Translate this page into:
Enhanced production of mosquitocidal cyclic lipopeptide from Bacillus subtilis subsp. subtilis
Reprint requests: Dr A.M. Manonmani, Scientist ‘E’, Unit of Microbiology & Immunology, Vector Control Research Centre (ICMR), Medical Complex, Indira Nagar, Puducherry 605 006, India e-mail: ammanonmani@yahoo.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
A cyclic lipopeptide, surfactin produced by a strain of Bacillus subtilis subsp. subtilis (VCRC B471) was found to exhibit activity against both the larval and pupal stages of mosquitoes. The present study was aimed at increasing the production of the mosquitocidal metabolite by modifying the conventional medium.
Methods:
Enhancement of mosquitocidal metabolite production was attempted by replacing the existing micronutrients of the conventional NYSM and supplementing the medium with additional amounts of glucose. The LC50 value of culture supernatant (CS) against the larval and pupal stages of Anopheles stephensi was determined. Crude mosquitocidal metabolite (CMM) was separated from the CS, identified by MALDI-TOF analysis and its LC50 dosage requirement for the pupal stage of the above mosquito species determined.
Results:
The medium containing a new composition of micronutrients and glucose up to 1 per cent resulted in increased metabolite production. The LC50 value of the CS obtained in the improved medium against larvae and pupae of An. stephensi was 5.57 and 0.71 μl/ml, respectively. The yield of CMM was doubled in the improved medium. MALDI-TOF analysis revealed that the CMM was surfactin.
Interpretation & conclusions:
The new improved medium enhanced the production of mosquitocidal metabolite as the dosage required for inciting 50 per cent mortality among the pupal stages of mosquitoes was only half of that required when the metabolite was produced in the conventional medium. The mosquitocidal metabolite was identified as surfactin, a cyclic lipopeptide and biosurfactant.
Keywords
Anopheles
Bacillus subtilis
cyclic lipopeptide
mosquitocidal metabolite
surfactin
Among various microbial pesticides being advocated for the control of mosquitoes, those containing Bacillus thuringiensis subsp. israelensis and B. sphaericus enjoy top priority1–3. However, reports indicate development of resistance by certain species of mosquitoes to certain strains of B. sphaericus45. Though resistance development has not been a major problem encountered with B. thuringiensis till date, the stability, solubility and insecticidal activity of the crystal toxins of B. thuringiensis are known to be affected by pH of the habitat67 and by exposure to sunlight8. These drawbacks call for the development of new mosquitocidal bacterial agents which can overcome the above limitations.
We have isolated a Gram-positive, aerobic, spore-forming bacterium from mangrove forests of Andaman & Nicobar islands, India. It was identified to be a strain of Bacillus subtilis subsp. subtilis (VCRC B471) based on biochemical parameters, 16S ribosomal DNA and gyrA gene sequencing. The culture supernatant (CS) of this bacterium was found to kill the larval and pupal stages of three species of mosquitoes viz., Anopheles stephensi, Culex quinquefasciatus and Aedes aegypti9. The production of the mosquitocidal metabolite from this strain was optimized using Nutrient Yeast Salt Medium (NYSM)910. In this study, this medium was modified by replacing and/or supplementing various media constituents reported for increasing the yield of this metabolite.
Material & Methods
Microorganism: The B. subtilis subsp. subtilis strain (VCRC B471) isolated from soil samples collected from the mangrove forests of Andaman & Nicobar islands was used for production of the mosquitocidal metabolite. The production strain has been deposited in Microbial Type Culture Collection (MTCC), Chandigarh, India (Accession Number No. 5368). Stock cultures were stored in 10 per cent glycerol (Sigma, USA) at -80°C. The strain was maintained on Nutrient Yeast Salt Medium (NYSM) [glucose 5 g, peptone 5 g NaCl 5 g, beef extract 3 g, yeast extract 5 g, MgCl2 203 mg , MnCl2 10 mg and CaCl2 103 mg (Hi-Media, India) per liter of water] agar slants11 at 4°C.
