Translate this page into:
Endometrial serous carcinoma: A retrospective review of histological features & their clinicopathological association with disease-free survival & overall survival
For correspondence: Dr Kedar K. Deodhar, Department of Pathology, Tata Memorial Hospital, Homi Bhabha National Institute, Mumbai 400 012, Maharashtra, India e-mail: kedardeodhar@hotmail.com
-
Received: ,
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Endometrial serous carcinoma (ESC) is a high-grade epithelial neoplasm with increased risk for metastasis and recurrence. This study was aimed to assess various histomorphological features of ESC and their clinicopathological association with disease-free survival (DFS) and overall survival (OS).
Methods:
A total of 205 slides (belonging to 120 patients) diagnosed as ESC from January 2009 to December 2015 were reviewed. Receiver operating characteristics (ROC) curves were established for the diagnostic performance of depth of invasion (DOI), tumour-free distance (TFD) to serosa and percentage myometrial invasion (MI%). OS and DFS were generated by Kaplan-Meier curves and prognostic significance by Cox regression analysis.
Results:
The mean age at diagnosis was 61.8 yr and the mean tumour size was 4.01 cm. Majority of the females were multiparous (84%; n=94) and postmenopausal (89.2%; n=107). On histopathology, <50 per cent of MI was identified in 37 of the 104 (35%), while 62/104 (59.61%) patients had ≥50 per cent MI. Seven (6.7%) patients had full-thickness invasion with serosal involvement, while five (4.8%) patients had no microscopic MI (minimal uterine serous carcinoma). Information about MI was not available in 16 patients. TFD ≥7.0 mm, DOI ≥6.0 mm and MI% ≥40 were significant variables in univariate analyses for OS; however, on multivariate analysis; none of these turned out to be an independent predictor in terms of OS. For DFS, DOI (≥6.0 mm) and MI% (≥40%) showed a significant association, in univariate as well as multivariate analysis; however, TFD (≤7.0 mm) did not show any significant association with DFS. Follow up data were available in 111 of the 120 (92.5%) patients with a five-year OS and DFS of 22.2 and 17.2 per cent, respectively.
Interpretation & conclusions:
Conventionally calculated DOI (less than or more than half thickness) did not show significance in the present study. Thus, calculating the actual myometrial DOI, MI% and TFD to serosa have the potential for contributing meaningfully to prognostication of ESC.
Keywords
Endometrium
ESC
DOI
MI%
myometrial invasion
MI%
prognosis
serous carcinoma
tumour-free distance
Endometrial cancer (EC) is the most common gynaecologic malignancy and the fourth most common cancer in women worldwide, comprising six per cent of female cancers in developed countries. It is the third most common malignancy in Indian women1-3. Endometrial serous carcinoma (ESC) or serous uterine cancer is an aggressive subtype of endometrial carcinoma with a unique epidemiologic profile and clinicopathologic behaviour. It is capable of metastasis and extrauterine spread even as endometrial intraepithelial carcinoma (EIC) (in situ serous carcinoma). The estimated five-year overall survival (OS) for patients with ESC is 18-27 per cent, and approximately 60-70 per cent of women with ESC present with disease outside the uterus3,4. Therefore, its management differs from the endometrioid type adenocarcinomas. The present study was aimed to assess various histomorphological features of ESC and their clinicopathological association (whether any parameter can be a predictor) with disease-free survival (DFS) and OS.
Material & Methods
The present retrospective study was carried out in the department of Surgical Pathology, Tata Memorial Hospital, Mumbai, India. A total of 205 slides (belonging to 120 patients), diagnosed as ESC from January 2009 to December 2015 were retrieved and reviewed after approval from the Institutional Review Board. A waiver of consent was granted since this was a retrospective slide review and checking available medical records with no intervention or risk to patient safety.
Apart from age, clinical parameters extracted were parity, menstruation status, systemic diseases, presenting complaints, family history of malignancy, radiology findings (endometrial thickness), tumour marker (CA-125) levels, Pap smear findings, etc. Patients were considered menopausal if there were no menstrual cycles for at least 12 months. Serum cancer antigen 125 (CA 125) levels of more than 35 IU/ml were considered elevated. Pap smear result of glandular cell abnormality and above was taken as a positive result. Various histological parameters were assessed, such as myometrial invasion (MI) – less than half or equal to or more than half of myometrial thickness, depth of myometrial invasion (DOI; absolute and in percentage), tumour-free distance (TFD) to serosa, lymphovascular emboli, perineural invasion, lymph node status and stage. The fimbrial end of the fallopian tube was sampled in all the cases; however, extensive sectioning of all fallopian tubes using the Sectioning and Extensively Examining the Fimbriated End (SEE-FIM) Protocol5 was not carried out. Available immunohistochemistry slides were also reviewed. Electronic medical records and charts were examined for clinical details and follow up. All patients diagnosed other than serous carcinoma on histology were excluded from the study including grade 3 endometrioid adenocarcinoma based on histology and supportive immunohistochemistry; however, mixed histology with serous component >10 per cent was included in the study.
The depth of myometrial invasion (DOI) was defined as the distance between the endometrial junction and the deepest point of myometrial invasion (Fig. 1). TFD indicates distance between the deepest myometrial invasive focus and the closest serosal surface (Fig. 1), while the percentage of myometrial invasion (MI%) was calculated as MI%=DOI×100/(DOI+TFD).
