Translate this page into:
Estimation of biofilm, proteinase & phospholipase production of the Candida species isolated from the oropharyngeal samples in HIV-infected patients
Reprint requests: Dr. Lahari Saikia, Department of Microbiology, Assam Medical College & Hospital, Dibrugarh 786 002, Assam, India e-mail: mdrlamch@yahoo.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Candida, the most common opportunistic infection in acquired immunodeficiency syndrome (AIDS), attributes its pathogenicity to its virulence factors, mainly the biofilms, the proteinases and the phospholipases. There is a significant interplay of these factors during the HIV infection. This study was aimed to estimate the biofilm, proteinase and phospholipase production in Candida species isolated from the oropharyngeal samples in the HIV-infected patients.
Methods:
A total of 126 consecutive HIV-positive patients were screened for Candida growth using oropharyngeal swabs. Identification was done by Gram staining, germ tube test, chlamydospore identification, chromagar and biochemical tests on Vitek 2. Biofilm production was observed on Sabouraud's dextrose broth with glucose, phospholipase production in egg yolk agar medium and proteinase production in bovine serum albumin agar medium.
Results:
Of a total of 126 patients, 53 (42.06%) showed Candida growth: Candida albicans (n=46, 86.8%) was most common followed by the non-albicans Candida (NAC) (n=7, 13.93%). Of a total 33 (62.3%) biofilm positive isolates, significant production was observed in the NAC species (P <0.05). C. albicans reported the highest phospholipase (n=37/41, 90.24%) and proteinase (n=37/43, 86%) activities in a total of 41 (77%) phospholipase positive and 43 (81.1%) proteinase positive isolates.
Interpretation & conclusions:
Although C. albicans was the most common Candida species identified in HIV positive patients, the emergence of NAC was of special concern. Virulence factors such as biofilms, proteinases and phospholipases were noted in both these groups. Further research is required for better understanding of the pathogenic role of Candida species so as to aid in therapeutic interventions.
Keywords
Biofilm
Candida albicans
HIV
non-albicans Candida
phospholipase
proteinase
The retrovirus – human immunodeficiency virus (HIV) 1 and 2 is responsible for causing the pandemic of acquired immunodeficiency syndrome (AIDS)1. Among the many opportunistic infections, oropharyngeal candidiasis is the most common and is an independent predictor of immunodeficiency in this condition23. The virulence of the Candida species is attributed to a wide variety of mechanisms including production of biofilm, secretion of enzymes such as proteinases and phosphatases besides hyphae production and phenotypic switching4.
The expression of these virulence factors may vary depending on the infecting species, geographical origin, type of infection, the site and stage of infection, and host reaction5. Therefore, the knowledge of these factors is important to understand the pathogenesis of candidiasis in HIV-positive patients and in addition, it will also help to explore new antifungal drug targets for improved therapeutic regimens. Various studies from India have explored these virulent attributes of Candida species56789. However, studies are lacking from the northeastern part of India. Therefore, this study was aimed to estimate the biofilm, proteinase and phospholipase production of Candida species isolated from oropharyngeal samples in HIV-infected patients.
Material & Methods
A cross-sectional study on 126 consecutive patients was carried out in the department of Microbiology, Assam Medical College and Hospital, Dibrugarh, Assam, India, from April 2012 to March 2013. All patients who attended the Integrated Counselling and Testing Centre of Assam Medical College and Hospital during the study period and were found to be HIV seropositive, were included in the study and classified as per WHO clinical staging. HIV seropositive patients on highly active antiretroviral therapy (HAART) and HIV-seronegative patients were excluded. Informed written consent from the patients and ethical clearance from the Institutional Ethical Committee were taken.
Specimen collection and processing
Oropharyngeal swabs were collected from the patients under proper aseptic measures. The swabs were cultured in Sabouraud's dextrose agar (SDA) (HiMedia, Mumbai) with and without chloramphenicol (HiMedia). The organisms were isolated and identified by germ tube test, morphology on cornmeal agar (HiMedia), urea hydrolysis tests (HiMedia), chromagar media (HiMedia) and growth at 45°C, which were done as per standard microbiological protocols10. Vitek 2 YST identification card (bioMerieux, France) was also used for yeast identification as per manufacturer's instructions.
Determination of biofilm production
The method of Branchini et al11 for Candida biofilm detection was used with modification. Briefly, a loopful of culture isolate from the SDA plate was inoculated into a tube containing 10 ml Sabouraud's liquid medium (HiMedia) supplemented with glucose (HiMedia). The tubes were incubated at 37°C for 24 h after which the broth was aspirated out, and the walls of the tubes were stained with safranin (HiMedia). Biofilm formation was scored as negative (0+), weak positive (1+), moderate positive (2+) or strong positive (3+). Staphylococcus epidermidis ATCC 35984 (American Type Culture Collection, Manassas, VA, USA) was used as the positive control and S. epidermidis ATCC 12228 as the negative control for biofilm detection and grading.
