Translate this page into:
Environmental management through sluice gated bed-dam: a revived strategy for the control of Anopheles fluviatilis breeding in streams
Reprint requests: Dr K. Gunasekaran, Scientist F, Vector Control Research Centre (ICMR), Medical Complex, Indira Nagar, Puducherry 605 006, India e-mail: k_guna@yahoo.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Integrated vector management (IVM) emphasizes sustainable eco-friendly methods and minimal use of chemicals. In this context, the present study highlights the environmental control of breeding of Anopheles fluviatilis, the primary malaria vector, through water management in a natural stream in Koraput district, Odisha, India.
Methods:
The District Rural Development Agency (DRDA), Koraput, constructed two bed-dams across streams, one in Barigaon and the other in Pipalapodar village. The bed-dam in the former village was fitted with two sluice gates whereas the bed dam constructed in the latter village was without the sluice gate. The sluice gates were opened once in a week on a fixed day to flush out the water from the dam. Anopheles immatures were sampled systematically in the streams using a dipper for density measurement and species composition.
Results:
There was a reduction of 84.9 per cent in the proportion of positive dips for Anopheles larvae/pupae and a reduction of 98.4 per cent in immature density (number/dip) of An. fluviatilis in the experimental downstream compared to the control following opening of the sluice gates.
Interpretation & conclusions:
Our findins showed that opening of sluice gates of the bed-dam regularly once in a week resulted in the control of vector breeding in the downstream due to the flushing effect of the water released with a high flow from the bed-dam that stagnated water in the upstream. The outcome of the study encourages upscaling this measure to other areas, wherever feasible.
Keywords
Anopheles fluviatilis
bed dam
environmental management
malaria
sluice gate
stream
Malaria is a major public health problem in India and contributes significantly to the overall malaria burden in Southeast Asia1. The National Vector Borne Disease Control Programme (NVBDCP) in India reported 0.88 million malaria cases and 440 deaths due to malaria in 20132. Odisha is one of the severely affected States with malaria in the country. The State, with about 4 per cent of India's population, accounts for a quarter of the disease burden and 15.2 per cent of the total malaria deaths reported in the country during 20132. The Odisha State has been reporting high proportion (93.0%) of Plasmodium falciparum malaria which is known to cause complications and mortality. The ecological and geographical conditions of this State favour various ecotypes of malaria with Anopheles fluviatilis being the predominant vector transmitting P. falciparum. Two rounds of indoor residual spraying with DDT by the National Programme have not produced the adequate level of control of the vector population3.
In such a situation, bioenvironmental method of vector control could be an alternative or supplementary option, wherever feasible. Moreover, this strategy is a holistic approach besides its cost effectiveness and eco-friendly nature. Streams are the most preferred habitat for breeding of An. fluviatilis and intensity of breeding was high during winter and early summer when the transmission of malaria was also high4. The Government of Odisha has executed numerous water resource development schemes. Under one such scheme, bed-dams are constructed across streams for irrigation purpose. Any water resource development scheme is likely to influence the vector-borne diseases if it interferes with the breeding habitats of the vector species5. It is expected that the construction of bed-dam across a stream leads to stagnation of water at one point, resulting in reduced flow of water in downstream compared to upstream as the downstream receives only the overflowing water from the bed-dam. To minimize the vector breeding in the downstream, it was proposed to the district administration to introduce sluice gates in bed dams and open them once in a week to facilitate flushing of immature of the vector mosquito present in the downstream. Here we present the results of a study that assessed the breeding of An. fluviatilis from May 2010 to April 2011 in a stream across which a sluice gated bed-dam was constructed by the district administration, Koraput in Odisha State, India.
Material & Methods
Study area: The study was carried out in Laxmipur Community Health Centre (CHC) of Koraput district, Odisha. The district is situated in the southern part of the State and is one of the hilly, forested and highly malarious districts, predominantly inhabited by tribes6. The terrain is highly undulating and the villages are located on hilltops or on the slopes of hillocks, criss-crossed by streams. The climate of the district is characterized by hot summer (March-June), rainy (July-October) and winter (November-February) seasons. The monsoon generally breaks during the later part of June each year. Average annual rainfall recorded during 2012 was 1533.3 mm, of which >80 per cent occurred during July to September under the influence of south-west monsoon. Maximum rainfall was in August. The mean minimum temperature varied between 14.0°C (January-February) and 27.5°C (April-May) and mean maximum temperature between 27.0°C (December) and 41.5°C (May) in 20077. P. falciparum is the predominant malaria parasite and deaths due to cerebral malaria are high. An. fluvialitis ‘S’ has been incriminated as the major malaria vector3 and it prefers to breed in slow flowing streams4. Two streams, one each in Barigaon and Pipalpodar villages of the Laxmipur CHC were selected for the study. The two villages are situated 5 km away from each other. In both the villages, the proximity of the stream to the village was 100 m. The upper portion of the stream from bed dam was the upstream and lower portion was the downstream.