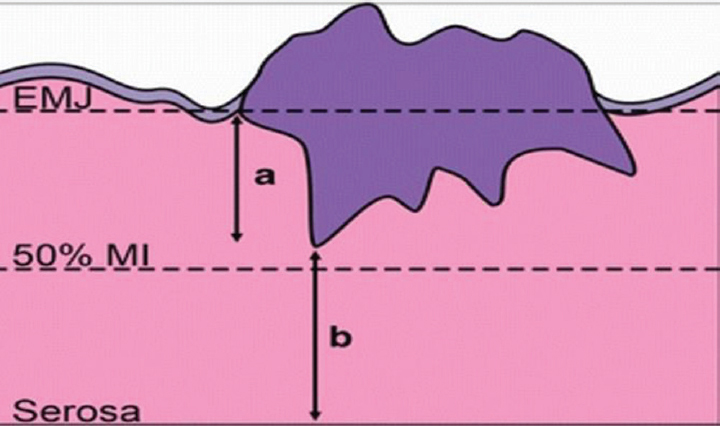
-
Figure showing pictographic representation of depth of invasion (DOI); tumour-free distance (TFD) and myometrial invasion percentage (MI%). a, depth of invasion; b, tumour-free distance from serosa; EMJ, endomyometrial junction.
Recurrence of disease was defined as presence of any abdominal, pelvic or distant disease confirmed by radiological imaging and/or pathologic examination. DFS was calculated in months from the date of primary tumour removal surgery to the date of recurrence or date of disease-free follow up. The OS and DFS were calculated from the date of the surgery; these two variable were the primary endpoints evaluated. Receiver operating characteristic (ROC) analyses were conducted to evaluate the diagnostic performance of DOI, TFD and MI% for the prediction of recurrent disease and OS.
Statistical analysis: All statistical analyses were performed using the Statistical Package for the Social Sciences version 20.0 (SPSS Inc., Chicago, IL, USA). Associations between categorical variables were evaluated using Chi-squared test, and for continuous variables; the t test or the Mann-Whitney U test was used. The independent effects of pathologic and treatment variables on OS was determined by Cox regression analysis and Fisher’s exact test. OS and DFS were estimated using Kaplan-Meier curves.
Results
Clinical parameters: A total of 120 patients of ESC were reviewed. The mean and median age at diagnosis was 61.8 and 63 yr, respectively, with 90.8 per cent (n=109) patients over 50 yr of age. ESC was more common in multiparous women (n=94; 84%). Majority of the women were postmenopausal (n=107; 89.2%). Vaginal bleeding was the most common presentation in 74 of the 120 (61.66%) patients. Other complaints included pain in abdomen in 30 of 120 (25%) patients, vomiting in 27 (23%), vaginal discharge in 13 (11%), ascites in five (4.2%) and supraclavicular lymph node enlargement in one patient. Concurrent ovarian malignancy was present in three (2.5%) patients. The patients’ characteristics, their frequency and recurrence information are given in Table I.
| Variables | Frequency, n (%) | Recurence | P | Death | P |
|---|---|---|---|---|---|
| Age (yr) (n=120) | |||||
| <50 | 11 (9.2) | 5 | 0.161 | 5 | 0.190 |
| ≥50 | 109 (90.8) | 69 | 65 | ||
| Parity (n=120) | |||||
| Multiparous | 94 (84) | 59 | 0.891 | 30 | 0.195 |
| Nulliparous | 9 (8) | 4 | 4 | ||
| Uniparous | 9 (8) | 6 | 5 | ||
| Information not available | 8 (6.67) | ||||
| MH (n=120) | |||||
| Postmenopausal | 107 (89.2) | 66 | 0.966 | 62 | 0.826 |
| Premenopausal | 13 (10.8) | 8 | 8 | ||
| Serum CA125 levels (n=35) | |||||
| Raised serum CA125 levels (Normal range: 0-35 units/ml) | 35 (29.1) | 15 | 0.846 | 5 | 0.885 |
| Abnormal cytology (n=42) | |||||
| Positive | 20 (47.6) | 10 | 0.404 | 10 | 0.764 |
| Negative | 22 (52.4) | 12 | 11 | ||
| ET (n=66) (mm) | |||||
| ≤4 | 10 (15.1) | 5 | 0.565 | 3 | 0.464 |
| >4 | 56 (84.8 | 36 | 23 |
ET, endometrial thickness; MH, menstrual history
International Federation of Gynaecology and Obstetrics (FIGO) stage: FIGO stage information was available in 116/120 (96.6%) patients. Staging and its relation to recurrence, DFS, and OS are given in Table II. A significant association was observed between stage IV disease and DFS and OS, with P<0.05 (Table II).
| DFS | OS | |||||
|---|---|---|---|---|---|---|
| FIGO stage | Events (n=recurrence) | Median DFS (months) | P | Events (n=dead) | Median OS (months) | P |
| I | 16/35 | 29.733 | 0.181 | 14/35 | 31.939 | 0.505 |
| II | 2/7 | - | 0.181 | 2/7 | 0.505 | |
| III | 25/37 | 19.975 | 0.887 | 23/37 | 14.050 | 0.288 |
| IV | 31/37 | 8.181 | 0.002 | 31/37 | 5.773 | 0.001 |
| Overall | 74/116 | 17.183 | 0.001 | 70/116 | 16.982 | 0.001 |
DFS, disease-free survival; OS, overall survival
Histological parameters: The mean tumour size was 4.01 cm (range: 0.2-8 cm.). The most common location of tumour was the body, followed by fundus and isthmus of the uterus. In about 30 per cent (n=36) of the patients, there was simultaneous involvement of the fundus, body and isthmus. Information about the gross appearance of the tumour was available in 91 patients. Majority of the tumours were of proliferative (47, 51.6%) type, followed by polypoidal (29, 23%) and infiltrative (13, 14.2%) variety. On microscopy, various tumour growth patterns were observed, the most common being mixed pattern (58.3%) followed by pure papillary pattern (34%), solid (5.8%) and diffuse glandular patterns (1.6%). In our study, 81 of the 120 cases (67.5%) showed pure serous histology and 39 (32.5%) showed mixed histology, of which serous carcinoma with endometrioid component was seen in 34 of 39 (87.1%), while serous carcinoma with clear cell carcinoma was present in five of 39 (13%) (more than 10% of the other component was taken as mixed carcinoma). Histology (pure serous histology or mixed histology) and nuclear grade (high or moderate) had no significant association with DFS and OS (Fig. 2).
