Translate this page into:
Estimation of radiation dose to patients from 18FDG whole body PET/CT investigations using dynamic PET scan protocol
Reprint requests: Dr B.S. Dwarakanath, Sri Ramachandra University, Porur, Chennai 600 116, India e-mail: dwarakanathdrbs@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
There is a growing concern over the radiation exposure of patients from undergoing 18FDG PET/CT (18F-fluorodeoxyglucose positron emission tomography/computed tomography) whole body investigations. The aim of the present study was to study the kinetics of 18FDG distributions and estimate the radiation dose received by patients undergoing 18FDG whole body PET/CT investigations.
Methods:
Dynamic PET scans in different regions of the body were performed in 49 patients so as to measure percentage uptake of 18FDG in brain, liver, spleen, adrenals, kidneys and stomach. The residence time in these organs was calculated and radiation dose was estimated using OLINDA software. The radiation dose from the CT component was computed using the software CT-Expo and measured using computed tomography dose index (CTDI) phantom and ionization chamber. As per the clinical protocol, the patients were refrained from eating and drinking for a minimum period of 4 h prior to the study.
Results:
The estimated residence time in males was 0.196 h (brain), 0.09 h (liver), 0.007 h (spleen), 0.0006 h (adrenals), 0.013 h (kidneys) and 0.005 h (stomach) whereas it was 0.189 h (brain), 0.11 h (liver), 0.01 h (spleen), 0.0007 h (adrenals), 0.02 h (kidneys) and 0.004 h (stomach) in females. The effective dose was found to be 0.020 mSv/MBq in males and 0.025 mSv/MBq in females from internally administered 18FDG and 6.8 mSv in males and 7.9 mSv in females from the CT component. For an administered activity of 370 MBq of 18FDG, the effective dose from PET/CT investigations was estimated to be 14.2 mSv in males and 17.2 mSv in females.
Interpretation & conclusions:
The present results did not demonstrate significant difference in the kinetics of 18FDG distribution in male and female patients. The estimated PET/CT doses were found to be higher than many other conventional diagnostic radiology examinations suggesting that all efforts should be made to clinically justify and carefully weigh the risk-benefit ratios prior to every 18FDG whole body PET/CT scan.
Keywords
Cumulated activity
effective dose
18FDG
PET/CT
residence time
Positron emission tomography/computed tomography (PET/CT) is an imaging modality that acquires functional (PET) and anatomical (CT) information of a patient within a single examination12. However, PET/CT investigations lead to exposure of patients from the internally administered PET radiopharmaceutical and externally from the X-rays generated by the CT1. Due to this fact, it is a challenging area of radiation safety in diagnostic radiation medicine3. The radiation dose to the patient from a PET/CT scan depends on the PET/CT protocol, the patient's size and physiology, amount of injected activity and the make and model of the PET/CT scanner4. The combined PET/CT examination results in an increased radiation dose to patients as compared to stand alone components of PET/CT scan and also other conventional diagnostic radiology examinations25. The effective doses from PET/CT investigations are reported to be 25 mSv1, and 13.45 - 31.91 mSv for female patients and 13.65 – 32.18 mSv for male patients from three different PET/CT protocols2.
Amongst all the radiopharmaceuticals developed so far for PET imaging, 18F-fluorodeoxyglucose (18FDG) has widespread application and is most commonly used. Although several dose estimates in humans have been reported from internal administration of 18FDG, most of these are based on the bio-distribution studies in animals6789 or by combining data from animals and measurements in humans10111213141516. Kinetics of 18FDG distribution has also been reported using segmented dynamic PET scan protocols, whole body PET scans at different time intervals or using thermoluminescent dosimeters (TLD)17181920212223. However, Niven et al18 have provided comprehensive data/information on 18FDG kinetics and radiation dosimetry using dynamic PET scan protocol for both the genders involving many patients. The objective of the present study was to study the kinetics of 18FDG distributions in male and female patients, to estimate the internal dose from administered 18FDG, external dose from the CT component and total effective dose from PET/CT investigations.
Material & Methods
The clinical PET/CT studies were performed on a 16-slice PET/CT system (Discovery STE, M/s GE, USA) installed at Division of PET Imaging, Institute of Nuclear Medicine and Allied Sciences, Delhi, India, during May 2009 to June 2012. The PET/CT system has Lightspeed 16 as the model of the CT system. The study protocol was approved by the Ethical Committee of the Institute and the study was carried out after obtaining written informed consent of the patients. Effective dose from PET/CT investigations have been reported based on thermo luminescent dosimetry (TLD) measurements on anthropomorphic phantoms and by using established coefficients for 18FDG12. A couple of studies have reported effective dose from CT component using softwares like WinDose and internal dose from 18FDG by using medical internal radiation dose (MIRD) method either by using established biokinetic models or by obtaining kinetics of 18FDG distribution using dynamic PET protocols1822. In the present study, the effective dose from PET/CT investigations was estimated based on kinetics of 18FDG distribution in patients obtained using dynamic PET scan protocol and CT dose using the software CT-Expo (Version 2.0, Medizinische Hochschule, Hannover, Germany).