Determination of proteinase production
A modification of the method as proposed by Staib12 was followed for detection of the proteinases. The yeast suspension (10 μl) was aliquoted into the wells punched onto the surface of the bovine serum albumin medium (HiMedia) and incubated at 37°C for two days. The plates were fixed with 20 per cent trichloroacetic acid and stained with 1.25 per cent amido black (HiMedia) followed by decolourisation with 15 per cent acetic acid. The plates were examined for opaqueness of the agar, corresponding to a zone of proteolysis around the wells. The diameter of unstained zones around the well was considered as a measure of proteinase production. The proteinase activity (Pz) was determined in terms of the ratio of the diameter of the well to the diameter of the proteolytic unstained zone. When Pz=1, no proteinase activity was detected in the strain. Candida albicans ATCC 10231 was used as a positive control.
Determination of phospholipase production
For phospholipase detection, the method proposed by Samaranayake et al13 was used with modification. Aliquots of the yeast suspension (10 μl) were added to wells punched onto the surface of the egg yolk agar medium (HiMedia). The diameter of the precipitation zone around the well was measured after incubation at 37°C for two days. Phospholipase activity (Pz value) was determined as previously described13. When Pz=1, no phospholipase activity was detected in the strain. C. albicans ATCC 10231 was used as a positive control.
Statistical analysis
Data entry, database management and analysis were done using SPSS software, version 16.0 (SPSS Inc., Chicago, IL, USA). Statistical analysis was done by Chi-square test and Fisher's exact test wherever applicable.
Results
Of the 126 patients included in this study, the majority were in the 21-40 yr age group (n=86, 68.24%, P <0.05) with male preponderance (n=69, 54.7%). Most of them were educated up to 10th standard (n=115, 91.26%) and unemployed (n=81, 64.28%) and some of them had their own business (n=22, 17.46%). Rural population (n=90, 71.42%), mostly married (n=101, 80.15%, P <0.05) constituted most of the cases. History of alcohol intake (n=44, 34.92%) and smoking (n=41, 32.53%) were also seen. The HIV virus was mainly transmitted by the heterosexual route (n=81, 64.28%) with most of the patients presenting with World Health Organization clinical stage I disease (n=66, 52.38%) followed by stage III (n=34, 26.98%) and stage II (n=25, 19.84%). Seventeen patients (13.49%) suffered from HIV-tuberculosis co-infection with one patient presenting with Cryptococcus meningitis.
The mean CD4 count in the study group was 211.81±129.62 cells/μl with oropharyngeal lesions being observed in 28 patients. The presence of oropharyngeal lesions in patients with CD4 counts ≤200 cells/μl was found to be significant (n=21/33, P <0.05).
Of the 126 patients, 53 (42%) of the isolates were Candida isolates: C. albicans (n=46/53, 86.07%) was the most common followed by the non-albicans Candida (NAC) species, namely, Candida parapsilosis (5.66%), Candida krusei (3.77%), Candida glabrata (1.88%) and Candida lipolytica (1.88%) (Table).
