three genotypes were equally distributed (50% each) (Supplementary Table).
-
hr-HPV infection status in men: hr-HPV were detected in eight per cent (16/200) urine and five per cent (10/200) oral gargle by qPCR (Table I). Among those with HPV-positive urine samples, 56 per cent (9/16) were infected with a single hr-HPV genotype and the rest had multiple genotypes. In oral gargle samples, 20 per cent (2/10) were positive for single hr-HPV type, and the rest had multiple genotypes. Among those with multiple genotypes in urine, 28.6 per cent (2/7) had two genotypes, 57.1 per cent (4/7) had three genotypes, and 14.3 per cent (1/7) were with four genotypes. Similarly, the two and three genotypes were equally distributed: 37.5 per cent (3/8) for each oral sample(1/8) followed by 12.5 per cent each with four and five strains (Supplementary Table & Supplementary Fig.1).
High-risk HPV genotype profile in urine and oral gargle sample using qPCR:
hr-HPV detection in women's urine and oral gargle samples: HPV 16 was the most frequent genotype detected (33.3%) in urine and in 42.9 per cent of oral gargle samples (Fig. 2). Variations of genotypes were observed for urine and oral. In urine, HPV 39 and 45 were 19.6 per cent each followed by HPV 68 at 17.6 per cent, HPV 35 and 58 at 11.8 per cent each, the other HPVs 18, 59, 33, 52, 56, 31, 51, 66 were distributed in descending order (Supplementary Fig. 2). Similarly for oral gargle samples, the other strains detected in descending order were HPV 68 (28.6%), HPV 39 & 45 (14.3% each) and HPV 59, 35 and 58 (7.1% each).
hr-HPV detection in men's urine and oral gargle samples: The most frequent genotypes detected were HPV 35 and 33 (37.5% each) in urine, and HPV 33 (80%) in oral gargle samples. In urine, the frequency of occurrence in descending order were HPV 68 (31.25%), HPV 16 (25%), HPV 39 (12.5%) and HPV 31, 45, 56, 59 (6.25% each). For oral gargle samples, the highest infection rate with HPV 33 was followed by HPV 35 and 68 (40% each), HPV 45 (30%) and HPV 56 (20%) and HPV 31 and 59 (10 per cent each). hr-HPV 18, 51, 52, 58 and 66 strains were not detected in any sample.
Comparison of hr-HPV detection in different samples by different techniques using Cohen’s kappa:
hr-HPV detection in urine and oral gargle samples of women by qPCR: The overall concordance of hr-HPV detection between urine and oral gargle was moderate (κ=0.50). Furthermore, for comparison between HPV genotypes, between samples, the quality of agreement was moderate for HPV 16 (κ=0.45). However, no agreement could be established for the other type-specific hr-HPV infection status between the paired urine and gargle sample (κ value <0.20; Table I).
hr-HPV detection in urine and oral gargle samples of men by qPCR: Overall, the concordance of hr-HPV detection between urine and oral gargle samples was good (κ=0.754). While comparing the specific genotypes between samples, quality of agreement between the two sample types were moderate for HPV 33 (κ=0.556) and fair for two genotypes HPV 16 (κ=0.324) and HPV 35 (k=0.385). No agreement could be established for the remaining hr-HPV genotypes between the paired samples (κ value<0.20; Table I).
The Cohen kappa scores between the two different techniques (Pap and HC2 for HPV detection) in paired cervical samples: Moderate agreement was observed for hr-HPV detection in cervical samples by HC2 as compared to Pap (κ=0.42; Table II).
The Cohen kappa scores between the different techniques (qPCR in reference to HC2 and Pap for HPV detection) with two different biological samples of Women: No agreement could be established for hr-HPV detection in urine by qPCR as compared to HC2 used for HPV detection in cervical samples (κ=0.06). However, fair agreement could be observed when HPV detection in urine by qPCR was compared to that of Pap (κ=0.28; Table II).