Internal dosimetry:
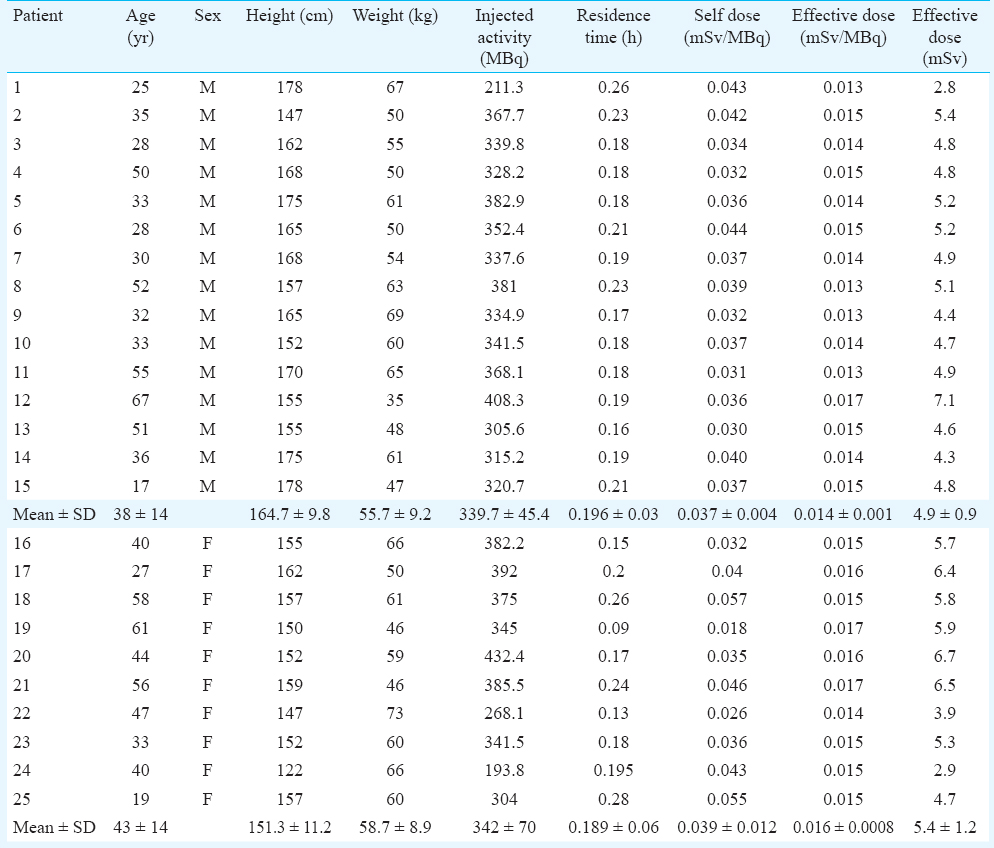
Subjects - A total of 49 patients (27 male, 22 female) were included in this study. The mean age of male patients was 40.85 ± 16.4 yr (age range 17-67 yr) and for female patients was 43.4 ± 13.2 yr (age range 19-68 yr). The average weight and height of the male patients were 58 ± 9.2 kg and 165 ± 8 cm, respectively and for female patients 57.1 ± 9.4 kg and 152.5 ± 8.9 cm, respectively. The patients were oncological cases in remission who could comfortably lie down on the couch for the study period of 75 min without movement. As per the clinical protocol, the patients were refrained from eating and drinking for a minimum period of 4 h prior to their studies.
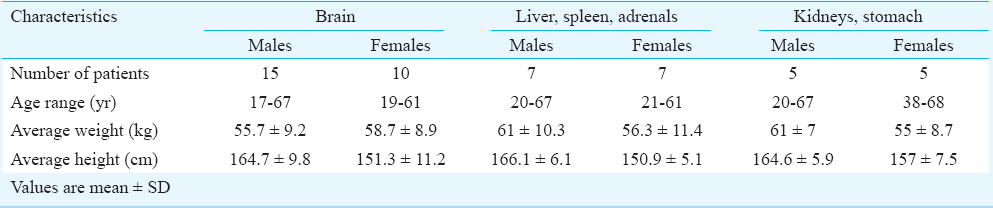
PET scanning protocol - The 16-slice PET/CT system has a spatial resolution of 5.0 mm in all directions and an axial field of view (AFOV) of 15 cm. It operates in 2D as well as 3D mode. For the present study, the data were acquired in 3D mode. A radioactive pin source of 68Ge (55.5 MBq) was used for system calibration and daily quality control. PET protocol involving dynamic acquisition of temporal images was used. Dynamic PET images were acquired in three different regions of the body for 75 min at five min per frame so as to study variation in 18FDG activity with respect to time in six organs namely brain, liver, spleen, adrenals, kidneys and stomach. The images were reconstructed using a fully 3D ordered subset expectation maximization (3D-OSEM) algorithm with all corrections (scatter, random, dead time, attenuation and normalization) incorporated into the iterative reconstruction scheme. The data were not decay corrected. Since the axial field of view (AFOV) of the PET scanner was only 15 cm, it was not possible to obtain uptake in all the six organs simultaneously. The study was divided into three sets of patients in which only brain; liver, spleen and adrenals together and kidneys and stomach together were studied. The characteristics of the male and female patients on whom dynamic PET scans were performed are described in Table I.