Bacterial growth conditions: For the production of mosquitocidal metabolite(s), the bacterium was grown aerobically in NYSM. Tubes containing 10 ml NYSM broth were inoculated with a loopful of bacterial cells from the slant culture. The tubes were incubated overnight on a rotary shaker (New Brunswick Scientific Co. Inc., USA) at 28 ± 2°C and 250 rpm. After incubation, cultures were inoculated to fresh 50 ml of NYSM broth and incubated again for a further period of 7 h to synchronize the growth. From this young culture, 5 ml was added to flasks containing 100 ml of the various media (Table I) and incubated with shaking for 72 h. Bacterial cells were removed from the medium by centrifugation at 9000 × g for 25 min at 4°C in a Sorvall Evolution RC superspeed centrifuge (Kendro Lab. Products, Asheville, NC, USA) using SLA-1500 rotor. The culture supernatant (CS) obtained was used for bioassay.
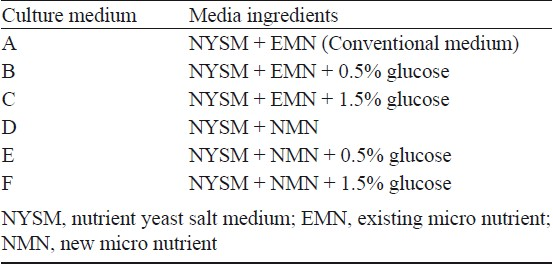
Media used: A total of six media were prepared in 2 sets using NYSM as the basal medium, by varying the micronutrient and glucose concentration. Media set 1 was prepared with Existing Micro Nutrient (EMN) [EMN: MgCl2 203 mg, MnCl2 10 mg and CaCl2 103 mg per litre of water] and media set 2 was prepared with New Micro Nutrient (NMN) [NMN: MgSO4 592 mg, KH2PO4 1.36 g, MnSO4 2 mg, FeSO4 2 mg, CaCl2 1 mg per litre of water12 pH 7.0±0.2]. The concentration of glucose was varied from 1 - 2 per cent in both the sets (Table I).
Source of mosquito: Third instar larvae and freshly emerged pupae of An. stephensi were obtained from a laboratory colony maintained at the Rearing and Colonization Unit of the Vector Control Research Centre, Puducherry, and used for bioassay of the mosquitocidal metabolite(s).
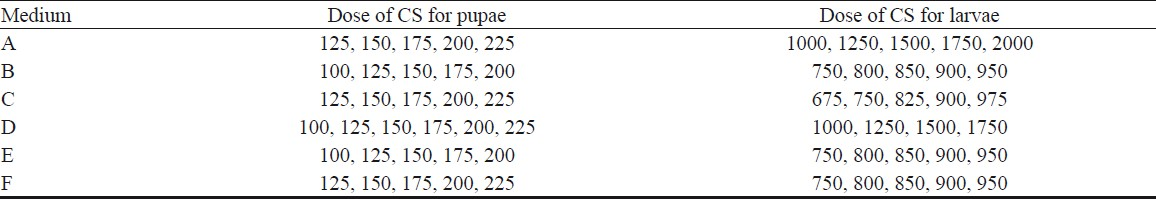
Bioassay: CS obtained from the different sets of media was used for bioassay following WHO standard protocols13. To 200 ml capacity, disposable wax-coated paper cups, 150 ml of chlorine-free tap water was added and 25 numbers of larvae or freshly emerged pupae of An. stephensi were transferred to each cup. For experimental treatment, five concentrations of CS were used. The dosages used for testing the larvicidal and pupicidal activity are given in Table II. Each experiment was performed using four replicates per dose and having an equal number of controls. The treated and control cups were held at 28 ± 2°C, 80-90 per cent relative humidity, and a photoperiod of 12 h light followed by 12 h dark. Bioassay cups used for testing the pupicidal activity were covered with mosquito net cloth to prevent the escape of emerging adults, if any. The mortality of the pupae was scored after 24 h of exposure by counting the number of live ones present in the bioassay cup. The dead pupae in the four replicates were combined and expressed as percentage of pupal mortality for each concentration. Dead pupae included those found at the bottom of the bioassay cups as straightened pupae after losing their typical coma shape as well as those which had moulted to adults but were unable to come out of the pupal exuviae. The experiments were repeated twice. In cases where the control mortality was between 5 and 20 per cent, the observed percentage mortality was corrected using Abbott's formula14. Data from all replicates were pooled for analysis.