Construction of bed-dams: The District Rural Development Agency (DRDA) of Koraput constructed a reinforced cement concrete bed-dam across the selected stream in Barigaon and also in Pipalapodar village. The bed-dam across the Barigaon stream was 15 m long with a height of 4.5 m and two sluice gates were fixed in the bed-dam. The bed-dam, constructed across the Pipalapodar stream was a conventional one, without any sluice gates; it was 15 m in length and 1.5 m in height. The residents of the Barigaon village selected a volunteer to operate the sluice gates (opening and closing) weekly once on a fixed day to let out water from the bed-dam. Hereafter, the stream with sluice gated bed-dam is referred to as experimental arm and the stream with conventional bed-dam (without sluice gates) as control arm.
Initially, the study was planned for one year from May 2010 to April 2011. Accordingly, sampling was initiated from May 2010. The district administration started the construction work of sluice gated bed-dam in June 2010 and completed by September 26, 2010. The first post-construction sampling was done on September 28, 2010. The sampling period during pre-construction period was five months and during post-construction period was seven months. The study could not be continued beyond April 2011 because of complete drying of the downstream as a result of bed-dam construction.
Immature sampling: Immature of Anopheles mosquitoes were sampled systematically8, taking one sample (one dip) at every 10 m along the edges on both sides of the stream, using a dipper (10 cm diameter, 300 ml capacity). Prior to the construction of bed-dam (i.e. from May 2010 to September 2010) immature were sampled at fortnightly interval while weekly sampling was done during post-construction period (October 2010 to April 2011). On each survey, 100 dips were taken over a stretch of 500 m from upstream and an equal number of dips also from downstream. The total number of dips taken, number of positive dips for Anopheles immature and number of immature collected were recorded. The immature were brought to the laboratory of Vector Control Research Centre, Field Station, Koraput (Odisha State) and reared to adults for species identification. Percentage of dips positive for Anopheles immature (Number of positive dips/Total dips taken X 100) and per dip density of An. fluviatilis immature (Total number of An. fluviatilis immature/Total number of dips taken) were calculated.
Data analysis: The percentage values of positive dips for Anopheles immature obtained from upstream and downstream of both experimental and control arms during pre- and post-intervention periods were transformed to arcsine values for further analysis. The values of per dip density of An. fluviatilis (y) in upstream and downstream of experimental and control arms during pre- and post-intervention periods were subjected to log transformation after adding “1” i.e. ln (y + 1). The difference in mean percentage of positive dips and per dip density of An. fluviatilis between upstream and downstream of experimental and control arms during pre- and post-intervention periods were compared using Student’t’ test. Reduction in percentage of positive dips for Anopheles immature and in per dip density of An. fluviatilis immature after the construction of sluice gated bed-dam were calculated using the formula of Mulla9.
Results
A total of 13 Anopheles species were identified from the immature samples collected from the experimental and the control streams. In the experimental streams, prior to bed-dam construction, the percentage of dips positive for Anopheles immature varied from 5.0 to 10.3 and 7.0 to 12.7 in upstream and downstream, respectively; the mean values did not differ significantly between the two segments, indicating an equal status of breeding throughout the stream. The corresponding values in the control stream varied from 6.5 to 15.0 and 8.5 to 16.0; with no significant difference in the mean values between the upstream and the downstream (Figs 1, 2). There was also no significant difference in the percentage of positive dips for Anopheles immature between the upstream and downstream of experimental and control arms over months before bed-dam construction.