Description of measurements - Regions of interest (ROI) were drawn around the source organs for all planes that contained them. The volume of the source organs was estimated by using CT volume rendering technique. The average uptake of radioactivity in terms of Bq/ml was obtained for each frame. The total uptake of radioactivity (in Bq) was obtained by multiplying this with the estimated volume of the organs for each subject. Time activity curves were produced for entire organ volumes. To obtain cumulated activity in the source organs, time activity curves were integrated using the trapezoidal rule1824. 18FDG was assumed to be fixed in the patient at completion of the scan and that it decayed only by its physical half-life thereafter182526. Analytical integration was performed on this single exponential decay and then summed with the numerical integration to yield the total cumulated activity in the source organ as follows:
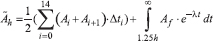
Where Ãh = cumulated activity in the source organ (MBq-h); Ai = activity in the source organ (in MBq) at the ith time frame; Af = activity in the source organ (in MBq) at the last time point of measurement (1.25 h); and λ = physical decay constant.
The cumulated activity for the remainder of the body was calculated as the difference between the cumulated activity in the total body and the sum of the cumulated activities in the source regions. Assuming instantaneous uptake and an effective half-life of 1.83 h, the cumulated activity in the total body was calculated as18: Ãtb =1.443 A0 × Teff, where Ãtb = cumulated activity in the total body (in MBq h); A0 = injected activity (in MBq); and Teff = effective half-life (in h). Thus, the cumulated activity for the remainder of the body was: 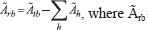 cumulated activity for the remainder of the body (in MBq h); and Ãh = cumulated activity in the source organs (in MBq h).
cumulated activity for the remainder of the body (in MBq h); and Ãh = cumulated activity in the source organs (in MBq h).
The residence time, τh, defined as the total number of disintegrations per unit administered activity was calculated by dividing the cumulated activity Ãh by the injected activity A0. Thus.
injected activity A0. Thus 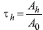
Absorbed dose estimates - The absorbed dose to the organs was estimated using Java based Organ Level Internal Dose Assessment/Exponential Modelling (OLINDA/EXM) Code (Version OLINDA/EXM 1.0, Vanderbilt University, Nashville, TN, USA). The input parameters required by the OLINDA software for the estimation of the absorbed dose are the type of radionuclide, a phantom (or phantoms), and the number of disintegrations per unit administered activity (or residence time). The type of radionuclide chosen for the present study was 18F and the adult male and female phantoms were used for absorbed dose estimation. The residence time calculated as per the methodology described above was used as input for the source organs and remainder of body. The volume of the source organs estimated using CT volume rendering technique was used to estimate the subject specific mass of the source organs by multiplying the measured volume of the source organs of each subject with their respective specific gravities2728. The S-values found in the OLINDA software are specific to adult male and female phantoms having total body weight of 73.7 kg and 56.9 kg, respectively. Since the estimated mass of the source organs and total mass of the subjects differed from the standard phantoms used in the software, the masses were accordingly modified for all the subjects for the purpose of dose estimation.
CT dosimetry
The methodology for CT dosimetry was essentially according to Kaushik et al23. Briefly, the radiation dose from the CT component was calculated using the software CT-Expo (Version 2.0, Medizinische Hochschule, Hannover, Germany) for the low dose CT protocol for which the scan parameters used were 120 kV, 110 mA, rotation time 0.8 sec, beam collimation 10 mm, pitch 1.75 and the slice thickness was 0.625 mm. The scanner output was measured in terms of weighted Computed Tomography Dose Index (CTDIw) using CTDI phantom and ionization chamber (M/s RADCAL Corporation, USA). Volume Computed Tomography Dose Index (CTDIvol), Dose Length Product (DLP) and effective dose were derived from the measured parameters for the scanned length of 90 cm (6 PET bed positions) and 102 cm (7 PET bed positions).
Results
Internal dosimetry:
Brain - Of the 49 patients, dynamic PET scans of brain were performed on 15 male and 10 female patients (Table I). Considering the specific gravity of 1.03 g/cm3, the average mass of the brain of the male patients was estimated to be 1242.9±100.3 g whereas the average mass of the brain of females was estimated to be 1101.7±57.7 g27. The relative weight of the brain to total body was estimated to be 2.3 ± 0.5 per cent for males and 1.93±0.4 per cent for females. A time-activity curve depicting the uptake of activity for an adult male and female brain is shown in Fig. 1. 18FDG got rapidly incorporated in brain and its average uptake was observed to be 7.41±0.99 per cent in male brain and 6.965 ± 2.39 per cent in female brain. The average residence time for males and females brain was estimated to be 0.196±0.03 h and 0.189±0.059 h, respectively. Based on the physical half-life of 1.83 h for 18FDG and assuming no elimination from the body, the total body residence time for 18FDG was calculated to be 2.64 h. This yielded average residence time for the remainder of the body to be 2.44±0.027 h for males and 2.45±0.06 h for females. The average self dose to the brain of male subjects was 0.037±0.004 mSv/MBq and to female brain was 0.039±0.012 mSv/MBq. The average total dose to brain that includes contribution from remainder of body was 0.039±0.004 mSv/MBq for males and 0.043±0.012 mSv/MBq for females. Patient characteristics, biokinetic data in brain and the associated radiation dose received by this group of patients is provided in Table II.