The Cohen kappa scores between urine and oral gargle samples of men and women by qPCR: The quality of agreement for hr-HPV detection in urine and oral gargle samples by qPCR was moderate (κ=0.50) in women and good (κ=0.75) in men (Table II).
| Comparison | Type of paired sample | Participant | Number of samples | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | k |
|---|---|---|---|---|---|---|---|---|---|
| *Pap &1HC2 | C & C | F | 200+200 | 91.7 | 87.2 | 31.4 | 99.4 | 87.5 | 0.41 |
| 2HC2 & qPCR | C & U | F | 200+200 | 85.7 | 87.3 | 58.8 | 96.6 | 79.5 | 0.06 |
| Pap & qPCR | C & U | F | 200+200 | 91.7 | 78.7 | 21.6 | 99.3 | 79 | 0.28 |
| qPCR & qPCR | U & O | F | 200+200 | 27.5 | 100 | 100 | 80.1 | 81.5 | 0.5 |
| qPCR & qPCR | U & O | M | 200+200 | 62.5 | 100 | 100 | 96.8 | 97 | 0.75 |
| qPCR & qPCR | U & O | M & F | 400+400 | 35.8 | 100 | 100 | 88.6 | 89.3 | 0.48 |
P*<0.001 was obtained by fisher exact test; PPV, positive predictive value; NPV, negative predictive value. Pap positive was any sample with cytology reports including ASCUS, LSIL and HSIL. 1HC2 compared with Pap smear as gold standard. 2qPCR of urine was compared with HC2 of cervical sample as a gold standard.
Performance characteristics of different techniques
Table II and Figure 2 summarize the performance characteristics of Pap, HC2 and qPCR for hr-HPV detection in paired cervical, urine and oral gargle samples of women (n=200), and urine, and oral gargle samples of men (n=200). The comparison model within biological samples (oral, with urine and urine with cervical), and within techniques (Pap, HC2, and qPCR) were assessed for their performance. Pap was considered as the ‘gold standard’ in this study.
Performance of HC2 with reference to Pap (gold standard): The sensitivity, specificity, PPV, NPV and accuracy of HC2 as a screening tool were 91.7, 87.2, 31.4, 99.4 and 87.5 per cent respectively.
Performance of qPCR with reference to HC2, and Pap (gold standard): The sensitivity, specificity, PPV, NPV and accuracy of qPCR for HPV detection in urine were 85.7, 87.3, 96.6 and 79.5 per cent, respectively, in comparison to HC2. In reference to Pap-Cytology, the sensitivity, specificity, PPV, NPV and accuracy of qPCR for urine was 91.7, 78.7, 21.6, 99.3and 81.5 per cent, respectively.
Performance of qPCR for different samples (urine and oral gargle) of men and women as prospective candidates for viable biological samples for screening of hr-HPV infection: The sensitivity, specificity, PPV, NPV and accuracy of qPCR used for hr-HPV detection in oral gargle samples when compared with urine were 27.5, 100, 100, and 81.5 per cent respectively in females and 62.5, 100, 100 and 97 per cent in men. When the performance of qPCR was compared for HPV detection in oral gargle and urine samples in both men and women, the sensitivity, specificity, PPV, NPV and accuracy were 35.8, 100, 100, 88.6 and 89.3 per cent, respectively (Table II).
Performance accuracy of the multi-layered comparison algorithm: The ROC and AUC was evaluated for each assay (HC2 and qPCR) compared to Pap (Fig. 3). The AU-ROC was 0.895 (95 per cent CI 0.8, 0.989) for HC2 for hr-HPV detection in cervical, 0.852 (95 per cent CI 0.753, 0.951) for qPCR for hr-HPV detection in urine, and 0.596 (95 per cent CI 0.411, 0.78) for qPCR for hr-HPV detection in oral gargle samples. The AUC for both HC2 and qPCR urine was close to 1, establishing a good measure for hr-HPV detection. The AUC was lower for qPCR for oral samples (0.59).