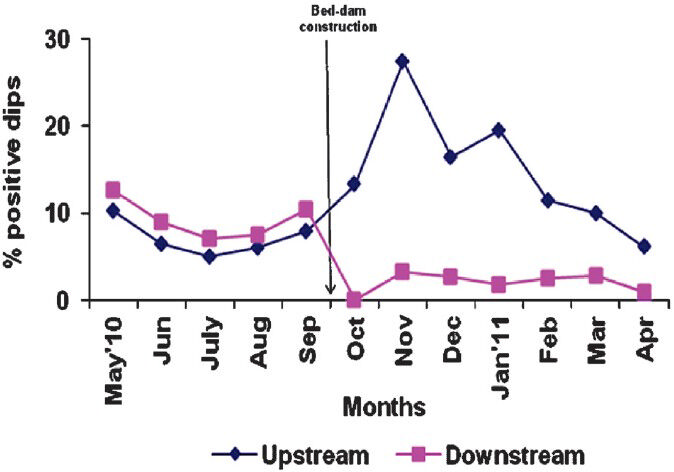
- Proportion (%) of dipper samples (dips) found positive for Anopheles immature (larvae and/or pupae) in experimental up and down stream.
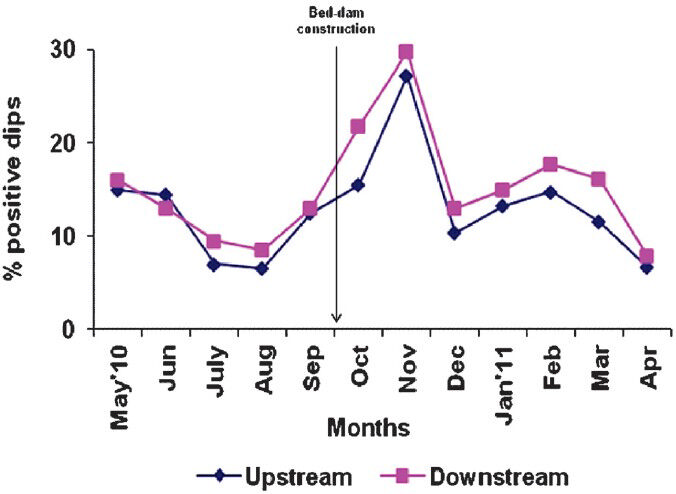
- Proportion (%) of dipper samples (dips) found positive for Anopheles immature (larvae and/or pupae) in control up and down stream.
During post-construction period, the percentage of dips positive for Anopheles immature in the experimental stream varied from 6.1 to 27.5 and 0 to 3.3 in upstream and downstream, respectively; the mean percentage value was significantly lower in the downstream than that in the upstream (P<0.00). In the control stream, after the construction, the percentage of positive dips for Anopheles immature varied from 6.7 to 27.3 and 7.9 to 29.8 in upstream and downstream, respectively, but there was no significant difference in the mean values. While there was no significant difference in the mean percentage values between the upstream of experimental and control streams, the mean percentage of positive dips was significantly lower (P<0.00) in the downstream of experimental arm over months than that of the control arm (Figs 1, 2).
The survey conducted during the post-construction period detected only five older (stage III and IV) instars of larvae and zero pupae of Anopheles species in the downstream of the experimental stream, while the number of the corresponding stages recorded in the downstream of the control arm was high (301 and 43). Prior to the construction of bed-dam, the immature density (number per dip) of An. fluviatilis varied from 0 to 0.05 and 0.02 to 0.04, respectively, in upstream and downstream of the experimental arm. The corresponding values for the control stream were 0.01 to 0.03 and 0 to 0.02. There was no significant difference in the mean density between upstream of experimental and control stream as well as between the downstream of the two arms (Figs 3, 4).
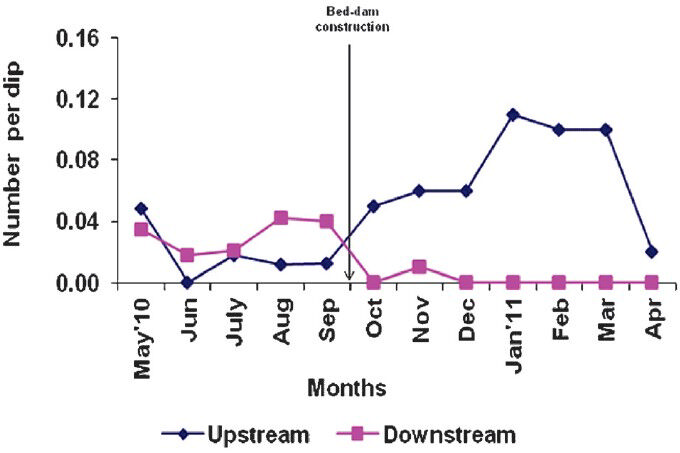
- Density (number per dip) of An. fluviatilis immature (larvae and/or pupae) in experimental up and down stream.