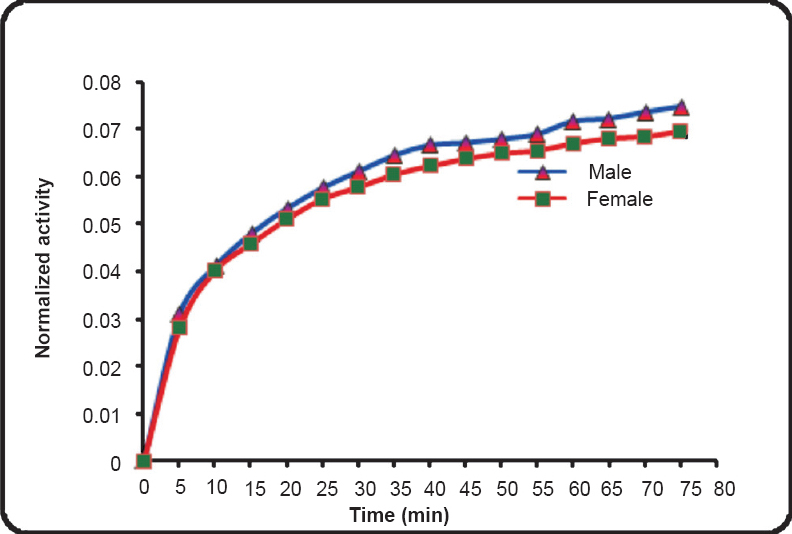
- Representative time activity curves depicting the uptake of activity in adult male and female brain.
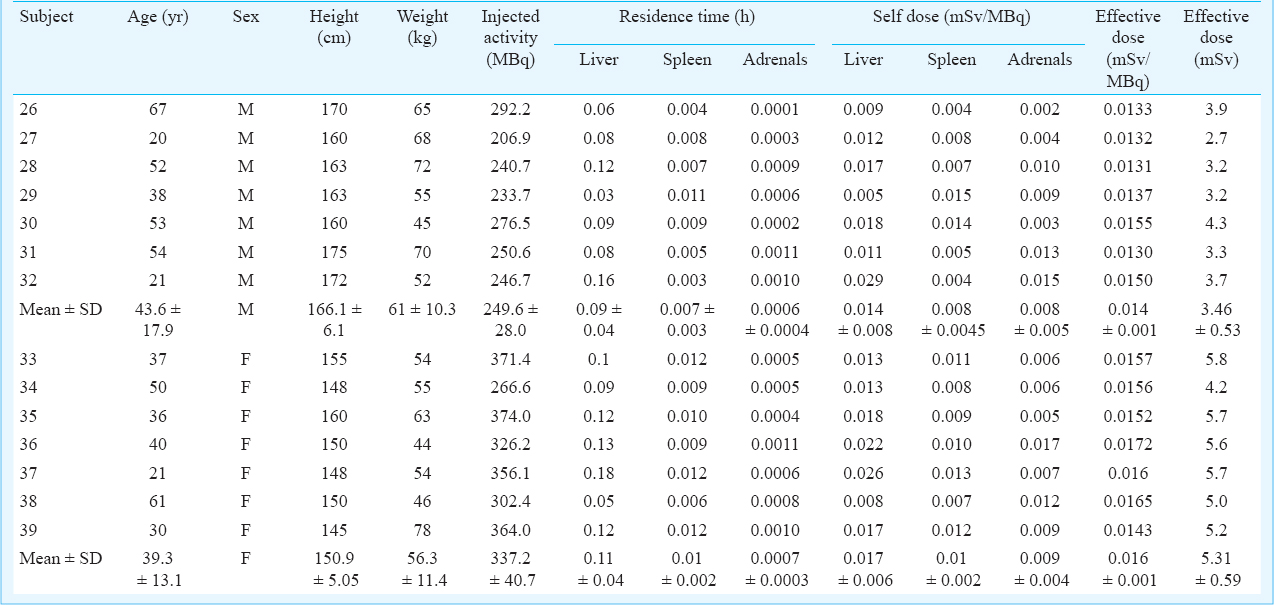
Liver, spleen and adrenals - Dynamic PET scans were performed on seven males and seven females so as to obtain uptake of 18FDG activity with respect to time in liver, spleen and adrenals (Table I). Given densities of 1.053 g/cm3 for the liver28, 1.06 g/cm3 for the spleen and 1.02 g/cm3 for the adrenals27, the organ volumes determined from the planar images were converted to organ masses. The average organ masses of the male subjects were estimated to be 1392.8±198 g for liver, 158.5±28.3 g for spleen and 11.8±1.7 g for adrenals and for females were estimated to be 1551.1±157.03 g for liver, 190.7±27.98 g for spleen and 12.54±2.13 g for adrenals. The average residence times calculated for the adult males were 0.09±0.04, 0.007±0.003 and 0.0006±0.00041 h for the liver, spleen and adrenals, respectively. The average residence times calculated for the adult females were 0.11±0.04, 0.01±0.002 and 0.0007±0.0003 h for the liver, spleen and adrenals, respectively and was 2.54±0.04 and 2.52±0.04 h for the remainder of the body for the males and females, respectively. The time-activity curves of 18FDG in liver, spleen and adrenals in males and females are depicted in Fig. 2a and 2b, respectively. The percentage uptake in male liver ranged from a maximum value of 8.4 to 3 per cent and in female liver ranged from a maximum value of 6.35 to 3 per cent, in spleen from a maximum value of 0.5 to 0.15 per cent in males and 0.7 to 0.3 per cent in females and in adrenals from 0.02 to 0.007 per cent in males and 0.03 to 0.015 per cent in females. Patient characteristics, biokinetic data in adult males and females for liver, spleen and adrenals and associated radiation dose received by this group of patients are provided in Table III.