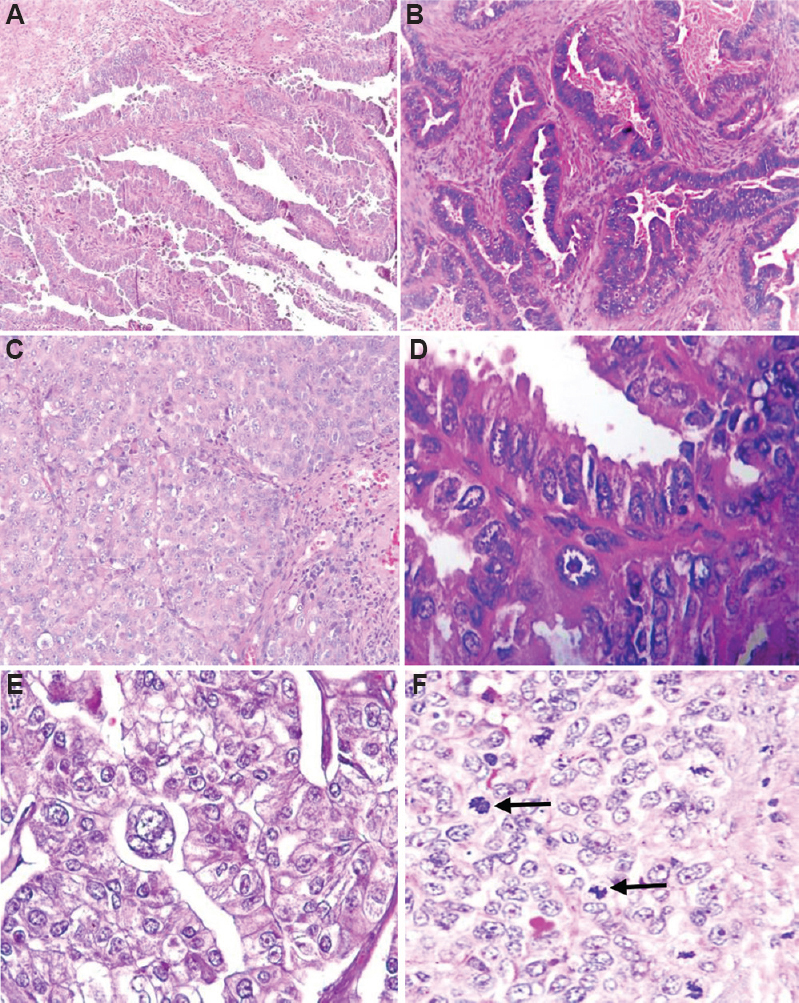
- Histological pattern (A) papillary (H & E, x100); (B) glandular (H & E, x200); (C) solid (H & E, x200); (D) nuclear pleomorphism; (E) multinucleate giant cells and; (F) mitosis (black arrow; H & E, x400).
Associated endometrial polyp was seen in 39 of the 120 (32.5%) cases. Significant association (P<0.05) was found between EIC and polyp. Of the total 48 cases with EIC, in situ carcinoma arising in the background of polyp was seen in 52 per cent (n=25) patients. (Fig. 3). Other histological features are summarized in Table III.
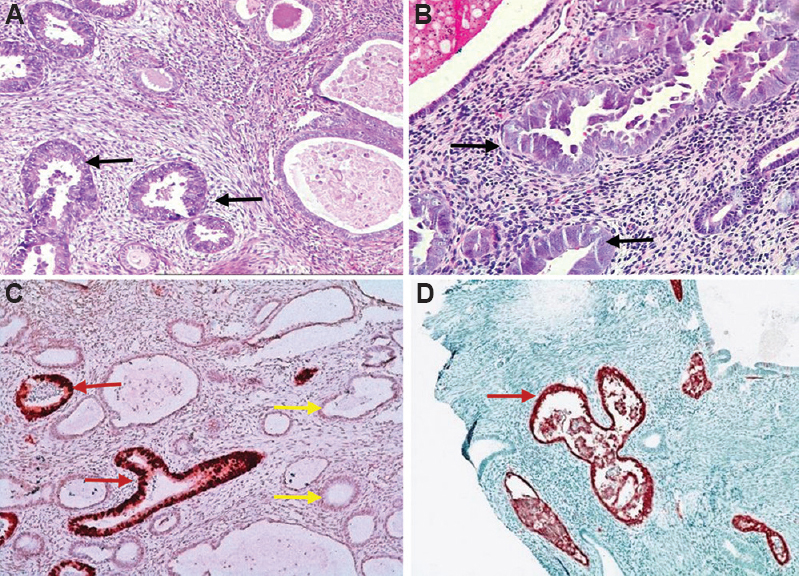
- (A and B) Endometrial intraepithelial carcinoma arising in an endometrial polyp (black arrow; H & E, x200); (C and D) strong expression of p53 protein in the neoplastic glands (red arrow). Weak staining pattern is noted in the benign glands (yellow arrow).