Separation of the mosquitocidal metabolite: Three batches of the 2 sets of media (NYSM + EMN and NYSM + NMN) were prepared on different days. The crude mosquitocidal metabolite (CMM) was precipitated from the culture supernatant obtained from the six media using 6N HCl and the precipitates were collected by centrifugation (9000 × g rpm for 25 min at 4°C)10. The CMM was re-suspended in water, adjusted to pH 7.0, lyophilized (Freeze dryer Modulyo Edwards, B.O.C. Ltd., Crawley, England) and tested against the pupae of An. stephensi, which was found to be the most susceptible among the three mosquito species studied9.
Matrix assisted laser desorption ionization - Time of flight (MALDI-TOF): MALDI-TOF mass spectra were recorded by using a Bruker Daltonik Autoflex MALDI-TOF (Technische Universitaet Berlin, Berlin, Germany) instrument equipped with a 337-nm nitrogen laser for desorption and ionization. For mass spectrometric analysis, 2 μl portions of CMM obtained from the conventional medium was mixed with an equal volume of matrix medium [a saturated solution of alpha-cyano-4-hydroxycinnamic acid in 70% aqueous acetonitrile) containing 0.1% trifluoroacetic acid (v/v)], spotted onto the target and air dried for measurement. Positive-ion detection and reflector mode were used. The acceleration and reflector voltages were 19.0 and 20 kV in pulsed ion extraction mode. A molecular mass gate of 350 kDa improved the measurements by filtering out most of the matrix ions.
Statistical analysis: Data obtained from the bioassay experiments were pooled and LC50 and LC90 values were calculated from a log dosage-probit mortality regression line using SPSS 10.0 (SPSS, India) yielding a level of effectiveness at 50 and 90 per cent mortality and 95 per cent confidence intervals. The LC50 values obtained with different media were compared using 95 per cent confidence interval. LC50 values with non overlapping confidence interval were considered to be significant at P<0.05.
Results
Effect of CS of different production media on An. stephensi: The CS of VCRC B471 grown in six different culture media were bioassayed against larvae and pupae of An. stephensi and results are presented in Tables III and IV. Among the combinations NYSM + EMN and NYSM + NMN, the metabolite produced in the latter medium was more effective. Pupicidal and larvicidal activity of the CS obtained from NYSM + EMN was 1.31 and 10.6 μl/ml whereas with NYSM + NMN it was 0.97 and 9.27 μl/ml. The replacement of EMN with NMN in NYSM showed significant difference in mosquitocidal activity (P<0.05).