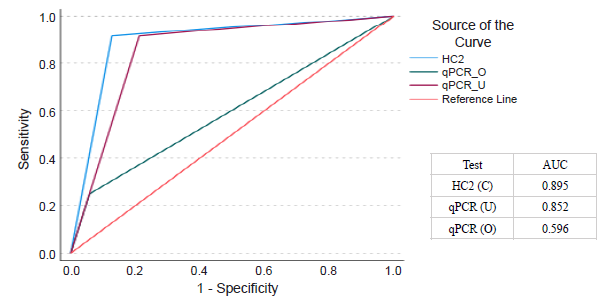
- Comparison of receiver operating characteristic (ROC) curves for Hybrid Capture 2 (HC2) assays for cervical (C) and quantitative real time PCR (qPCR) for urine (U) and oral gargle sample (O) with reference to Pap as gold standard technique. AUC, area under curve.
Discussion
This comparative report from the North-Eastern region of India for the first time, elicits that urine and oral gargle samples and qPCR as a technique for detecting hr-HPV may be considered for screening programs related to cervical and oral malignancies.
Cytological abnormality was detected in 6 per cent of the participants, while 17.5 per cent women tested positive for hr-HPV using HC2.This was comparable to previous isolated studies reporting Pap abnormalities of 8 per cent in Uttar Pradesh, Central India13 and 16 per cent hr-HPV positivity in a study conducted in Assam, North East India14. Several Indian studies conducted at different geographical location (Northern, Eastern, and Southern India) have reported the hr-HPV positivity rates between 6-18 per cent by HC2 in both asymptomatic and symptomatic women14-16. The variations could be attributed to study settings, testing techniques and ethnic and geographical differences in HPV prevalence.
Genital hr-HPV was detected in 25 per cent urine of women by qPCR. This positivity rate was considerably higher compared to a study of Karnataka, India, reporting 0.4 per cent of genital HPV infection using nested PCR in 201617. The efficacy of HPV-DNA testing on urine has been reported in a few studies17,18. However, its validity in comparison to that assessed by cervical samples from the same individual has not been thoroughly investigated. Our study compared the HPV infection rates in urine and oral gargle samples by qPCR and cervical samples by HC2 from the same group. A trend of higher genital hr-HPV detection in qPCR than by HC2 was observed, as reported earlier19.Among women with HPV-positive urine samples, women infected with a single genotype (62.7%) were comparably greater than those with multi-genotype infection (37.5%), which was similar to those reported by Nilyanimit et al20.
Oral HPV infection in women (7%) was found to be of similar magnitude with that in previously studies (7.5%)21. Different studies across India have reported HPV positivity in Head and Neck Cancers ranging from 7-78 per cent using different modalities of detection, with the wide range of findings attributed to the variation in study population. Some studies were reports on clinically diagnosed cancer patients22. Consistent to previous studies, hr-HPV 16 was the most predominant genotype for both urine and oral gargle samples23,24.
When compared to Pap-cytology, a higher sensitivity and specificity for HC2, 91.7 and 87.2 per cent respectively were observed by us than the study by Kulmala et al19 reporting sensitivity and specificity of 85.2 and 67.2 per cent respectively for HC225. Different studies on HPV detection using urine reported sensitivity of 90 per cent and a specificity of 70 per cent in identifying female genital infection17,26. Similar were the findings of the present study confirming that HPV detection was possible (sensitivity 91.7 per cent and specificity of 78.7 per cent respectively) by qPCR in urine of females.