| Histological parameters | DFS | OS | |||||
|---|---|---|---|---|---|---|---|
| n=120, n (%) | Recurrence | Median DFS (months) | P | Death | Median OS (months) | P | |
| Macro nucleoli | |||||||
| Present | 115 (96) | 73 | 21.979 | 0.906 | 73 | 24.914 | 0.92 |
| Absent | 5 (4.2) | 2 | 2 | 14.752 | |||
| Giant cells | |||||||
| Present | 96 (80) | 62 | 22.538 | 0.662 | 62 | 24.914 | 0.925 |
| Absent | 24 (20) | 14 | 8.082 | 13 | 24.575 | ||
| Necrosis | |||||||
| Present | 94 (79) | 68 | 15.93 | 0.030 | 64 | 24.214 | 0.371 |
| Absent | 26 (21.6) | 08 | 11 | 37.224 | |||
| Psammoma bodies | |||||||
| Present | 68 (56.6) | 53 | 15.737 | 0.002 | 53 | 21.421 | 0.001 |
| Absent | 52 (43.3) | 23 | 48.394 | 22 | 40.411 | ||
| Mitosis | |||||||
| ≤20/10 hpf | 56 (46.7) | 32 | 25.955 | 0.842 | 33 | 21.979 | 0.800 |
| >20/10 hpf | 64 (53.3) | 43 | 24.575 | 43 | 19.975 | ||
| EIC | |||||||
| Present | 48 (40) | 32 | 17.183 | 0.061 | 32 | 22.603 | 0.020 |
| Absent | 72 (60) | 44 | 24.214 | 43 | 36.735 | ||
| Polyp | |||||||
| Present | 39 (32.5) | 25 | 22.702 | 0.678 | 23 | 24.914 | 0.727 |
| Absent | 81 (67) | 51 | 21.979 | 52 | 25.658 | ||
| LVI (n=104) | |||||||
| Present | 63 (60.5) | 43 | 19.975 | 0.472 | 43 | 24.246 | 0.207 |
| Absent | 41 (39.4) | 24 | 24.214 | 20 | 40.411 | ||
| Lymph nodes (n=58) | |||||||
| Involved | 34 | Pelvic LN | 20 | Pelvic + para-aortic LN | 14 | ||
| Not involved | 24 | ||||||
| Depth of invasion | n=120, n (%) | Correlation of LVI with depth of invasion | Present | Absent | |||
| Non-invasive | 5 (4.2) | 1 | 4 | ||||
| <50% MI | 37 (30.08) | 10 | 26 | ||||
| ≥50% MI | 62 (51.67) | 48 | 13 | ||||
| Unknown (not included in statistical analysis) | 16 (13.3) | 4 | 5 | ||||
LVI, lymphovascular invasion; EIC, endometrial in situ carcinoma; LN, lymph node; MI, myometrial invasion; DFS, disease-free survival; OS, overall survival
Immunohistochemistry: Immunohistochemistry revealed strong and diffuse positivity for p53 in around 84 per cent (n=94) of the patients, while four patients (3.6%) had null type expression. Heterogeneous staining pattern was observed in some, which corresponded to mixed histology. Four patients showed moderate intensity positivity in few cells and this corresponded to wild-type staining pattern of p53; P16 was available in five patients which showed diffuse positivity in all cases (Fig. 4 and Table IV).
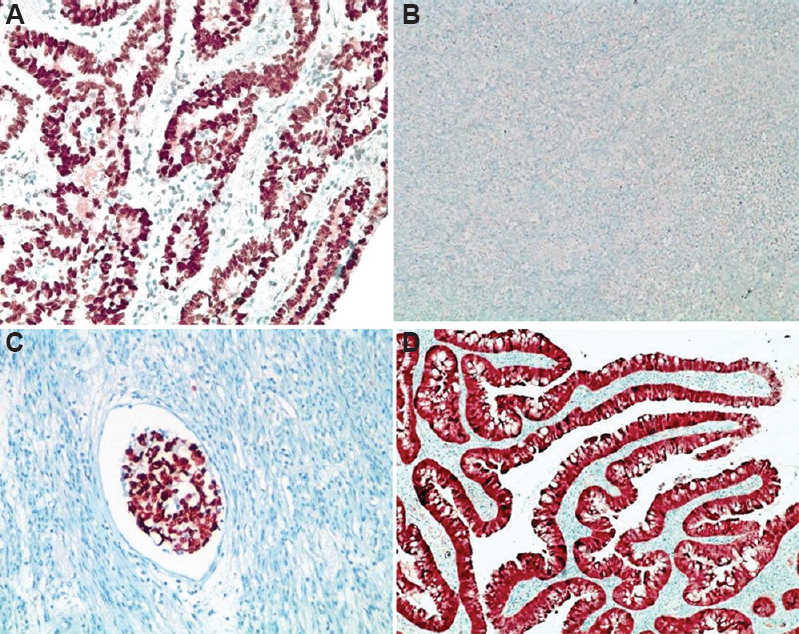
- (A) Diffuse and strong nuclear positivity for p53 protein (mutation type); (B) Absent staining of p53 protein (null type); (C) strong p53 expression in lymphatic emboli; (D) diffuse nuclear and cytoplasmic staining for p16.
| Immunohistochemistry | Percentage of positive cells, n (%) |
|---|---|
| P53 (n=112) | |
| Strong positive | 94 (83.9) |
| Absent staining (null type) | 4 (3.6) |
| Heterogeneous pattern | 10 (8.9) |
| Wild type | 4 (3.6) |
| P16 (n=5) | 5 (100) |
| WTI (n=80) | |
| Negative | 62 (80.5) |
| Focal weak positive | 15 (19.4) |
| Diffuse weak positive | 3 (3.8) |
| ER (n=81) | |
| Negative | 57 (70.3) |
| Focal weak positive | 16 (19.7) |
| Diffuse positive | 8 (10) |
| PR (n=67) | |
| Negative | 56 (83.5) |
| Focal weak positive | 9 (13.4) |
| Diffuse moderate positive | 2 (3) |
| MIB-1 (n=34) | Median-60 |
PR, progesterone receptor; ER, estrogen receptor; MIB-1, MIB E3 ubiquitin protein ligase 1; WT1, Wilms’ tumour 1
Myometrial invasion: The DOI was noted in 104 of the 120 (86.6%) patients (Table IV). Non-invasive tumour or minimal uterine serous carcinoma was seen in five of 104 (5%) patients, of which three (60%) patients showed distant metastasis and recurrence. There was a significant association between the DOI (≥50%) and lymphovascular invasion (P<0.001).