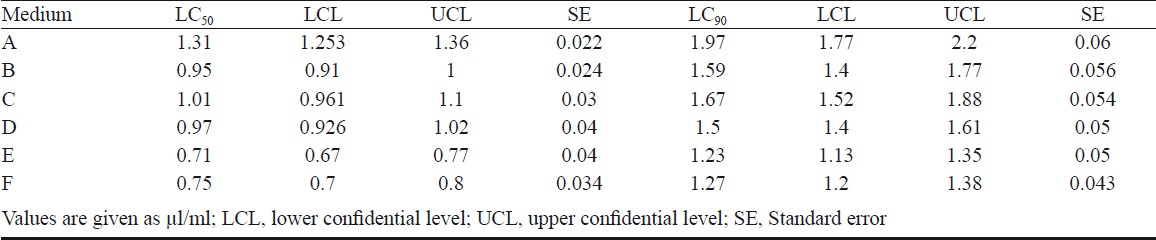
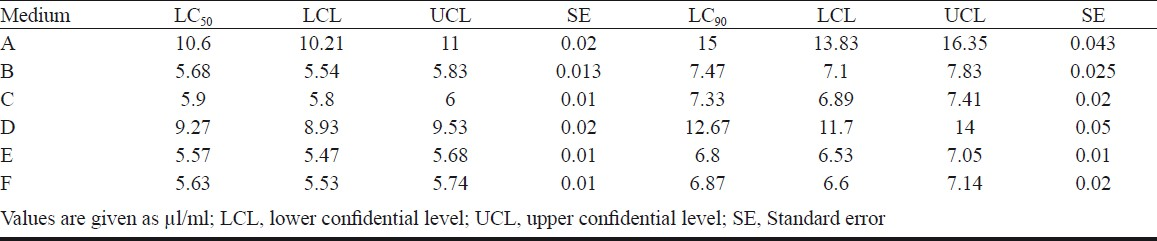
With the further addition of 0.5 and 1.5 per cent glucose to both the above media (NYSM + EMN and NYSM + NMN), there was significant difference in the production of mosquitocidal metabolites (P<0.05), as evident from the LC50 dosage. However, there was no significant difference in the 95 per cent confidence interval between the media containing 1 and 2 per cent glucose indicating that higher levels of glucose did not have a significant impact on the production of the mosquitocidal metabolite.
Among the six media used, the combination NYSM + NMN + 1 per cent glucose was found to be optimal for the production of the metabolite. In this medium, the LC50 dose required for larva (5.57 μl/ml) and pupa (0.71 μl/ml) of An. stephensi was almost half of that required when the metabolite was produced in the original medium i.e., NYSM + EMN (LC50 values 10.6 μl/ml and 1.3 μl/ml). However, the LC50 dose requirement of NYSM + NMN + 1 per cent glucose, for inciting 50 per cent mortality in the larvae (5.57 μl/ml) of An. stephensi was found to be 7.84 times higher than that required for the pupae of the same species (0.71 μl/ml).
Production of CMM in different culture media: It was observed that upto a concentration of 1 per cent glucose in the medium the production of mosquitocidal metabolites kept increasing. This trend was same in NYSM + EMN and NYSM + NMN. The yield of the metabolite in the media containing NYSM + EMN was 1.62 g/l compared to 2.05 g/l in the combination, NYSM + NMN. Among the different concentrations of glucose used, 1 per cent glucose gave the maximum yield of CMM, 2 g/l with the combination, NYSM + EMN and 2.48 g/l with the combination, NYSM + NMN. The LC50 dose of the CMM produced in the latter medium was found to be 2.2 g/l as against 2.6 g/l required for that produced in the same combination containing EMN (Fig. 1).
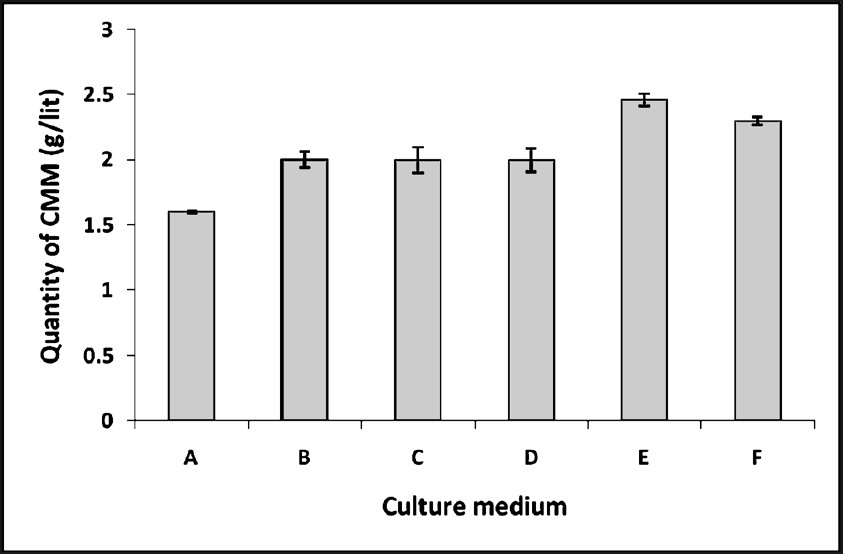
- Production of crude mosquitocidal metabolite (CMM) in different culture media by B. subtilis subsp. subtilis.