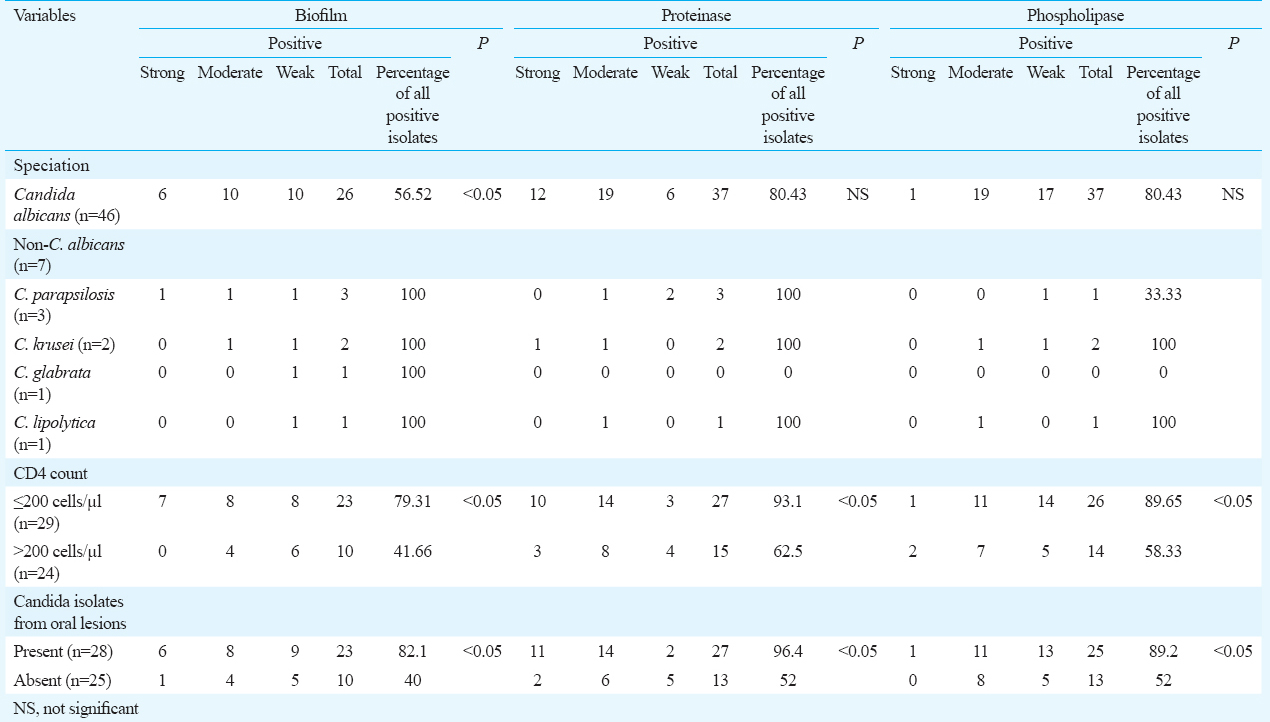
Biofilm production was seen in 62 per cent (n=33/53) of the cases with a significant association (P <0.05) being observed in the NAC species. Its increased expression was also found to be significant (P <0.05) in patients with CD4 counts ≤200 cells/μl and with oropharyngeal lesions (Table).
Forty three isolates of 53 (81.1%) showed proteinase activity with C. albicans representing the major producer (n=37/43, 86%) followed by the NAC species. There was no association between the species of Candida isolated and the proteinase expression. However, significant association (P <0.05) was found with its expression in cases with CD4 counts ≤200 cells/μl and with oropharyngeal lesions (Table).
The production of phospholipase was seen in 41 cases of 53 (77%) and it was mainly produced by C. albicans (n=37/41, 90.24%). No significant association was found in the expression of this virulence factor to the species of the Candida isolated. However, with CD4 counts ≤200 cells/μl and the presence of oropharyngeal lesions, its increased expression was significant (P <0.05) (Table).
Discussion
In the present study, the biofilm, proteinase and phospholipase production of Candida species in the oropharyngeal swabs of the HIV-infected population was studied. Adherence of the fungus to host cells by biofilms initiates its colonization or disease1415. NAC species was the major biofilm producer in the study, a finding in accordance with previous studies67.
Extracellular phospholipase lyses host cells to facilitate adhesion and penetration16. Phospholipase enzyme breaks down the phospholipids of the cell membrane causing cell lysis, thereby direct host cell damage and lysis has been proposed as a major mechanism contributing to microbial virulence16171819. C. albicans was found to be the most frequent phospholipase producer followed by the NAC species namely C. krusei, C. parapsilosis and C. lipolytica. This finding was similar to that reported earlier820.
Secreted aspartic proteinases damages the surface proteins (albumin, keratin) and degrades the locally protective IgA and C3 component2122. This facilitates tissue invasion and resistance to the antimicrobial attack by the host21. The highest proteinase expression was seen in C. albicans in our study, followed by the NACspecies as has been reported in previous studies820.
The CD4 count denotes the immune status of the individual1. With decreasing CD4 counts (CD4 counts ≤200 cells/μl), the expression of biofilm, proteinase and the phospholipase increased. This has been shown by Jin et al15 and De Bernardis et al23. The reason may be due to the fact that the preferential selection of strains with a higher overall level of these virulence factors increases with the advancing stages of HIV infection924.
Oropharyngeal candidiasis is the most common manifestation of AIDS23. Its presence indicates the inability of the body's immune system to combat the microbe2. However, it indirectly leads to xerostomia facilitating increased cell adherence and thereby aiding biofilm formation1525. Increased cell adherence also allows the phospholipases and the proteinases to act on the membrane phospholipids and target proteins, respectively.
Three major virulence factors of Candida species were measured in HIV-infected individuals. In this study, almost 30 per cent of Candida species associated with the oropharyngeal lesions were found not to produce any of these virulence factors. This finding was similar to the study by Kumar et al26.