ROC curve for DOI, MI% and TFD for predicting recurrence of disease: The optimal cut-off value (OCOV) for DOI was 6.5 mm, with 89 per cent sensitivity and 74 per cent specificity; OCOV for MI% was 40 per cent with 90 per cent sensitivity and 80 per cent specificity and OCOV for TFD was 7.5 mm with 85 per cent sensitivity and 62 per cent specificity. Absolute values of 6 and 7 mm were recorded instead of 6.5 and 7.5 mm, as a measurement of 0.5 mm was not feasible in a practical setting. The accuracy of these cut-off values was analyzed by dividing the population into two groups: patients with <6.0 mm and ≥6.0 mm DOI and for MI% <40 and ≥40 per cent and, TFD divided ≤7.0 and >7.0 mm TFD. By using dichotomous values for DOI, MI% and TFD, the results could be compared with the conventional 50 per cent MI in correlation analyses with survival parameters.
In terms of OS, TFD ≥7.0 mm, DOI ≥6.0 mm and MI% ≥40 per cent were significant variables in univariate analyses; however, on multivariate analysis, none of the above-mentioned factors were independently associated in terms of OS. In terms of DFS, DOI (≥6.0 mm) and MI% (≥40%) showed a significant association, in univariate as well as multivariate analysis; however, TFD (≤7.0 mm) did not show any significant association with DFS (Fig. 5 A-H).
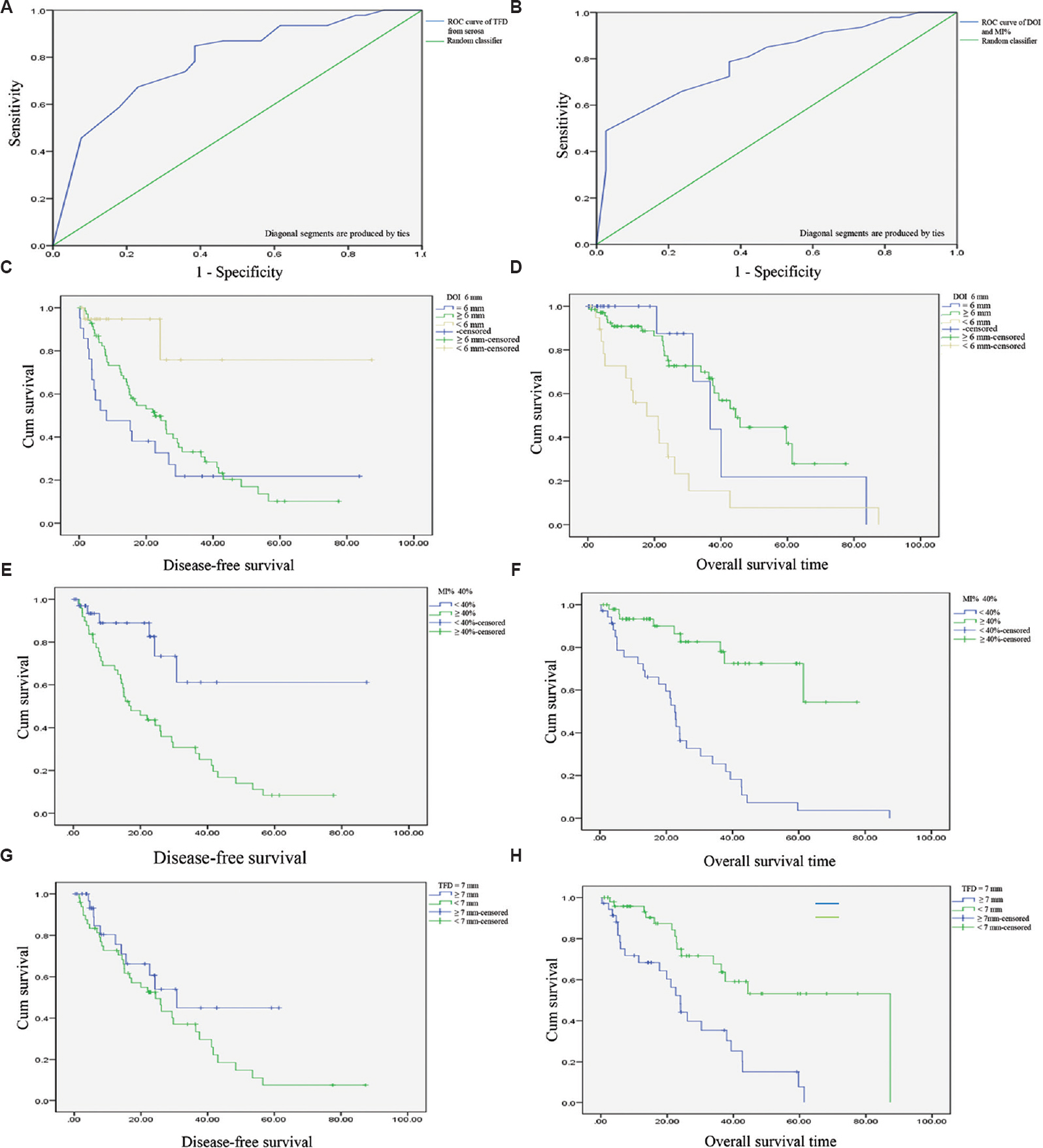
- ROC curve of (A) tumour-free distance from serosa predicting overall survival of disease; (B) depth of invasion and absolute percentage myometrial invasion predicting survival of disease; Kaplan-Meier curve for (C) depth of invasion ≥6.0 mm vs. disease-free survival; (D) overall survival vs. depth of invasion ≥6.0 mm; (E) absolute percentage myometrial invasion ≥40% vs. disease-free survival; (F) overall survival vs. percentage myometrial invasion ≥40%; (G) tumour-free distance ≤7.0 mm vs. disease-free survival; (H) tumour-free distance ≤7.0 mm vs. overall survival.