MALDI-TOF mass spectrometric analysis: MALDI-TOF spectrum of CMM showed well-resolved groups of peaks at m/z values between 1000 and 1080 (Fig. 2). The mass peaks obtained were at m/z 1030.6, 1046.7, 1044.6, 1052.6, 1058.7, 1060.7, 1066.7 and 1074.8.
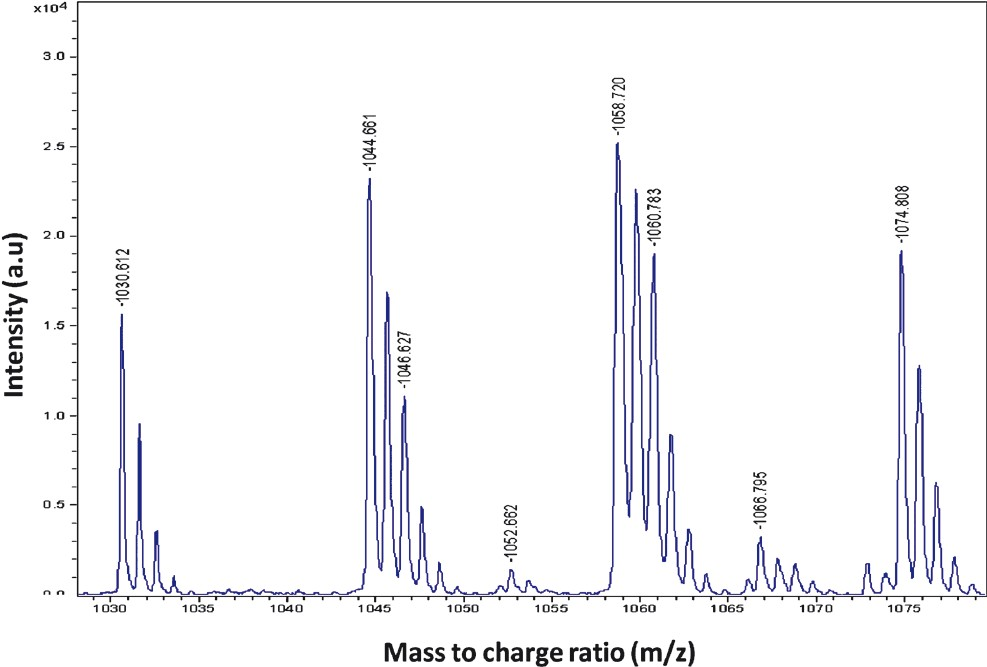
- MALDI-TOF spectrum of crude mosquitocidal metabolite (CMM) produced by B. subtilis subsp. subtilis
Discussion
Very few reports exist on the anti-mosquitocidal activity exhibited by strains of B. subtilis1516. Unlike in the case of B. thuringiensis var. israelensis and B. sphaericus, where the spore crystal complex is known to be larvicidal, the cyclic lipopeptides (CLPs) present in the culture supernatant of B. subtilis have been reported to be responsible for mosquito larvicidal property16. The CLPs of our strain however, were found to be effective both on the larval as well as pupal stages of various species of mosquitoes9.