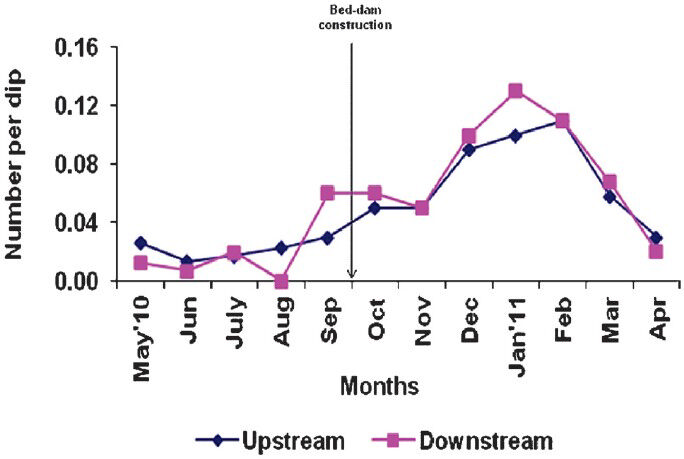
- Density (number per dip) of An. fluviatilis immature (larvae and/or pupae) in control up and down stream.
After the construction, in the experimental stream, the density of An. fluviatilis varied from 0.02 to 0.11 and 0.0 to 0.01 in up- and downstream, respectively, with a significant difference in the mean density (P<0.00) between the two sections. In the control stream, the immature density of An. fluviatilis varied from 0.03 to 0.11 and 0.02 to 0.13, respectively in up- and downstream, without any significant difference in the mean density between the two segments. When the density was compared between the upstream of experimental and control arms, there was no significant difference, but in the downstream of the experimental arm the immature density of An. fluviatilis was significantly lower than that of the control arm (P<0.00) (Figs 3, 4).
Overall, following the construction of the sluice gated bed-dam across the stream in the Barigaon village, there was 84.9 per cent reduction of the positive dips for Anopheles immature and 98.4 per cent reduction of the immature density of An. fluviatilis in downstream of the experimental stream.
Discussion
The current study demonstrated the dual benefit of the sluice gated bed-dam constructed across the stream; controlling vector breeding as well as stagnating water for irrigating the rice fields. The water is stored up to a level and above which it overflows to the downstream. The streams are the preferential breeding habitat of An. fluviatilis, the major malaria vector in the study area. When sluice gates were fixed to the bed-dam, besides ensuring water storage, these facilitated release of water with high flow that flushed out the immature of An. fluviatilis present in the downstream. The sluice gates were kept opened only for half an hour to release the entire water from the dam and then closed. Such operation for a short period of time once in a week did not interfere with the primary objective of the bed-dam, to stagnate water for irrigation. After the gates were closed, water got stagnated to the original level within 6 to 12 h and was used for irrigation until opening again the next week.
The two indicators (% of dips positive for Anopheles immature and immature density of An. fluviatilis) used in the current study to measure the flushing effect were in the same level in upstream of experimental and control arms during the pre- and post-construction of the bed-dam. In the case of downstream, both the indicators were comparable in experimental and control arms only during pre-construction period. Whereas, during the post-construction period both the indicators were significantly reduced in downstream of experimental arm compared to the control arm, indicating the flushing effect of the high flow of water released from the bed-dam through the sluice gates on immature of the vector species. The significant reduction of older instars and pupae during the post-construction period in the downstream of the experimental arm confirmed the washing effect. The flushing effect was observed up to a distance of 500 m, beyond which the sampling was not possible as the downstream merged with a river. However, since the average flight range of An. fluviatilis was reported to be 500m10, the distance of flushing effect (500 m) as recorded in the current study was adequate to control the vector breeding.
Flushing the stream using sluice gates is not a new concept. In Asian countries, knowledge on the breeding preference of malaria vectors in streams led to making efforts to reduce their abundance through engineering actions11. In India, such trials were carried out during the first half of 20th century before the extensive use of insecticides came to dominate vector control measures. Similar to the current study, the earlier approach used was to construct a dam with sluice gates across a stream and suddenly release the water confined in the reservoir created behind the dam12. The advantage of having the hand operated sluice gates is that the entire water in the reservoir could be released at once, when needed. While the hand operated sluices were mainly used in India12, automated siphon sluices were the option in Sri Lanka13, Malaysia14, and later in West Bengal15.