Although a moderate agreement was observed for overall hr-HPV detection in urine and oral gargle samples of women by qPCR (k=0.5), there were discordant results among the three techniques. The level of agreement was considerably low for hr-HPV detection in urine by qPCR when compared the cervical samples tested by Pap or HC2 (k<0.2). The two evaluated assays showed similar performance characteristic for HC2 assay (AUC=0.895), and qPCR for urine (AUC=0.852) for the detection of hr-HPV confirming to have good measure of separability as compared to that of qPCR (oral gargle samples). The performance for HC2 was similar to a study report (AUC=0.858)25.
The overall hr-HPV infection rates in men was 8 per cent in urine and 5 per cent in oral gargle samples, comparable to previously reported 9 per cent for urine27 and 4.8 per cent for oral samples28. However, unlike the other studies reporting multi-genotype infection, and HPV 16 as most predominant type27 single infection, HPV 35 and 33 were the most predominant types detected in both urine and gargle sample of men. Performance characterization could not be done for urine and oral gargle samples for lack of gold standard for comparison, and a good agreement was observed for overall hr-HPV detection in urine and oral gargle samples of men by qPCR (k=0.75).
This study demonstrated the presence of HPV 16, 39, 45, 68, 35, 58 as the most common hr-HPV genotypes, thus highlighting the need for more thorough HPV genotyping to tailor preventive measures including prioritizing vaccination. Different detection techniques in different samples have various implications, including sensitivity and specificity, HPV genotypes, multiplexing, sample types, cost and resources, validation, and clinical decision-making. Techniques like HC2 target pooled HPV genotypes, while multiplex PCR allows simultaneous detection of multiple HPV types in a single sample. Sample types, such as cervical samples, may require different collection and testing methods. When compared with Pap, qPCR and HC2 showed greater positivity rate for hr-HPV detection. The study limitations included the socio-cultural and ethno-geographical influences, inability to do hr-HPV genotyping of cervical samples, cross-sectional study design and inability to examine hr-HPV persistence and lack of gold standard for oral cancer. The study strengths included sample size and quality controls for every stage of the HPV-DNA assays. HC2 and qPCR were conducted on separate biological specimens, and differences in the buffer used for cervical and urine/oral gargle samples may have led to analytical limitations that couldn’t be adjusted for.
Sikkim is one of the foremost States implementing HPV vaccination programme, targeting females (9-14 yr). The outcomes of this study should prompt medical practitioners to implement readily accessible and cost-effective screening modalities to improve early detection and management of HPV infection-associated malignancies.
Acknowledgment
The authors thank Prof. (Dr.) Bhudev Das, Director, Amity Institute of Molecular Medicine & Stem Cell Research, Amity, and ICMR-National Institute of Cancer Prevention and Research, Noida, India for technology transfer through training of the research scholars on molecular techniques.
Financial support & sponsorship
The authors are thankful to the Indian Council of Medical Research Project (79/7/NE/PROJ/2016/NCD-III), Biotech Hub project, Department of Biotechnology- North Eastern Region-Biotechnology Programme Management Cell (BT/04/NE/2009) and Sikkim Manipal Institute of Medical Sciences (SMIMS) for the financial and infrastructure support.
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript, and no images were manipulated using AI.
References
- 2022. Pinkbook, HPV Epidemiology of Vaccine Preventable Diseases, CDC. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/hpv.html, accessed on April 12, 2023.
- 2020. Human Papillomavirus (HPV) Infection. Available from: https://www.cdc.gov/hpv/about/index.html, accessed on October 14, 2022.
- Human Papillomavirus. Available from: https://hpvcentre.net, accessed on October 14, 2022.
- The human papillomavirus infection in men study: Human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:2036-43.
- [Google Scholar]
- Human papillomavirus as a driver of head and neck cancers. Br J Cancer. 2020;122:306-14.
- [Google Scholar]
- New recommendations for screening and treatment to prevent cervical cancer. Available from: https://www.who.int/news/item/06-07-2021-new-recommendations-for-screening-and-treatment-to-prevent-cervical-cancer, accessed on April 15, 2023.
- Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579-88.