Trace elements and carbon level in the culture medium have been reported to increase the lipopeptide production in strains of B. subtilis1217. Using a statistical experimental design (Taguchi method), Wei et al12 have optimized the trace element composition for surfactin production by B. subtilis. These trace elements (micronutrients) were incorporated into the production media (NYSM) used in this study. As observed by them, replacement of the new trace elements (NMN) was found to increase the yield of the metabolite with subsequent increase in the mosquitocidal activity. Among various carbon substrates, glucose is reported to increase the yield of lipopeptide17. Hence, the conventional medium NYSM was supplemented with glucose at 1 and 2 per cent level. It was observed that metabolites produced in NYSM + NMN + 1 per cent glucose, showed highest mosquitocidal activity and highest metabolite production. Since the metabolites produced in the six culture media were not quantified and tested for mosquitocidal activity, the LC50 values are given in terms of μl/ml. In the next step, this needs to be done. The effect of glucose on surfactin production is rather indirect. Phosphotransacetylase (pta) is an enzyme required for the conversion of the end product acetyl coA to acetyl phosphate in the glycolysis pathway. The latter compound is a phospho donor responsible for activating the ComA protein required for the transcription of the srf operon which is essential for the production of surfactin by B. subtilis strains18. The expression of this pta enzyme was found to require high levels of glucose in the growth medium which might be the reason for the enhancement in the production of mosquitocidal metabolite in media containing high levels of glucose.
The purified mosquito pupicidal metabolite from B. subtilis subsp. subtilis was earlier identified as a cyclic lipopeptide, surfactin by IR, NMR and MALDI-TOF analysis19. This study also showed that the CMM when subjected to MALDI-TOF analysis exhibited peaks indicative of surfactin. The group of peaks could be attributed to the isoform ensembles of surfactins produced by the mosquitocidal strain. It is clear that the cyclic lipopeptide surfactin is the main component produced by our strain. Surfactin is one of the most powerful biosurfactants known for reducing the surface tension of water from 72 to 27 mN/m and reduction to levels 41-31 mN/m have been found to result in total pupal mortality172021. Pupae of mosquitoes depend on atmospheric oxygen for respiration. Due to reduction in surface tension of water by surfactin, the pupae were unable to come up to the surface of water for oxygen and had to remain submerged in water, followed by death22. Hence, the mosquito pupal mortality observed in our study could be primarily by reduction in the surface tension of the water caused by the biosurfactant, surfactin. However, the possibility of its action on the cuticle of the pupae cannot be ruled out as there are reports on the action of surfactin on biological membranes23–25.
The larvicidal cyclic lipopeptides produced by B. subtilis is reported to be non-toxic to fishes and aquatic organisms16. Toxicological tests viz. acute oral toxicity/pathogenicity tests conducted in Wistar rats and primary skin irritation test conducted in rabbits using CMM showed that it is non-toxic and safe to mammals as there was no adverse effect on body weight, food consumption, rectal temperature, haematological and biochemical parameters. This part of the study was done by outsourcing at International Institute of Biotechnology and Toxicology (IIBAT), Padappai, Tamil Nadu, India (VCRC, unpublished data). This bacterium assumes great importance as the first ever reported spore forming bacterium possessing activity against the pupal stage, a non feeding stage in the life cycle of a mosquito. Further, unlike the toxins of the commonly used biolarvicides, B. thuringiensis subsp. israelensis and B. sphaericus, this mosquitocidal metabolite was found to withstand wide range of water temperatures, pH, exposure to long hours of sunlight, UV radiation as well as moist heat, upto 121°C26. However, for use in operational control programmes it has to be made into a suitable formulation and currently studies are underway for designing an appropriate formulation as well as for developing a cheap production medium based on inexpensive and locally available raw materials.
Authors acknowledge Dr P. Jambulingam, Director, Vector Control Research Centre for the support and the facilities provided, and thank Dr Joachim Vater, Institut für Chemie, Arbeitsgruppe Biochemie und Molekulare Biologie, Technische Universität Berlin for MALDI-TOF analysis of the sample. This study was supported by a research grant from the Department of Science and Technology (DST), New Delhi. The Senior Research Fellowship provided by DST to the third author (SB) is gratefully acknowledged.
References
- Mosquito control potential of Bacillus thuringiensis subsp. israelensis and Bacillus sphaericus. ICMR Bull. 1995;25:45-51.
- [Google Scholar]
- Development of a Bacillus sphaericus tablet formulation and its evaluation as a larvicide in the biological control of Culex quinquefasciatus. Mem Inst Oswaldo Cruz. 2005;100:431-4.