The conventional DOI was also noted in the study, however, no significant association was found in terms of survival analysis in these groups of the DOI (Table V).
| Histological parameters | Recurrence | Median DFS | P | Death | Median OS | P |
|---|---|---|---|---|---|---|
| DOI (6.0 mm) (n=99) | ||||||
| <6.0 | 2 | 26.2 | 0.016 | 40 | 44.3 | <0.001 |
| ≥6.0 | 49 | 8.1 | 5 | 36.7 | ||
| MI% (40%) (n=99) | ||||||
| <40 | 6 | 17.1 | 0.002 | 30 | 22.6 | <0.001 |
| ≥40 | 40 | 25.9 | 9 | 37.5 | ||
| TFDS (7.0 mm) (n=99) | ||||||
| ≤7.0 | 35 | 24.4 | 0.160 | 15 | 87.3 | <0.001 |
| >7.0 | 11 | 30.1 | 24 | 22.6 | ||
| Conventional DOI (n=99) | ||||||
| <50%MI (n=37) | 17 | 15.9 | 0.537 | 16 | 30.3 | 0.552 |
| ≥50% MI (n=55) | 36 | 22.5 | 18 | 59.1 | ||
| Serosal involvement (n=7) | 5 | 25.9 | 2 | 36.7 |
Cases without MI (n=5) and those in which MI could not be calculated (n=16) were excluded. MI, myometrial invasion; DFS, disease-free survival; OS, overall survival; DOI, depth of invasion; TFDS, tumour free distance to serosa
Locoregional spread: Locoregional spread was present in 76 of 104 (73%) patients. Of these 23 (30%) had adnexal involvement, two (3%) had vaginal involvement, 20 patients (26%) showed pelvic lymph-node involvement and 14 (18%) had additional para-aortic lymph-node involvement. Cervix was involved in 42.3 per cent (44/104) patients, microscopically.
The lymph node dissection was done in 58/104 (56%); 20/58 (34%) patients showed pelvic nodes metastasis, while 24 per cent (14/58) patients had metastasis to both pelvic and para-aortic lymph nodes. Peritoneal deposits were seen in eight (10.5%) and direct involvement of urinary bladder and bowel was present in nine patients (11.8%).
Sixty three per cent (65/103) patients had distant metastasis of tumour to one or more organs, omentum being the most preferred site in 21 (32%) patients. Liver and lung metastases were found in five (7.6%) and four (6%) out of the 65 patients, respectively. Supraclavicular nodes were involved in three out of 65 (4.6%) patients. Overall locoregional spread to the peritoneal organs and distant metastasis as well as omental involvement was found to be significant in terms of DFS and OS.
Treatment characteristics: Of the total 120 patients, 95 (79.1%) patients underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy. Adjuvant chemotherapy and radiotherapy were given in 54 (45%) patients. Longer survival (median 19 months) was noted in patients with stage IV disease who received chemotherapy (with or without radiotherapy) compared to the non-chemotherapy group (median three months) (P<0.05). However, no benefits were noted in lower stage disease.
Follow up information: Follow up information was available for 111 of the 120 patients. All patients were followed up every three months in the first year of the disease, followed by six-monthly follow up. In case of any adverse events, the patients were asked to report immediately for appropriate management. The overall mean and median follow up period was 23.1 months and 24.3 months, respectively (range: 0-87.3 months; median follow up time was 26 months). On follow up, 74/111 (67%) patients had a recurrence, while 37/111 (33.3%) did not show any recurrence. Recurrence was assessed on imaging studies. Recurrence pattern was disseminated/widespread involvement of more than one organ such as small intestine, multiple lymph nodes, omentum, vaginal wall and bones in majority of the cases. OS of the patients was affected by the presence and absence of recurrence.
Survival analysis: The mean DFS was 29.3 months and median was 19.7 months (95% confidence interval, 2-87.3 months); and mean and median OS was 31 and 26 months, respectively. Seventy out of 111 (63%) patients ended up with death as a terminal event. Forty one (37%) patients were alive until the date of the last follow up. Five year OS and DFS was 22.2 and 17.2 per cent, respectively.
Using a Cox proportional hazards model, on multivariate or univariate analysis for DFS, MI% (40%), DOI (6.0 mm), overall FIGO stage, psammoma bodies and locoregional spread were all independent predictors of poorer survival. However, distant metastasis, omentum involvement and necrosis were not significant independent prognostic factors for DFS.
Using a Cox proportional hazards model on multivariate analysis for OS, presence of psammoma bodies (P<0.04), EIC (P<0.050) and recurrence were (P<0.015) independent predictors of poorer survival. However, MI% (40%), DOI (6.0 mm), TFD (7.0 mm), FIGO stage group and locoregional spread, peritoneal washing and overall distant metastasis were not significant independent prognostic factors.
Discussion
Endometrial serous carcinoma commonly occurs in elderly, postmenopausal, and multiparous women, with mean age ranging from 59 to 72 year1-3 similar to the findings of the present study. Abnormal cervical cytology was recorded in 62 per cent (20/42) patients in our study, a finding similar to that noted in a study comprising 101 high-grade ECs including ESC6. Raised CA 125 have been described to have a significant association with worse outcomes (OS and DFS) in the study by Gupta et al7. However, in the present study, such association was not observed, which could be due to fewer occurrence in this study. Similarly, ET ≥4 mm also did not show any significant association with survival outcomes.