In conclusion, C. albicans was found to be the most common Candida species isolated from HIV infected patients. Biofilm production, proteinase and phospholipases were seen in both C. albicans and NAC. Further studies need to be done to understand the pathogenic role of Candida species in HIV positive patients and to device therapeutic modalities.
Acknowledgment
This study was funded by the Department of Biotechnology, Government of India (DBT Sanction No, BT/Mcd/l5/Vision-NER/2011, November 2, 2011). The authors acknowledge the Multidisciplinary Research Unit (ICMR), Assam Medical College, Dibrugarh, Assam, India for technical and infrastructure support. The authors also thank Shri Chakrapani Hazarika for statistical assistance and Shri Rajesh Sonar for technical assistance during the study.
Conflicts of Interest: None.
References
- Human immunodeficiency virus: AIDS. In: Kapil A, ed. Textbook of microbiology (9th ed). Hyderabad: Universities Press; 2013. p. :571-4.
- [Google Scholar]
- HIV/AIDS with an emphasis on Africa. In: Gilks CF, ed. Manson tropical diseases of medicine (21st ed). London: British Council, ELST with Saunders; 2003. p. :401-22.
- [Google Scholar]
- Virulence factors contributing to pathogenicity of Candida tropicalis and its antifungal susceptibility profile. Int J Microbiol. 2014;2014:456878.
- [Google Scholar]
- Distribution of Candida species in different clinical samples and their virulence: Biofilm formation, proteinase and phospholipase production: A study on hospitalized patients in Southern India. J Glob Infect Dis. 2011;3:4-8.
- [Google Scholar]
- Biofilm production by clinical isolates of Candida species. Med Mycol. 2006;44:99-101.
- [Google Scholar]
- Proteinase and phospholipase activity as virulence factors in Candida species isolated from blood. Rev Iberoam Micol. 2008;25:208-10.
- [Google Scholar]
- Biofilm production in oral Candida isolates from HIV-positive individuals from Pune, India. Mycoses. 2013;56:182-6.
- [Google Scholar]
- Laboratory methods in basic mycology. In: Weissfeld AS, ed. Bailey & Scott's diagnostic microbiology (12th ed). Missouri: Mosby Elsevier; 2007. p. :698-704.
- [Google Scholar]
- Genotypic variation and slime production among blood and catheter isolates of Candida parapsilosis. J Clin Microbiol. 1994;32:452-6.
- [Google Scholar]
- Serum-proteins as nitrogen source for yeast like fungi. Sabouraudia. 1965;4:187-93.
- [Google Scholar]
- Phospholipase B enzyme expression is not associated with other virulence attributes in Candida albicans isolates from patients with human immunodeficiency virus infection. J Med Microbiol. 2005;54(Pt 6):583-93.
- [Google Scholar]
- Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95-108.
- [Google Scholar]
- Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J Clin Microbiol. 2003;41:2961-7.
- [Google Scholar]
- Virulence factors that damage the host. In: Salyers A, Witt D, eds. Bacterial pathogenesis: A molecular approach. Washington, DC: ASM Press; 1994. p. :47-62.
- [Google Scholar]
- Cloning and disruption of caPLB1, a phospholipase B gene involved in the pathogenicity of Candida albicans. J Biol Chem. 1998;273:26078-86.
- [Google Scholar]
- The cytochemical localization of phospholipase in Candida albicans infecting the chick chorio-allantoic membrane. Sabouraudia. 1977;15:29-35.
- [Google Scholar]
- Phospholipase and proteinase activities in different Candida species isolated from anatomically distinct sites of healthy adults. Jpn J Infect Dis. 2007;60:280-3.
- [Google Scholar]
- High levels of hydrolytic enzymes secreted by Candida albicans isolates involved in respiratory infections. J Med Microbiol. 2003;52(Pt 11):971-4.
- [Google Scholar]
- Degradation of humoral host defense by Candida albicans proteinase. Infect Immun. 1995;63:984-8.
- [Google Scholar]
- Elevated aspartic proteinase secretion and experimental pathogenicity of Candida albicans isolates from oral cavities of subjects infected with human immunodeficiency virus. Infect Immun. 1996;64:466-71.
- [Google Scholar]
- Increased expression of Candida albicans secretory proteinase, a putative virulence factor, in isolates from human immunodeficiency virus-positive patients. J Clin Microbiol. 1995;33:2543-9.
- [Google Scholar]
- Inhibition of Candida albicans yeast-hyphal transition and biofilm formation by Solidago virgaurea water extracts. J Med Microbiol. 2012;61(Pt 7):1016-22.
- [Google Scholar]
- Phospholipase and proteinase activities of clinical isolates of Candida from immunocompromised patients. Mycopathologia. 2006;161:213-8.
- [Google Scholar]