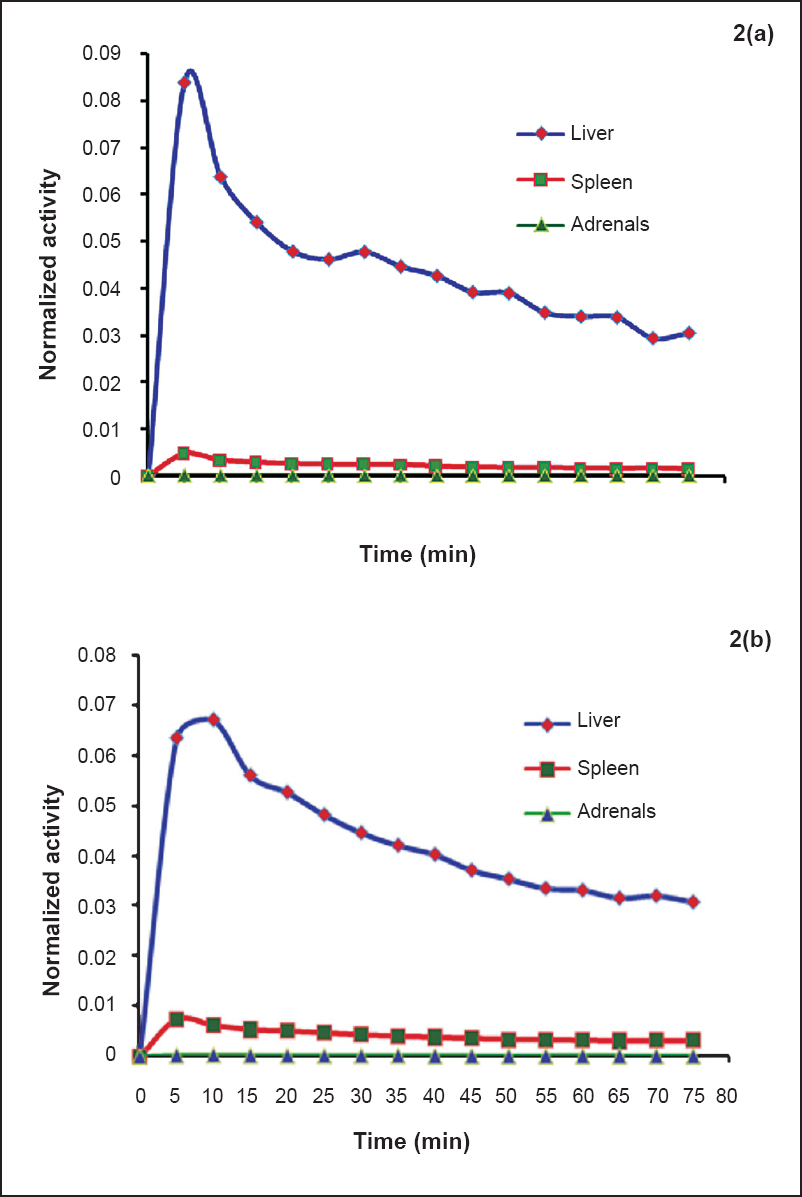
- Time activity curves normalized to the administered activity for adult males (2a) and females (2b) liver, spleen and adrenals.