Mixed histology with the presence of ESC as a minor component as <10 per cent can be associated with a poorer prognosis when compared with tumours of pure grade 3 endometrioid histology1,8. However, the present investigation displayed similar aggressive behaviour whether pure serous or mixed serous carcinoma (no significant difference in DFS as well as OS). We recognize that the definition of mixed carcinomas has changed and even a serous carcinoma component of five per cent or more will constitute a label of mixed carcinoma9. There were 39 cases with mixed histology in our study which included endometrioid carcinoma (n=34) and clear cell carcinoma (n=5). In a study by Hagemann et al10 on 934 women with uterine serous carcinoma; the group demonstrated that the presence of a non-serous component in a mixed histology did not alter the survival of the patients and it was the serous component which drove the prognosis/survival of the patients. Similarly, Kaban et al11 analyzed 93 cases of ESC including mixed histology and reported that pure serous histology had the worst prognosis.
Hendrickson et al8 described psammoma bodies in one third of the cases of uterine serous carcinomas. Psammoma bodies were also frequently seen at the metastatic sites in ESC cases in a study by Nag et al12. In the present study, psammoma bodies were seen in >50 per cent of the patients (P<0.05) with poor DFS and OS in univariate analysis but not on multivariate analysis.
Sherman et al4 and Kawata et al13 suggested that EIC was the major precursor of serous endometrial carcinoma seen in 100 per cent of ESC with concurrent EIC. In the present study, we observed 48 out of 120 patients with EIC in the adjacent endometrium as well as five cases of non-invasive carcinoma. Three of these five patients (60%) showed distant metastasis without demonstrable invasion in the myometrium. Dunton et al14 reported surgically identifiable metastasis in 30-63 per cent of EIC cases.
The World Health Organization has included EIC as a separate entity (subtype) in carcinoma group1. In our study, EIC also showed a significant association with OS in both univariate and multivariate analyses. Hence, it is an independent factor for survival. Notably, 64 per cent (25/39) of our cases with in situ carcinoma developed in an endometrial polyp. A significant association (P<0.05) was found between the occurrence of EIC and polyp. Hui et al15 reported minimal uterine serous carcinomas involving the endometrial polyp in 88 per cent of the cases and those were confined to the polyp in 53 per cent. Thus, a significant majority of stage IA uterine serous carcinomas are limited to the endometrial polyp.
TFD (≥7.0 mm), DOI (≥6.0 mm) and MI% (≥40%) were significant variables in univariate analyses for OS; however, these were not independent predictors in multivariate analysis. For DFS, DOI (≥6.0 mm) and MI% (≥40%) showed a significant association, in univariate as well as multivariate analysis; however, TFD (≤7.0 mm) did not show any significant relation with DFS. Geels et al16 and Ozbilen et al17 also maintained that DOI was a better predictor of survival performance than TFD and strongly correlated with clinicopathologic parameters than TFD and 50 per cent MI. This assessment, however, could be difficult in cases with irregular endomyometrial junction, exophytic tumour growth, adenomyosis, extensive leiomyomas and different patterns of myometrial invasion. Cervical stromal involvement and regional lymph-node involvement did not show any significant association with DFS and OS in our study, similar to the studies by Kulhan et al18 and Carcangiu and Chambers19.
Distant metastasis was present in 63 per cent patients in our investigation. Both DFS and OS showed significance with distant metastasis as well as omental involvement in univariate analysis. Al Husaini et al20 mentioned that distant metastasis was the most common site of relapse and it significantly affected the OS and DFS. The presence of overall extrauterine disease had a significant correlation with DFS and OS in univariate analysis. However, on multivariate analysis, only DFS was affected by the regional spread.
Immunohistochemistry revealed strong and diffuse positivity for p53 in 84 per cent (94/112) of the patients, while four cases (3.6%) had absent staining (null type expression). Both these staining patterns are considered as capable of detecting mutation types. Heterogeneous (strong nuclear expression in some foci, whereas weaker expression in some foci) staining pattern was observed in some cases, which explained the mixed histology. Moll et al21 had 85 per cent tumours showing intense nuclear overexpression of p53 (abnormal staining), whereas six tumours were p53 wild type. We had four cases (3.6%) with p53 wild-type staining; p16 was done only in five cases and showed diffuse positivity in all cases.
Al Husaini et al20 and Acs et al22 reported diffuse and moderate to strong WT1 positivity in ovarian, tubal and primary peritoneal serous tumours. Hence, WT1 is useful in distinguishing ovarian serous carcinoma (diffuse strong nuclear positivity) from uterine serous carcinoma (usually negative). WT1 was performed in 80 (66.7%) cases and was negative in 62 (77.5%) cases. In the present study, 18 cases showed focal or diffuse weak positivity for WT1 which can be sometimes seen in ESC, similar to previous report23.
There was a significant association between DFS and OS with stage IV disease. In the present study, stage II had a better prognosis with a mean overall DFS of 43 months and OS of 44 months. It was also found that even patients with stage IA disease could have a poor outcome, as demonstrated by the fact that about 50 per cent (16/32) of the patients in this study with stage IA disease had recurrences/metastasis. Although patients with stage I-III and non-invasive tumours have a better prognosis than those with stage IV and more deeply invasive tumours24, our findings of a recurrence rate of 50 per cent in the stage IA group confirm that these tumours do not follow a slow-growing course4. Zhong et al25 in their retrospective study on ESC reported positive peritoneal cytology and myometrial invasion >50 per cent as independent prognostic factors for three-year DFS, recurrence and mortality.
Patients of stage IV disease receiving chemotherapy (with or without radiotherapy) had longer survival compared to the non-chemotherapy group (P<0.05). The median survival in our study for stage IV patients receiving chemotherapy was 19 months, as opposed to three months for those not receiving chemotherapy. Ramondetta et al26 demonstrated a 77 per cent overall response rate in patients with stage IV or recurrent ESC with paclitaxel alone. Slomovitz et al24 also recommend chemotherapy in patients of stage III and IV disease in their study on 129 patients. This was recommended by Geels et al16 as well. Systemic therapy had a survival benefit in patients with stage IV disease in our patients. For stage I, II or III diseases, the addition of chemotherapy did not affect DFS and OS. The patients who received radiotherapy showed a significant association with DFS; these findings are similar to the existing literature3,24. Holman et al27 studied 260 patients of advanced ESC and reported that those patients treated with a combination of surgery, chemotherapy and radiation, stage III disease and mixed histology had a better OS on multivariate analysis.