Integrated vector management (IVM) has been the new strategic approach to vector control since 20011617. The IVM, while emphasizing environmentally sound, inter-sectoral, selective, targeted, cost-effective and sustainable measures, envisages a minimum reliance on chemical insecticides1819. The current study was an example of inter-sectoral collaboration which involved the DRDA and the department of Minor Irrigation in implementation of vector control through construction of bed-dams with sluice gates. A recent study carried out in Sundargarh district, Odisha State, observed the impact of construction of small dam across a stream on breeding of An. fluviatilis. The study showed that the dam construction altered the water flow above and below the dam thereby making it unfavourable for the breeding of An. fluviatilis, and as a result there was a decline in malaria incidence5.
Use of chemical insecticides in streams for the control of An. fluviatilis breeding is not a feasible option, as stream water is used by the hill and foot-hill inhabitants and domestic and wild animals. Further, use of biological control agents in streams could not be sustained due to the gradient and water current. Under such circumstances, the only option would be implementation of environmental management methods such as introduction of sluice gates in bed-dams. Since, the current study has demonstrated the feasibility of water management through the sluice gated bed-dam for the control of vector breeding, it is recommended that the scheme of bed-dams with sluice gates under the water shed/minor irrigation projects could be upscaled for public health benefit, particularly in malaria endemic areas.
Acknowledgment
This study was carried out with the support of the district administration, Koraput. The authors thank Shri Rajesh Pravakar Patil, IAS, Collector and Shrimati Roopa Roshan Sahoo, IAS, the Project Director, DRDA, Koraput district for their cooperation. The technical assistance of the staff at the VCRC field station, Koraput, is acknowledged.
References
- Malaria in India: the center for the study of complex malaria in India. Acta Trop. 2012;121:267-73.
- [Google Scholar]
- Malaria situation in India. National Vector Borne Disease Control Programme, Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India. 2014. Available from: www.nvbdcp.gov.in
- [Google Scholar]
- DDT indoor residual spray, still an effective tool to control Anopheles fluviatilis-transmitted Plasmodium falciparum malaria in India. Trop Med Int Health. 2005;10:160-8.
- [Google Scholar]
- Breeding habitats of malaria vectors: A. fluviatilis, A. annularis and A. culicifacies, in Koraput district, Orissa. Indian J Malariol. 1990;27:209-16.
- [Google Scholar]
- Building small dams can decrease malaria: A comparative study from Sundargarh District, Orissa, India. Acta Trop. 2008;107:174-8.
- [Google Scholar]
- Acceptability, willing to pruchase and use long lasting insecticide treated mosquito nets in Orissa State, India. Acta Trop. 2009;112:149-55.
- [Google Scholar]
- Persistent foci of falciparum malaria among tribes over two decades in Koraput district of Odisha State, India. Malar J. 2013;12:72.
- [Google Scholar]
- Ecological methods with particular reference to the study of insect populations. (2nd ed). London: Chapman & Hall; 1978. p. :576.
- [Google Scholar]
- Control of chironomid midges in the recreational lakes. J econentomol. 1971;64:300-7.
- [Google Scholar]
- Studies on dispersal of malaria vectors in a hilly tract of Koraput District, Orissa State, India. Southeast Asian J Trop Med Public Health. 1993;24:508-12.
- [Google Scholar]
- Anopheles culicifacies breeding in Sri Lanka and options for control through water management. Acta Trop. 1998;71:131-8.
- [Google Scholar]
- Anti-mosquito measures with special reference to India. Health Bulletin No. 11. Malaria Bureau No 3. (6th ed). Delhi, India: Government of India Press; 1943.
- [Google Scholar]
- Anti-larval flushing of rivers and streams in Ceylon. J Malar Inst India. 1940;3:81-92.
- [Google Scholar]
- Case history on malaria vector control through the application of environmental management in Malaysia. Geneva, Switzerland: World Health Organization; 1988.
- [Google Scholar]
- An investigation on the use of automatic siphon sluices on a group of tea estates in Northern Bengal. J Malar Inst India. 1940;3:93-7.
- [Google Scholar]
- Global strategic framework for integrated vector management. WHO/CDS/CPE/PVC/2004. Geneva: WHO; 2004. p. :1-12.
- [Google Scholar]
- WHO position statement on integrated vector management. Wkly Epidemiol Rec. 2008;83:177-81.
- [Google Scholar]
- Integrated vector control. Seventh report of the WHO Expert Committee on Vector Biology and Control. In: WHO Tech Rep Ser No.688. Geneva: WHO; 1983. p. :1-72.
- [Google Scholar]
- Global malaria control and elimination: report of a technical review. Geneva: World Health Organization; 2008.
- [Google Scholar]