Kidneys and stomach - Dynamic PET scans were performed in five males and five female patients to obtain time course of activity in stomach and kidneys (Table I). Given densities of 1.05 g/cm3 for both stomach and kidneys27, the average mass of the kidney was estimated to be 153.5±14.7 g for the males and 140.6±19.8 g for the females and the average mass of stomach was estimated to be 138.9±20.97 g for the males and 117.2±13.7 g for the females. The time-activity curves for both the organs are shown in Fig. 3a and 3b. The average residence time values calculated for the adult males were 0.013±0.010 and 0.005±0.002 h for the kidneys and stomach, respectively. The average residence time values for the adult females were 0.02 ± 0.008 and 0.004 ± 0.001 h for the kidneys and stomach, respectively. The average residence times for the remainder of the body was estimated to be 2.622±0.011 and 2.616±0.007 h for the males and females, respectively. The average self-dose to the kidneys of males was estimated to be 0.0151±0.014 mSv/MBq and total dose to be 0.021±0.015 mSv/MBq. The average self-dose to the kidneys of females was estimated to be 0.025±0.013 mSv/MBq and total dose to be 0.039±0.0135 mSv/MBq. The average self-dose to the stomach of males was 0.0020±0.0009 mSv/MBq and total dose was 0.017±0.002 mSv/MBq. The average self-dose to the stomach of females was 0.0019±0.0005 mSv/MBq and total dose was 0.019±0.003 mSv/MBq. The percentage uptake in male kidneys ranged from 0.6 to 0.15 per cent and in female kidneys ranged from 0.9 to 0.4 per cent. An uptake of 0.24 per cent was observed in male stomach and 0.14 per cent in female stomach. Patient characteristics, biokinetic data in adult males and females for kidneys and stomach and associated radiation dose received by this group of patients are provided in Table IV.
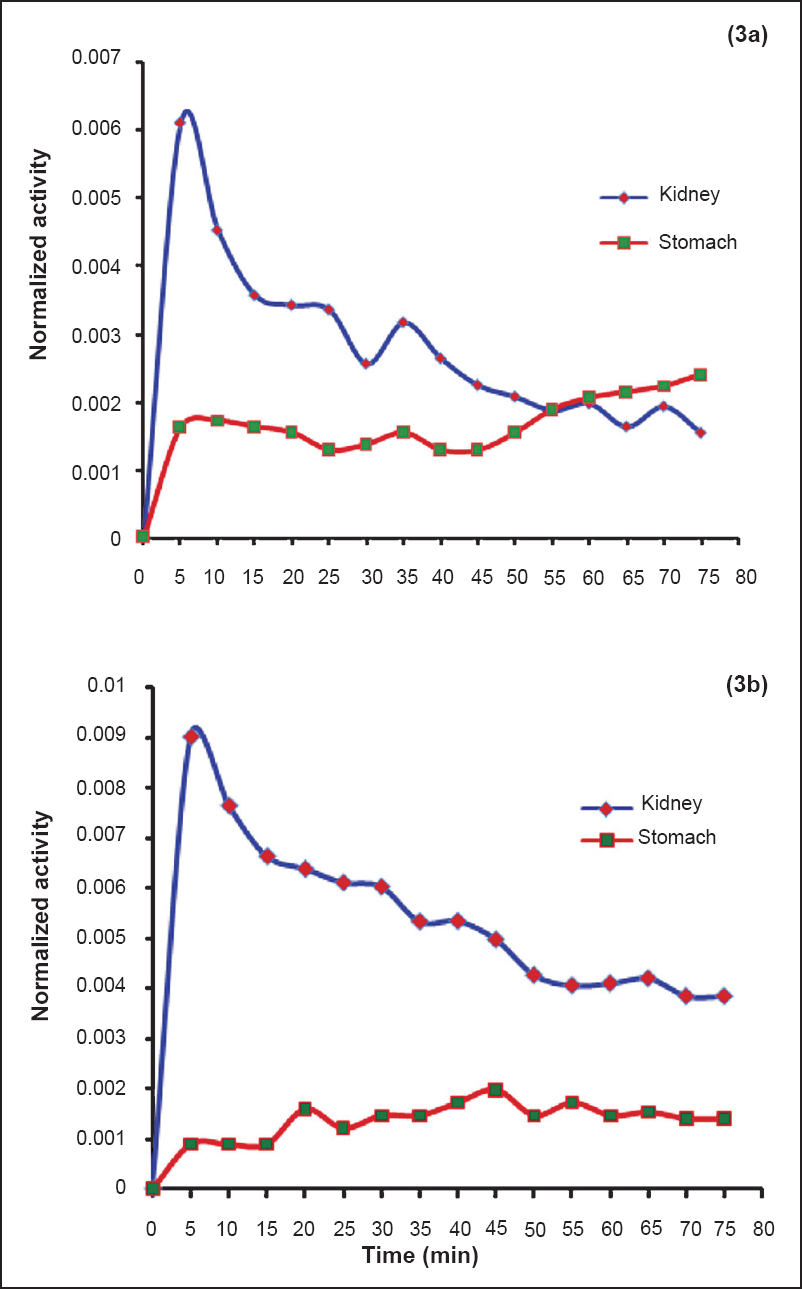
- Time activity curves normalized to the administered activity for adult males (3a) and females (3b) kidneys and stomach.

Effective dose - In addition to uptake in brain, liver, spleen, adrenals, kidneys and stomach as presented in this study, uptake in heart, lungs and urinary bladder is also reported to be high21. Effective dose was also estimated by using the residence times of 0.11, 0.079 and 0.26 h in these three organs, respectively along with the residence times in the six organs calculated in the present study. The effective dose coefficient was calculated to be 0.020 mSv/MBq in males and 0.025 mSv/MBq in females. For an administered 18FDG activity of 370 MBq, the effective dose was estimated to be 7.4 mSv for males and 9.25 mSv for females. The difference in residence time and radiation dose between males and females was calculated using a two-tailed t-test. This difference was not found to be significant for all the organs except spleen wherein significant difference (P<0.05) was observed in the residence times of males and females.