Overall median survival in the present study was 24.3 months, and overall five years survival was 22.2 per cent. The median DFS period was estimated to be 26 months with a five-year DFS of 17.2 per cent. del Carmen et al3 reported that the estimated five-year OS for patients with uterine serous carcinoma was 18-27 per cent, and even in cases where the disease was apparently confined to the corpus, the rate of recurrence was high (estimated to be 31-80%).
A retrospective design introduces bias in the present study. Many patients were operated outside our hospital. Imaging findings were not uniformly documented in files. A small sample size was also a limiting factor of our study. However, the strengths of the study include a relatively large number of cases of this uncommon variant, availability of electronic records for treatment and follow up information. Calculating myometrial invasion in a different way has shown interesting results, which could potentially change routine practice.
To conclude, ESC is an aggressive endometrial tumour and the present study did not show significance for the conventionally calculated DOI (less than half-thickness or more than half-thickness). Hence, reporting the actual DOI, MI% and TFD to serosa will be more useful for prognosis.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Female genital tumours:WHO classification of tumours (5th ed). Lyon: IARC; 2020.
- Gynecological cancers:A summary of published Indian data. South Asian J Cancer. 2016;5:112-20.
- [Google Scholar]
- Uterine papillary serous cancer:A review of the literature. Gynecol Oncol. 2012;127:651-61.
- [Google Scholar]
- p53 in endometrial cancer and its putative precursors:Evidence for diverse pathways of tumorigenesis. Hum Pathol. 1995;26:1268-74.
- [Google Scholar]
- Evaluation of SEE-FIM (Sectioning and Extensively Examining the FIMbriated End) Protocol in identifying fallopian tube precursor lesions in women with ovarian tumors. J Obstet Gynaecol India. 2019;69:153-9.
- [Google Scholar]
- Abnormal cervical cytology in the diagnosis of uterine papillary serous carcinoma:Earlier detection of a poor prognostic cancer subtype? Acta Cytol. 2011;55:255-60.
- [Google Scholar]
- Performance of serum CA125 as a prognostic biomarker in patients with uterine papillary serous carcinoma. Int J Gynecol Cancer. 2011;21:529-34.
- [Google Scholar]
- Uterine papillary serous carcinoma:A highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol. 1982;6:93-108.
- [Google Scholar]
- Endometrial cancer histopathology reporting guide. (3rd edition). Available from: http://www.iccr-cancer.org/ICCR/media/Documents/ICCR-Endometrium-3rd-ed-v3-1-bookmarked.pdf
- The presence of an endometrioid component does not alter the clinicopathologic profile or survival of patients with uterine serous cancer: A gynecologic oncology group (GOG/NRG) study of 934 women. Gynecol Oncol. 2021;160:660-8.
- [Google Scholar]
- Clinicopathologic and survival results in serous endometrium carcinoma and subgroup analysis for mixed serous and pure serous histology. J Turk Ger Gynecol Assoc. 2018;19:23-8.
- [Google Scholar]
- Bilateral primary fallopian tube papillary serous carcinoma in postmenopausal woman:Report of two cases. J Midlife Health. 2016;7:34-7.
- [Google Scholar]
- Serous endometrial intraepithelial carcinoma:Case report and literature review. J Clin Gynecol Obstet. 2017;6:49-52.
- [Google Scholar]
- Minimal uterine serous carcinoma:A clinicopathological study of 40 cases. Mod Pathol. 2005;18:75-82.
- [Google Scholar]
- Absolute depth of myometrial invasion in endometrial cancer is superior to the currently used cut-off value of 50%. Gynecol Oncol. 2013;129:285-91.
- [Google Scholar]
- Comparison of myometrial invasion and tumor free distance from uterine serosa in endometrial cancer. Asian Pac J Cancer Prev. 2015;16:519-22.
- [Google Scholar]
- Assessment of clinicopathological features, evaluation of treatment, and prognosis of clear cell and serous papillary endometrial carcinoma. Ginekol Pol. 2016;87:570-4.
- [Google Scholar]
- Uterine papillary serous carcinoma: A study on 108 cases with emphasis on the prognostic significance of associated endometrioid carcinoma, absence of invasion, and concomitant ovarian carcinoma. Gynecol Oncol. 1992;47:298-305.
- [Google Scholar]
- Evaluation of adjuvant therapy in women with uterine papillary serous cancer. Ann Saudi Med. 2012;32:27-31.
- [Google Scholar]
- Uterine papillary serous carcinoma evolves via a p53-driven pathway. Hum Pathol. 1996;27:1295-300.
- [Google Scholar]
- WT1 is differentially expressed in serous, endometrioid, clear cell, and mucinous carcinomas of the peritoneum, fallopian tube, ovary, and endometrium. Int J Gynecol Pathol. 2004;23:110-8.
- [Google Scholar]
- WT1, p53 and hormone receptor expression in uterine serous carcinoma. Histopathology. 2009;55:478-82.
- [Google Scholar]
- Uterine papillary serous carcinoma (UPSC):A single institution review of 129 cases. Gynecol Oncol. 2003;91:463-9.
- [Google Scholar]
- Prognostic factors of uterine serous carcinoma –A multicenter study. Int J Gynecol Cancer. 2018;28:1138-44.
- [Google Scholar]
- Treatment of uterine papillary serous carcinoma with paclitaxel. Gynecol Oncol. 2001;82:156-61.
- [Google Scholar]
- Factors prognostic of survival in advanced-stage uterine serous carcinoma. Gynecol Oncol. 2017;146:27-33.
- [Google Scholar]