- [Google Scholar]
- Cervical cancer screening: Less testing, smarter testing. Cleve Clin J Med. 2011;78:737-47.
- [Google Scholar]
- Human papillomavirus (HPV) DNA detection in self-collected urine. Int J Gynaecol Obstet. 2005;90:223-7.
- [Google Scholar]
- Genomic DNA extraction from different human tissue sample: Urine, oral gargle, blood, and cervical for real time amplification by qPCR. Ind J Clin Biochem. 2024;39:1-9.
- [Google Scholar]
- Qiagen. Digene HC2 high-risk HPV DNA test. 2021. Available from: https://www.qiagen.com/us/products/diagnostics-and-clinical-research/sexual-reproductive-health/cervical-cancer-screening/digene-hc2-high-risk-hpv-dna-test, accessed on December 30, 2022.
- AmpliSens. Real time PCR manual. Available from: https://www.pcrdiagnostics.eu/data/wysiwyg/Manu%C3%A1ly/HPV%20HCR%20genotype-titre-FRT_ME_240321.pdf, accessed on October 13, 2022.
- A study on cervical cancer screening using pap smear test and clinical correlation. Asia Pac J Oncol Nurs. 2018;5:337-41.
- [Google Scholar]
- Hybrid capture 2 assay based evaluation of high-risk HPV status in healthy women of North-East India. Asian Pac J Cancer Prev. 2014;15:861-5.
- [Google Scholar]
- Association of chlamydia trachomatis infection with human papillomavirus (HPV) & cervical intraepithelial neoplasia - A pilot study. Indian J Med Res. 2013;137:533-9.
- [Google Scholar]
- Prevalence of high-risk human papillomavirus and cervical intraepithelial neoplasias in a previously unscreened population–a pooled analysis from three studies. Int J Cancer. 2013;132:1693-9.
- [Google Scholar]
- Detection of genital HPV infection using urine samples: A population based study in India. Asian Pac J Cancer Prev. 2016;17:1083-8.
- [Google Scholar]
- The utility of urine-based sampling for cervical cancer screening in low-resource settings. Asian Pac J Cancer Prev. 2019;20:2409-13.
- [Google Scholar]
- Human papillomavirus testing with the hybrid capture 2 assay and PCR as screening tools. J Clin Microbiol. 2004;42:2470-5.
- [Google Scholar]
- Comparison of human papillomavirus (HPV) detection in urine and cervical swab samples using the HPV GenoArray diagnostic assay. Peer J Inc. 2017;5:e3910.
- [Google Scholar]
- Oral sex practices, oral human papillomavirus and correlations between oral and cervical human papillomavirus prevalence among female sex workers in Lima, Peru. Int J STD AIDS. 2011;22:655-8.
- [Google Scholar]
- Prevalence and impact of human papillomavirus on head and neck cancers: Review of Indian studies. Indian J Surg Oncol. 2018;9:568-75.
- [Google Scholar]
- Role of HPV genotype, multiple infections, and viral load on the risk of high-grade cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2019;28:1816-24.
- [Google Scholar]
- HPV Genotypes distribution in Indian women with and without cervical carcinoma: Implication for HPV vaccination program in Odisha, Eastern India. BMC Infect Dis. 2017;17:30.
- [Google Scholar]
- Human papillomavirus testing with the hybrid capture 2 assay and PCR as screening tools. J Clin Microbiol. 2004;42:2470-5.
- [Google Scholar]
- HPV type-specific prevalence using a urine assay in unvaccinated male and female 11- to 18-year olds in Scotland. Br J Cancer. 2011;104:1221-6.
- [Google Scholar]
- Human papillomavirus prevalence in urine samples of asymptomatic male sexual partners of women with sexually transmitted diseases. Int J Environ Res Public Health. 2021;18:11706.
- [Google Scholar]
- Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199:1263-9.
- [Google Scholar]