CT dosimetry: The effective dose from the low dose CT protocol used in PET/CT investigations was found to be 6.8 mSv for male patients and 7.9 mSv for female patients.
PET/CT dosimetry: The total radiation dose from 18FDG whole body PET/CT investigations ranged from 10.1 to14.5 mSv for male patients and 10.7 to 14.5 mSv for female patients. Based on the residence times in source organs calculated in this study and published values in heart, lungs and urinary bladder21, the PET/CT doses were estimated to be 14.2 mSv for males and 17.2 mSv for females.
Discussion
A number of studies on absorbed dose to different organs from internally administered 18FDG based on kinetics of 18FDG distribution in humans have been reported1011121314151617181920212223. But there have been very few studies that provide the type of data on kinetics of 18FDG distribution in humans that is needed for internal dosimetry calculations for both the genders. A comparison between residence time and absorbed dose to male and female brains is made only in one study where significant sex difference in the residence times and absorbed dose to the brain from 18FDG has been reported18. In the present study, no significant difference in the residence time and absorbed dose to male and female organs was observed except for spleen.
In spite of similar biodistribution in males and females, females receive higher dose for the same administered activity due to a difference in the S-values29. In the present study, percentage uptake in male brain was observed to be higher than female brain and the residence time in male brain was also observed to be slightly higher than female brain. However, for a given amount of administered activity, the total dose to the female brain was slightly higher than the male brain. The difference in the residence times of activity in the male and female brain was not significant implying no difference in the biodistribution of 18FDG in the brains of males and females. This may be due to difference in the type of subjects/patients chosen for the study that may affect the kinetics of the radiopharmaceutical. The relative weight of the brain to the total body was 2.6 per cent for Japanese adults and 2 per cent for European and American adults, whereas it was estimated to be 2.3 per cent in the present study2730. It was interesting to note that although two male subjects included in the present study showed lesions in the brain, the uptake and hence, the residence time in the brain of these patients were not found to be substantially different from others. The normal brain itself has a high background accumulation of 18FDG on 18FDG-PET scanning and hence the amount of uptake in lesion was not significantly different from normal brains. The residence times in male and female liver were lower than the reported values though no study has reported the values for female patients1921. However, the absorbed doses to male and female liver were comparable to the values reported by International Commission on Radiological Protection (ICRP) (0.021 mGy/MBq)24. ICRP 106 has used an initial uptake of 18FDG as 5 per cent for liver21 whereas it was 8.4 per cent for males and 6.35 per cent for females in the present study. The residence times observed in male and female spleen in the present study were comparable to the reported values19, although the values were observed to be significantly different for males and females. Significant difference was not noted in the dose to male and female spleens but the dose to spleen was observed to be higher than the reported values21. The residence times in male adrenals and female adrenals could be compared with only one study where the reported value was 0.001 h14. The average dose to adrenals was observed to be higher than the published values1421.
The residence time in kidneys in the present study was observed to be lesser than reported value of 0.0319. The dose to kidneys was observed to be higher than the values reported in ICRP 10621. The residence time in stomach was reported in only one study14. The value of residence time of 18FDG in stomach in the present study was observed to be lesser than the reported value. However, the corresponding dose to stomach was observed to be higher21. The PET doses estimated in the study were patient specific and effective dose coefficients estimated were slightly higher as compared to the values reported in ICRP 10621.
Since it was not possible to adapt the scan length to body size of the individual patient, the CT doses estimated in the present study were for the axial CT range that could be set up only in integer multiples (6 or 7 PET bed positions) of the fixed AFOV (15 cm in the present study). The CT doses estimated in the study using the Software CT-Expo were validated by phantom measurements using an ionization chamber. The effective doses from the CT component of whole body PET/CT studies computed in this study (7-8 mSv) were comparable to the earlier findings122223. The total effective dose from the PET/CT scanning was slightly lower than the values reported earlier12 because of the differences in the CT scan parameters.
In conclusion, the present study did not demonstrate significant difference in the kinetics of 18FDG distribution in male and female patients. The PET/CT doses were found to be higher than many other conventional diagnostic radiology examinations suggesting that all efforts should be made to clinically justify and carefully weigh the risk-benefit ratios prior to every 18FDG whole body PET/CT investigation.
Acknowledgment
Authors thank Dr R.P. Tripathi, Director, INMAS for his support. Technical support provided by Shriyut Sanjiv Saw, Dinesh Singh and Santosh Pandey, of the division of PET Imaging is gratefully acknowledged. The work was supported by a grant from Defence Research and Development Organisation (DRDO), Government of India, New Delhi (TC/2519/INM-03/2010).
Conflicts of Interest: None.
References
- Radiation exposure of patients undergoing whole-body dual-modality [18] F-FDG PET/CT examinations. J Nucl Med. 2005;46:608-13.