- [Google Scholar]
- Long-lasting effects of a Bacillus thuringiensis serovar. israelensis experimental tablet formulation for Aedes aegypti (Diptera: Culicidae) control. J Econ Entomol. 2006;99:1590-5.
- [Google Scholar]
- Resistance in a laboratory population of Culex quinquefasciatus (Diptera: Culicidae) to Bacillus sphaericus binary toxin is due to a change in the receptor on midgut brush-border membranes. Eur J Biochem. 1995;228:206-10.
- [Google Scholar]
- Documentation of high-level Bacillus sphaericus 2362 resistance in field populations of Culex quinquefasciatus breeding in polluted water in Thailand. J Am Mosq Control Assoc. 2004;20:405-11.
- [Google Scholar]
- Factors affecting the insecticidal activity of δ-endotoxin of Bacillus thuringiensis. J Invertebr Pathol. 1977;29:162-9.
- [Google Scholar]
- Suppression of Bacillus thuringiensis δ-endotoxin activity by low alkaline pH. J Invertebr Pathol. 1992;60:47-52.
- [Google Scholar]
- Biolarvicides in vector control: challenges and prospects. J Vector Borne Dis. 2003;40:20-32.
- [Google Scholar]
- Characterisation of three mosquitocidal Bacillus strains isolated from mangrove forest. Biol Control. 2007;42:34-40.
- [Google Scholar]
- Mosquito pupicidal toxin production by Bacillus subtilis subsp. subtilis. Biol Control. 2008;44:242-7.
- [Google Scholar]
- Localization of a mosquito-larval toxin of Bacillus sphaericus 1593. Appl Environ Microbiol. 1980;39:1205-11.
- [Google Scholar]
- Using taguchi experimental design methods to optimize trace element composition for enhanced surfactin production by Bacillus subtilis ATCC 21332. Process Biochem. 2007;42:40-5.
- [Google Scholar]
- WHO. In: Guidelines for laboratory and field testing of mosquito larvicides. WHO/CDS/WHOPES/GCDPP/2005.13. Geneva: WHO; 2005.
- [Google Scholar]
- A method for computing the effectiveness of an insecticide. J Econ Entomol. 1925;27:265-7.
- [Google Scholar]
- Efficacy of Bacillus subtilis against mosquito larvae (Anopheles culicifacies) Z Angewandte Zool. 1989;6:85-91.
- [Google Scholar]
- Assessment of mosquito larvicidal potency of cyclic lipopeptides produced by Bacillus subtilis strains. Acta Trop. 2006;97:168-73.
- [Google Scholar]
- Enhanced production of surfactin from Bacillus subtilis by continuous product removal and metal cation additions. Appl Environ Microbiol. 1981;42:408-12.
- [Google Scholar]
- Involvement of acetyl phosphate in the in vivo activation of the response regulator ComA in Bacillus subtilis. FEMS Microbiol Lett. 2001;195:179-83.
- [Google Scholar]
- Identification and characterization of a mosquito pupicidal metabolite of a Bacillus subtilis subsp. subtilis strain. Appl Microbiol Biotechnol. 2010;86:1737-44.
- [Google Scholar]
- Ovicidal activity of aliphatic amines and petroleum oil against two species of mosquitoes. J Econ Entomol. 1968;61:510-5.
- [Google Scholar]
- Antimycoplasma properties and application in cell culture of surfactin, a lipopeptide antibiotic from Bacillus subtilis. Appl Environ Microbiol. 1997;63:44-9.
- [Google Scholar]
- Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus subtilis. Biologicals. 1997;25:289-97.
- [Google Scholar]
- Surfactin-triggered small vesicle formation of negatively charged membranes: A novel membrane-lysis mechanism. Biophys J. 2008;95:3840-9.
- [Google Scholar]
- Surfactin: a novel mosquitocidal biosurfactant produced by Bacillus subtilis sp. subtilis (VCRC B471) and influence of abiotic factors on its pupicidal efficacy. In: Lett Appl Microbiol. Vol 51. 2010. p. :406-12.
- [Google Scholar]