- [Google Scholar]
- Whole-body PET/CT scanning: estimation of radiation dose and cancer risk. Radiology. 2009;251:166-74.
- [Google Scholar]
- Radiation protection and dosimetry in PET and PET/CT. In: Valk PE, Delbeke D, Bailey DL, Townsend DW, Maisey MN, eds. Positron emission tomography. London: Springer; 2006. p. :41-62.
- [Google Scholar]
- International Atomic Energy Agency (IAEA) In: Radiation protection in newer medical imaging techniques: PET/CT. Safety Reports Series No. 58. Vienna, Austria: IAEA; 2008.
- [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Vol. 1. In: Sources – Report to the General Assembly, Scientific Annexes A and B. Vienna, Austria: UNSCEAR; 2008.
- [Google Scholar]
- Radiopharmaceuticals XXVII. 18F-labeled 2-deoxy-2-fluoro-D-glucose as a radiopharmaceutical for measuring regional myocardial glucose metabolism in vivo: tissue distribution and imaging studies in animals. J Nucl Med. 1977;18:990-6.
- [Google Scholar]
- The [18F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circ Res. 1979;44:127-37.
- [Google Scholar]
- [18F]fluorodeoxyglucose method for measuring local cerebral glucose metabolism in man: technique and results. Prog Nucl Med. 1981;7:138-48.
- [Google Scholar]
- Preliminary imaging results with 18F-2-fluoro-2-deoxy-D-glucose. J Comput Assist Tomogr. 1980;4:473-7.
- [Google Scholar]
- The radiation dosimetry of 2-[18F]fluoro-2-deoxy-D-glucose in man. J Nucl Med. 1982;23:613-7.
- [Google Scholar]
- Re: The radiation dosimetry of 2.[18F] fluoro-2-deoxy-D-glucose in man. J Nucl Med. 1983;24:447-8.
- [Google Scholar]
- International Commission on Radiological Protection (ICRP). Radiation dose to patients from radiopharmaceuticals. ICRP Publication 53. Ann ICRP. 1988;18:1-4.
- [Google Scholar]
- Estimation of absorbed doses in humans due to intravenous administration of fluorine-18-fluorodeoxyglucose in PET studies. J Nucl Med. 1991;32:699-706.
- [Google Scholar]
- Estimation of absorbed dose for 2-[F-18]fluoro-2-deoxy-D-glucose using whole-body positron emission tomography and magnetic resonance imaging. Eur J Nucl Med. 1998;25:565-74.
- [Google Scholar]
- Internal absorbed dose estimation by a TLD method for [18] F-FDG and comparison with the dose estimates from whole body PET. Phys Med Biol. 1999;44:595-606.
- [Google Scholar]
- International Commission on Radiological Protection (ICRP). Radiation dose to patients from radiopharmaceuticals (Addendum 2 to ICRP Publication 53) Ann ICRP. 1998;28(3):1-126. ICRP Publication 80. Canada: ICRP; 1998
- [Google Scholar]
- A mathematical model for the distribution of fluorodeoxyglucose in humans. J Nucl Med. 1999;40:1358-66.
- [Google Scholar]
- Absorbed dose to the adult male and female brain from 18F-fluorodeoxyglucose. Health Phys. 2001;80:62-6.
- [Google Scholar]
- MIRD dose estimate report no.19: radiation absorbed dose estimates from [18] F-FDG. J Nucl Med. 2002;43:210-4.
- [Google Scholar]
- Assessment of absorbed dose of PET (18F-FDG) scanning patients with MIRD and TLD methods. Ann Nucl Med. 2007;20:137-43.
- [Google Scholar]
- International Commission on Radiological Protection (ICRP). Radiation dose to patients from radiopharmaceuticals - addendum 3 to ICRP Publication 53. ICRP Publication 106. Ann ICRP. 2008;38:1-2.
- [Google Scholar]
- The determination of patient dose from 18F-FDG PET/CT examination. Radiat Prot Dosimetry. 2010;141:50-5.
- [Google Scholar]
- Estimation of patient dose in 18F-FDG and 18F-FDOPA PET/CT examinations. J Cancer Res Ther. 2013;9:477-83.
- [Google Scholar]
- MIRD pamphlet no. 16. Techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med. 1999;40(Suppl 2):37S-61S.
- [Google Scholar]
- Noninvasive determination of local cerebral metabolic rate of glucose in man. Am J Physiol. 1980;238:E69-82.
- [Google Scholar]
- International Commission on Radiological Protection (ICRP) In: Report on the task group on reference man. ICRP Publication 23 Ottawa, Canada: ICRP; 1975.
- [Google Scholar]
- International Commission on Radiation Units and Measurements (ICRU) In: Tissue substitutes in radiation dosimetry and measurement. ICRU Report 44. Bethesda, Md., USA: ICRU; 1989.
- [Google Scholar]
- Health concerns related to radiation exposure of the female nuclear medicine patient. Environ Health Perspect. 1997;105(Suppl 6):1403-9.
- [Google Scholar]
- Reference Japanese man - 1 Mass of organs and other characteristics of normal Japanese. Health Phys. 1979;36:333-46.
- [Google Scholar]






